Abstract
Nanotechnology is emerging as a promising modality for cancer treatment; however, in the realm of cancer prevention, its full utility has yet to be determined. Here, we discuss the potential of integrating nanotechnology in cancer prevention to augment early diagnosis, precision targeting and controlled release of chemopreventive agents, reduced toxicity, risk/response assessment, and personalized point-of-care monitoring. Cancer is a multistep, progressive disease; the functional and acquired characteristics of the early precancer phenotype are intrinsically different from those of a more advanced anaplastic or invasive malignancy. Therefore, applying nanotechnology to precancers is likely to be far more challenging than applying it to established disease. Frank cancers are more readily identifiable through imaging and biomarker and histopathologic assessment than their precancerous precursors. In addition, prevention subjects routinely have more rigorous intervention criteria than therapy subjects. Any nanopreventive agent developed to prevent sporadic cancers found in the general population must exhibit a very low risk of serious side effects. In contrast, a greater risk of side effects might be more acceptable in subjects at high risk for cancer. Using nanotechnology to prevent cancer is an aspirational goal, but clearly identifying the intermediate objectives and potential barriers is an essential first step in this exciting journey.
Keywords: nanotechnology, prevention, intraepithelial neoplasia, early detection, angiogenesis, imaging, premalignancy, nanomedicine, cancer, chemoprevention
Introduction
Nanotechnology by definition involves the study and use of materials between 1 and 100 nanometers (nm) in size (Figure 1). The idea of nanotechnology began as theoretical concepts posed by physicists and other scientists involving nanoscale assembly of materials(1, 2). The term nanotechnology was first used to describe semiconductor generation(3). The early discipline began with the invention of the scanning probe microscope and the discovery of molecular structures such as fullerenes(4, 5). The field has more recently expanded into various specialized areas of nanomaterials and nanomedicine(6-10).
Figure 1. Understanding the relative nanotechnology scale.
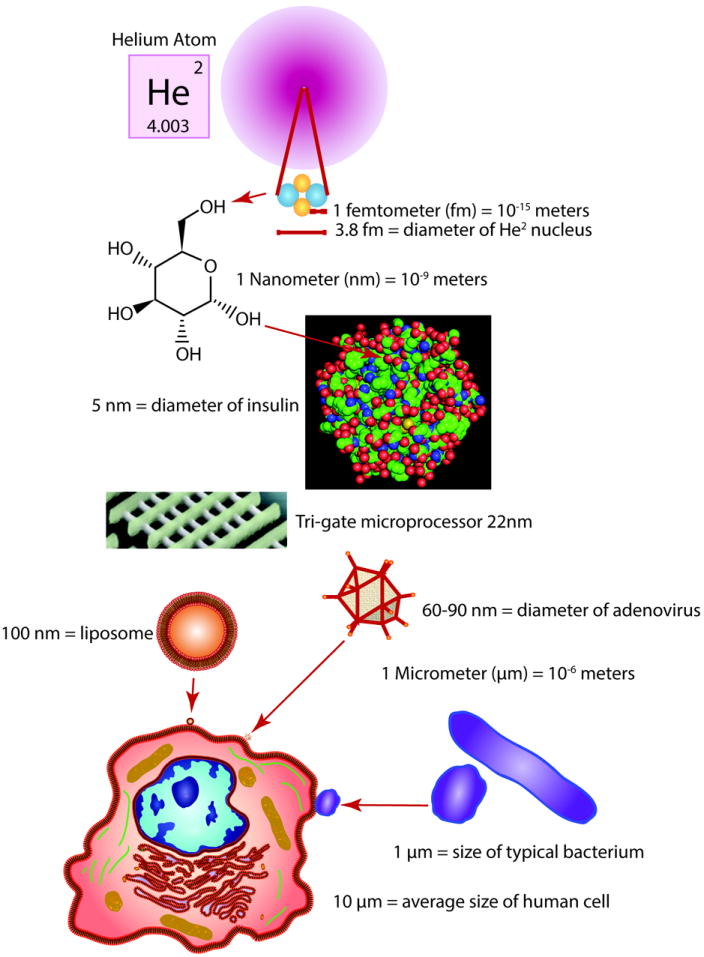
In terms of size, a nanometer (nm) equals one billionth of a meter (10-9 m). A proton in an elemental nucleus is 1 femtometer (fm). The nucleus of a Helium atom is 3.8 fm. The diameter of a glucose molecule is approaches 1 nm. The Insulin protein approaches 5 nm in size. A nanoparticle 10 nm in size is 1000 times smaller than the diameter of a human hair. A small microprocessor transistor is 22nm. An adenovirus particle is between 60-90nm. Liposome based nanoparticles are often 100nm in diameter. A typical bacterium is 1 μm in size. The average size of a human cell is 10 μm. As a reference, a billion inches is 15,783 miles, more than halfway around the earth.
Existing nanomaterials include liposomes, natural polymers (chitosan), synthetic polymers (PEG etc.), carbon, carbon fullerenes such as buckyballs and carbon nanotubes (CNT), graphene, ceramic, crystals, metal, silica and quantum dots (Figure 2, Table 1). They generally range is size between 5 and 200 nm, but some may exceed this range. When these technologies are coupled with molecularly based targeting methods, they can potentially achieve selective gene targeting. For example, coupling of chitosan with hydrogel technology and/or selective epitope targeting with siRNA based gene silencing are exciting developments in selective gene targeting(11, 12). The use of thioaptamer-based molecular epitope recognition also holds significant promise for selective targeting(13). The use of phage-display-based peptides as well can facilitate selectively “nanotargeting” cancers(14, 15). Selective targeting can also employ the use of specific promoter-based gene expression only in premalignant or malignant cells (16-20). Any single or combination approach for molecularly enhancing target recognition in conjunction with nanoscale payload delivery is likely to facilitate selectively targeting the various stages of cancer.
Figure 2. Nanomaterials and nanodelivery.
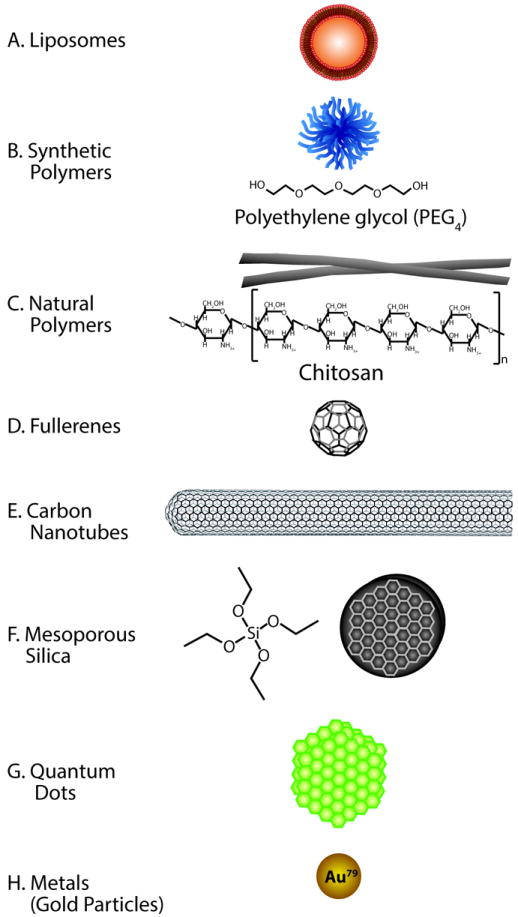
A. Liposomes are vesicles formed into a lipid bilayer. They are commonly made of bipolar phospholipids that generally contain an aqueou score. Liposomes carrying drugs can readily fuse with plasma membranes of cells. B. Synthetic polymers often begin with polyethylene glycol (PEG) backbones that exhibit a high degree of biocompatibility. PEG polymers can also be used in more complex multistage nanoparticles to improve biocompatabilty. C. Chitosan is a natural cationic polysaccharide made by the partial deacetylation of chitin. Chitosan is a positively charged hydrophilic polymer. Charge-based binding and release of drugs can involve physical or chemical stimuli, such as pH, ionic strength, temperature, and magnetic and biological molecules. D. Buckyballs are fullerenes that are made entirely of carbon. They form the shape of a hollow ball and are sparingly soluble in most solvents. They are often conjugated to amino acids like L-arginine and L-phenylalanine that enable amino acid transporters to bring them into cells. E. Nanotubes are cylindrical fullerenes made of carbon. They are often only a few nanometers in diameter but can be very long, from microns to millimeters in length. F. Mesoporous silica is a silicon-based molecule. It can be made from tetraethyl orthosilicate among other silicon-based molecules. Nanoparticles typically synthesized from silica generally have a large surface area of the pores that can be filled with a drug. Depending on the dimensions and synthesis process, silicon nanoparticles can be used in a multistage fashion to deliver other nanoparticles like liposomes or chitosan to target sites. G. Quantum dots are semiconductor crystals that exhibit electronic characteristics, which are closely related to the size and shape of each individual crystal. They can either be grown as crystals or made using lithography. H. Gold nanoparticles (colloidal gold) are produced using liquid chemical methods. Gold nanoparticles can be used to deliver drugs or to aid in non-invasive imaging.
Table 1.
Examples of nanoparticle targeting approaches in Pre-clinical or Clinical Studies
| Nanoparticle Type | Active Agent | Tumor | Clin. Trial | Deliv. | Target | Effect | Ref |
|---|---|---|---|---|---|---|---|
| Liposomes (lipid) | |||||||
| ONC-TCS liposome | Vincristine sulfate | Hu | Approved | IV | Non-Hodgkins Lymph. | Improved TI | (39) |
| DepoCyt liposome | Cytarabine ara-C | Hu | Phase III | IV | Lymphomatous meningitis | High Resp, QOL | (40) |
| Myocet liposome | doxorubicin citrate | Hu | Approved | IV | metastatic breast cancer | Improved TI | (37) |
| DaunoXome liposome | daunorubicin | Hu | Approved | IV | AIDS-related Kaposi’s sarc | skin necrosis | (38) |
| Liposomes (PEGylated/lipid) | |||||||
| Lipoplat-PEG-DSPE | cisplatin | Hu | Phase I | IV | Stage IV carcinomas | nephrotoxicity | (43) |
| Doxil PEG-lipo | doxorubicin | Hu | Approved | IV | AIDS-related Kaposi’s sarc | bone marrow tox | (42) |
| Thermodox PEG-lipo | DoxThermo-bubble | Hu | Phase III | IV | Liver, breast cancer | Improved TI | (41) |
| NanoDoxCurc PEG-lipo | Doxcurcumin comp | Hu | Pre-clinical | IV | Prostate cancer-PC3A | Cardiotoxicity | (44) |
| siRNA Liposomes | |||||||
| ALN-TTR01 & 2 | siRNA transthyretin | Hu | Phase I | IV | Transthyretin amyloidosis | transthyretin | (45) |
| ALN-PCS | siRNA kexin type 9 | Hu | Phase I | IV | Fam. hypercholesterolemia | LDL cholesterol | (46) |
| Albumin bound Nanoparticles | |||||||
| Abraxane Albumin-drug | paclitaxel | Hu | Approved | IV | Metastatic breast cancer | Improved TI | (48) |
| Polymeric Nanoparticles | |||||||
| PolyDL-lactide-PEG | paclitaxel | Hu | Phase II | IV | advanced refract malig | toxicity, OC | (50) |
| Oncaspar PEG-L-aspar | L-asparaginase | Hu | Approved | IV | acute lympho leukem(ALL) | remission | (49) |
| DACH diaminocyclohex | plat poly prodrug | Hu | Phase II | IV | Ovarian cancer | Tolerability, OC | (51) |
| BIND-014 polynanopart | docetaxel | Hu Mo | Phase II | IV | Animal Tumors, PK-human | PSA-Targeted | (52) |
| Chitosan | |||||||
| microcryst chitosan | Absorb GI fat | Hu | Approved | Oral | High LDL cholesterol | LDL cholosterol | (53) |
| glycated chitosan | laser immunotherm | Hu | Phase I | IV | metastatic breast cancer | Improved RR | (55) |
| Chitosan | Plasmid DNA | Mo | Pre-clinical | Lung | Lung uptake | In vivo luc | |
| Chitosan | Plasmid DNA | Mo | Pre-clinical | Oral | peanut-induced anaphylax | Improved RR | (56) |
| ExChitosan | siRNA | Mo | Pre-clinical | Nasal | Inhibit gene expression | Reduce EGFP Ex | (54) |
| Chitosan RGD-CH-NP | siRNA-periostin Int | Mo | Pre-clinical | IV | periostin ovarian tumors | Decrease T. Size | (12) |
| Fullerenes | |||||||
| C60(OH)24 | doxorubicin | Ra | Pre-clinical | IP | MNU Induced rat tumors | Dec hepato-tox | (58) |
| C60-PEI-folate | docetaxel | Mo | Pre-clinical | IV | Sarcoma S180 Balb/c | Decrease T. Size | (63) |
| C60-Hyaluronate | NA | Hu Mo | Pre-clinical | IV | HCT-116 | Decrease T. Size | (59) |
| Nanotubes | |||||||
| PEG-SWNTs | TNFR-(GITR) | Mo | Pre-clinical | IV | B16 melanoma | Intratumor T-regs | (61) |
| NGR-SWNTs-2ME | paclitaxel, taxol | Hu | Pre-clinical | IV | MCF-7 | Decrease T. Size | (62) |
| SWNT-lipid-PTX | 2-methox estradiol | Mo | Pre-clinical | IV | Sarcoma (S180) Balb/c | Decrease T. Size | (57) |
| O-MWNTs-PEG-ANG | doxorubicin | Ra/Mo | Pre-clinical | IV | C6 glioma /Balb/c | Decrease T. Size | (60) |
| Mesoporous Silica Nanoparticles | |||||||
| C-dots (Cornell-dots) | 124I-RGDY-PEG | Hu Mo | Phase I | IV | αvβ3 integrin–melanoma | real-time imaging | (64) |
| Mag-Dye@MSN | fluores, magnetic | Ra | Pre-clinical | IV | Rat tumors | Tumor imaging | (66) |
| MSV/EphA2 | siRNA Eph Apac | Hu | Pre-clinical | IV | Ovarian tumor | Decrease T. Size | (65) |
| Quantum Dots | |||||||
| QD-800 | anti-EGFRvIII | Hu | Pre-clinical | IV | Glioblastoma | Tumor imaging | (67) |
| QD-MUC1-DOX | Doxorubicin | Hu | Pre-clinical | IP | Human ovarian | Tumor imaging | (69) |
| QD-antiVEGFR2 | antiVGFR2 | Hu | Pre-clinical | IV | Human PC3 prostate | Tumor imaging | (68) |
| Metal nanoparticles | |||||||
| Gold, AuNPs | Gold photo-activ. | Ra | Pre-clinical | Cran | F98 glioma-bearing rats | Tumor imaging | (71) |
| Immuno-magnetic | EpCAM Mb | Hu | Pre-clinical | CTC | Identify CTCs | CTCs in BrCa | (72) |
| Gold, AuNP | EMT target | Hu | Pre-clinical | IP | Ovarian Cancer | Decrease T. Size | (70) |
| Gold, AuNP-TNF | TNF-α | Hu | Pre-Clinical | IV | Prostate Cancer | Improved Therapy | (73) |
Abbreviations: Multidrug resistance (MDR), single wall nanotubes (SWNT), multi wall nanotubes (MWNT), polyethylene glycol (PEG), circulating tumor cell (CTC), quantum dot (QD), Therapeutic Index (TI), Quality of Life (QOL), Outcome (OC), Response rate (RR), Human (Hu), Mouse (Mo), Rat (Ra)
Knowledge regarding uptake and distribution of nanoparticles comes from studies involving various types of environmental exposures(21). Nanoparticle internalization depends on the exposure time, carrier vehicle, mode of access, and tissue interface involved in a given exposure(21). Tissue interfaces include dermal surfaces, any exposed mucous membrane and the respiratory airways (21). The biodistribution of nanoparticles in the body typically depends on the material and its size, shape and charge(21-24). In the case of dermal exposures, nanoparticle penetration typically occurs at hair follicles(25) and in flexed (26) and broken skin(27). As one example, 10-50 nm sized TiO2 nanoparticles found in sunscreen, when suspended in oil-in-water emulsions, can penetrate hairy skin at the hair follicle sites or pores when compared to water based suspensions(28, 29). In the case of gastrointestinal mucous membranes, absorption depends on size, which diminishes for larger particles ranging from 50nm to 1000nm(30), exposure time, and enterocyte involvement (31). As a potential consequence, gastrointestinal uptake of dietary nanoparticles (100 nm-1000nm) may also influence chronic inflammation in the colon(32). In the case of aerosol delivery to the lung by contrast, noncationic nanoparticles larger than 34 nm are retained within the lungs while smaller nanoparticles enter the regional lymph nodes(33). In the same report, neutrally charged nanoparticles 6 nm or less entered the bloodstream from the alveolar airspaces followed by renal clearance(33). Similarly, blood-borne particles 10 nm or less in size usually undergo elimination by renal clearance, while particles 100 nm or greater in size are taken up by the reticuloendothelial system (34). The shape and surface properties of nanoparticles can also influence uptake and distribution, which can be experimentally optimized at the molecular level to enhance targeting properties (35, 36).
Pre-clinical and Clinical Nanotherapeutics
The relative success of nanoparticle pre-clinical and clinical use has evolved with the introduction of technology (Table 1). The use of nanoencapsulated agents helps reduce the toxicity of chemotherapeutic drugs. Many clinically approved approaches involved the early introduction of lipid liposomes, including ONC-TCS, DepoCyt, Myocet, and DaunXome(37-40). Similarly, a number of pegylated/lipid liposome-based approaches are either clinically approved or in trial, including Lipoplatin, Doxil and Thermodox(41-43). One approach to overcome multidrug resistance integrates therapy and preventive approaches by combining doxorubicin-curcumin into nanoparticles known as NanoDoxCurc (NDC) (44). The delivery of RNAi therapy using nanoparticles is another important use of nanotechnology. Alnylam Pharmaceuticals, for example, has two liposomal siRNA in non-cancer related clinical trials(45, 46). Alnylam also produced polymeric nanoparticles for in vivo delivery of siRNA to target endothelial cells, primary tumor growth and metastasis(47). Another nanoformulation consisting of albumin-based (Nab)-paclitaxel or Abraxane is the first drug delivery system approved for treatment of metastatic breast cancer, metastatic non-small cell lung cancer (NSCLC) and first-line treatment of patients with metastatic pancreatic cancer(48). A variety of synthetic polymeric nanoparticles are also in clinical settings, such as Genexol-PM, Oncaspar and Prolindac(49-51). In the targeted realm, BIND-14 is a nanoencapsulated composite that includes a controlled-release synthetic polymer containing docetaxel (DTXL). This nanoparticle composite binds to the prostate-specific membrane antigen (PSMA) targets for treating solid tumors(52). In contrast, chitosan is a natural polymer used orally to decrease high serum LDL cholesterol(53). Since chitosan was introduced, it has enjoyed numerous uses as a nanoparticle platform in pre-clincal and clinical studies(12, 54-56).
Inert carbon-based modalities are also receiving significant interest as drug, nucleotide and protein-protective delivery approaches. Fullerenes and nanotubes are nanoparticles that hold much promise in pre-clinical settings(57-63). Mesoporous silica is another biologically inert platform for building complex nanoparticle identification and delivery of drug, nucleotide and protein agents(64-66). Within the silica realm, Cornell (C)-dots received phase I approval for melanoma targeting(64). By contrast, uses of inert quantum dots are focused on targeted delivery and imaging due to their high quantum fluorescence yield (67-69).
In the heavy metal space, gold nanoparticles may be particularly useful for targeting tumors (70-73). In one study, unmodified gold nanoparticles inhibit tumor growth and metastasis through abrogation of MAPK signaling and reversal of the epithelialmesenchymal transition in two preclinical mouse models of ovarian cancer(70). The authors suggested that these findings laid the foundation for further research in the use of inorganic nanoparticles as a class of antitumor and antimetastatic agents(70). In addition to serving as drug carriers, gold nanoparticles are finding use as photothermal agents, contrast agents and radiosensitisers(74).
Other reports highlight some advantages of nanoparticles (75). Jain and his coworkers have applied nanodelivery methods to decrease the toxicity of therapeutic drugs (76). Inhalation of retinoids, steroids or certain therapeutics used to treat lung cancer may also be more effective if applied in a nanoized form (77-80). Since a primary goal of nanoprevention is sustained release along with significant reduction in toxicity, the use of low-dose nanoized chemotherapeutic drugs might be useful in high-risk preventive settings.
Toxicity: Delivery Modes, Mechanisms and Management
Identifying biocompatible non-toxic nanomaterials is vital for the application of nanotechnology in prevention. The mode of delivery greatly influences uptake and toxicity(81). Nanotherapies are often injected directly via the blood stream or peritoneum (Table 1 & Figure 3). Other common delivery methods include the skin, respiratory or gastrointestinal systems, as they are more acceptable for nanoprevention modalities(81). Mucous membrane surfaces of the eye, mouth, nose, upper GI tract and vaginal surfaces can also take up nanoparticles. The particulate nature of nanomaterials leads to local accumulation followed by systemic dissemination via the cardiovascular or lymphatic systems. Among a variety of toxic effects, acute responses can begin with the generation of reactive oxygen species coupled with inflammatory reactions(82).
Figure 3. Nanoparticles in the Blood or Lung.
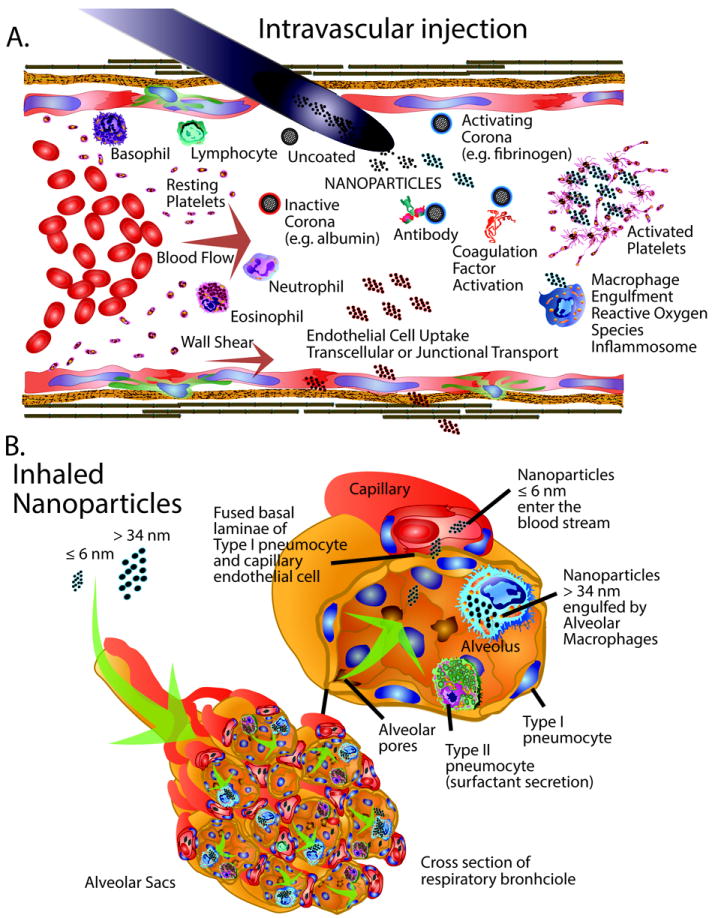
The biology and toxicity of nanoparticles are influenced by the mode of delivery. A. Intravascular delivery of nanoparticles can initiate a variety of interactions in the blood stream. Nanoparticles can interact with circulating proteins to form active or inactive protein coronas. Active protein coronas promote can trigger platelet activation. Activating interactions can also stimulate various immune cells like macrophages, lymphocytes, neutrophils, and eosinophils, as well as antibodies, complement proteins and coagulation factors. Nanoparticles with inactive coronas can undergo transcellular or transjunctional transport. B. Inhaled nanoparticles are dispersed throughout the alveolar sacs of respiratory bronchioles and undergo processing in the lungs depending on the material size and charge. Nanoparticles smaller than or equal to 6 nm can readily enter the blood stream via transcellular processes. Nanoparticles larger than approximately 34 nm are engulfed and processed by alveolar macrophages.
The blood stream is often used for delivering nanotherapy (Figure 3). It is a closed system that is not directly exposed to the environment. As a closed system, the blood stream has specific processes identifying and mitigating pathogens and foreign bodies, which are processed further by the liver or excreted by the kidneys(33, 83). Once in the blood stream, platelets are among the first responders to many foreign bodies that initiate emboli formation (84). Nanoparticles in flowing blood also interact with antibodies, circulating immune cells, coagulation factors and the surfaces of endothelial cells (85, 86). Nanomaterials can bind proteins through non-covalent interactions to form a protein corona(87, 88). This corona influences biological activity and interactions with platelets and probably liver clearance(87, 88). Chemically modifying CNT surfaces for example to preferentially bind albumin vs fibrinogen influences protein corona formation and platelet interactions (87). Similarly, nanoparticle protein coronas can activate the circulating macrophages and trigger inflammasome formation by immune cells(89). Preformed albumin nanoparticle coronas are another method of limiting toxicity in the blood by reducing complement activation(90). Other mechanisms of toxicity occur with metal and metal oxides that generate reactive oxygen species and pro-inflammatory oxidative stress (91, 92). Depending on the routes of exposure, metal and metal oxide nanoparticles can affect cells and organ pathophysiology (91, 92). Similarly, graphene-based (93) and other inorganic nanomaterials (94) exhibit various toxicities to consider in the exposure and safer design of these materials. Nanoparticles are also being used to facilitate vaccinations and immunotherapy(95). Functionalizing polymeric nanoparticles using membrane coating derived from cancer cells is a different approach to selectively target tumors but any effects on cardiovascular toxicity remain to be determined(96). The structure, size and surface properties that affect efficacy and toxicity should be carefully considered before using the blood stream as a portal for delivering nanomaterials.
Intraperitoneal (IP) injection is another common mode of nanoparticle delivery to a closed-body compartment (97, 98). Far less is known about IP uptake and toxicity associated with this delivery method. Among the various abdominal-cell targets are peritoneal macrophages (99). After IP injections in one study, silver nanoparticles accumulated most heavily in liver Kupffer cells and hepatocytes along with kidney podocytes and other cells in the peritoneum(100). These changes were accompanied by cell death and the infiltration of white blood cells, lymphocytes, granulocytes, and hemoglobin(100). Chemical modification of surface charges can minimize toxicity while enhancing biodistribution and tumor targeting of some IP injected nanoparticles(101).
In contrast to the blood and peritoneum, the lungs, skin and intestinal tract are in direct contact with the environment (102). The upper airway and lungs continuously interface with the atmosphere(103) (Figure 3). A well-developed immune system helps process inhaled atmospheric particulates(104). In the lung, inhaled nanoparticles initially encounter pulmonary surfactants and then pulmonary cells (105). The very thin air-blood barrier (<1 μm) combined with the collective lung-alveolar cell-surface area provides high systemic nanoparticle bioavailability (105). Once engaged by cell surfaces, caveolae-mediated endocytosis translocate nanoparticles into alveolar epithelial or immune cells(106). Depending on the type and size of inhaled nanoparticles (107), pyroptosis-based toxicity can occur (108). Pyroptosis is driven by alveolar macrophages that cause a specialized inflammasome-dependent form of cell death (108). Pulmonary effects are further influenced by the volumes of material inhaled and mass transfer (109). Clinical pathological responses involve follicular hyperplasia, protein effusion, alveolar lipoproteinosis and pulmonary capillary vessel hyperaemia that lead to fibrosis and emphysema with sustained exposure(110). Modification of nanoparticle size and charge can reduce toxicity while providing rapid sustained-release in the lung tissue thereby resulting in reduced dosing frequency and improved patient compliance(104, 111, 112). As long as toxicity and side effects are minimized, inhalation is a highly efficient mode of nanomaterial delivery for preventive treatments.
Skin, by contrast, is the largest organ and protective barrier of the body that can serve as a topical, regional and transdermal mode of drug delivery(113). The use of skin-based nanomedical delivery methods may help reduce toxicity, improve sustained release and penetration(113, 114). Skin exposure to nanoparticles as pollution, antibacterials, and sun screen each have some toxicity concerns(115-117). Nanoparticle uptake measurements using quantum dots showed that penetration into the dermal layer is limited to the uppermost stratum corneum layers and the hair follicles(118). Those nanoparticles that enter the blood stream accumulate in the liver and kidney with poor clearance rates(118). Toxicity can directly affect skin cells by forming reactive oxygen species along with autophagic vacuole accumulation and mitochondria damage(119). Localized inflammatory responses lead to macrophage-, monocyte-, and dendritic-cell responses that cause cytotoxicity(120). Additional oxidative stress, Ca2+ flux and decreased mitochondrial membrane potential are accompanied by production of IL-1beta and chemokine CXCL9(120). A variety of approaches hold promise for decreasing dermal toxicity and improving delivery as potential preventive modalities(121-123). Similar approaches hold promise for directly improving melanoma treatment(124).
Oral delivery of nanomaterials is the most common and well-tolerated method for delivering preventive or other agents(125). Other than distribution studies, the toxicology of nanoparticles is not well understood(126). Although oral delivery of nanomaterials is well integrated into the food and drug industry, their long-term fate in the GI tract remains unclear(127, 128). Nanoparticle delivery methods are showing improvements in stabilizing oral delivery and survival of in low gastric pH(129, 130). The intestinal tract maintains elaborate mechanisms for simultaneously taking in nutrients while preserving healthy gut flora and controlling micro- and nano-pathogens(131-134). The uptake and distribution of nanoparticles in the gut is also influenced by mucin produced as a protective barrier (135). The mucosal component of the GI tract includes the gut-associated lymphoid tissue that is responsible for antigen sampling (131, 136, 137). One of the primary endocytic pathways for pathogen sampling includes the follicle-associated epithelium of the Peyer’s patches found in the upper GI tract (Figure 4) (131-134). Peyer’s Patches contain specialized microfold (M) cells that actively internalize particulate material (131-134). This process involves endocytosis in clathrin-coated vesicles, actindependent phagocytosis or macropinocytosis(131-134). Once internalized M-cells form ‘intraepithelial pockets’ through an expanded basolateral domain. This transcytosis process culminates in shipment and presentation of pathogen-laden exosomes to immune cells (131-134). M-cell exosomal antigens are presented to subepithelial dendritic cells (DC)s or are directly sampled by LysoM+ dome DCs. These antigens are subsequently presented to T- and B-lymphocytes within Peyers’ Patches (138, 139). Some efforts are underway to specifically target M-cells(131).
Figure 4. Oral Delivery and Gastorintestinal Uptake.
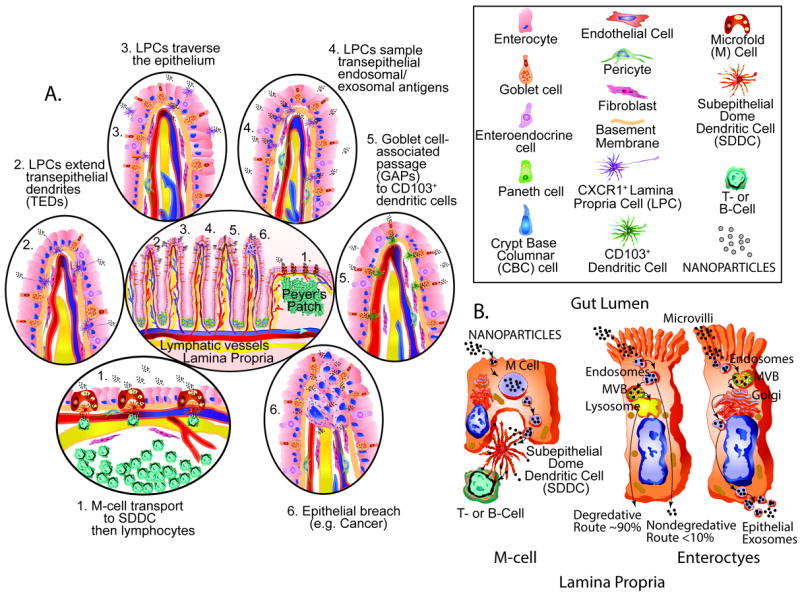
Oral delivery of nanoparticles depends primarily on GI uptake and sampling mechanisms. A. The GI tract contains 70% of the immune system and is heavily involved in uptake and processing of pathogens and foreign materials in preparation for defensive responses. Potential sampling of nanoparticles may include: 1. Microfold cell (M-Cell) transport to subepithelial dome cells (SDDC) then to lymphocytes is a key immune sampling process. 2. CXCR1+ lamina propria cell (LPC) is another sapling mechanism. 3. LPC movement across the epithelium is another sampling mechanism. 4. LPC sampling of transepithelal endosomal and exosomal antigens can also occur. 5. Goblet cell associated passage (GAPs) to CD103+ dendritic cells can also occur. 6. Pathologic breaches in the gut epithelium such as cancer formation can also allow for nanoparticle passage. B. Vesicle processing may be a key aspect of nanoparticle migration across the gut epithelium. M-cells form a series of prominent vesicles prior to transfer from SDDC to T- or B-lymphocytes. The uptake of nanoparticles by microvillar enterocytes is often followed by endosome formation, the genesis of microvessicular bodies (MVB) and fusion with lysosomes and then transfer to the lamina propria is another mode of passage. Another mechanism enterocytes use for transepithelial transport involves uptake into endosomes, MVB formation, fusion with golgi apparatus and the exosomal transfer to the lamina propria. Transport to the blood stream or lymphatic system can lead to further dissemination
In other areas of the gut, enterocytes absorb nutrients via microvilli of the brush border that can be disrupted by exposure to nanomaterials (140). Villous bearing areas of the gut also exhibit a different set of mechanisms for transepithelial antigen sampling or microbiota/antigen presentation to cells and vessels in the microenvironment of the lamina propria (Figure 3). This process can occur directly as CX3CR1+ LPCs cross the epithelium in a basolateral-to-luminal direction(141). Another mechanism involves direct sampling from the gut lumen by mucus-producing goblet cells(142). After the goblet cells have sampled the gut lumen, goblet-cell-associated antigen passages’ (GAPs) transfer various antigens to CD103(+) DCs in the lamina propria(142). These CD103(+) DCs within the lamina propria then induce T-cell responses(142). In other cases, CD11c+DCs or CXCR1+ LPC cells in the lamina propria can extend transepithelial dendrites (TEDs) between enterocytes directly into the gut to sample luminal contents and ensnare microbiota(143-145). As a completely different mechanism of transcytosis, vesicle passage into the lamina propria also occurs directly through intestinal epithelial cells(138, 146). Once exosomes enter the lamina propria they can gain access to the various cells, including myofibroblasts, pericytes and capillaries or lacteal vessels of the lymphatic system(147-150). Functionalizing the outer nanoparticle surface can make them an ideal vector suited to traverse brush borders, mucosal lymphoid tissue and other surfaces (140). Nanoparticles can also be used to deliver vaccines via the gut(151). Nanotargeting of chronic inflammation in the GI tract also holds promise for prevention(152). As with the other delivery methods, minimizing toxicity while maximizing tolerability are essential in generating acceptance of GI delivery approaches for prevention.
Distinguishing Premalignant from Malignant Lesions
Vital differences exist between various precancerous and cancer lesions that can profoundly influence how they are targeted using nanotechnology. Morphologic differences based on gross pathology and histology are the most obvious distinctions (153). The site of origin influences the morphologic characteristics and biological differences along with the unique characteristics of the individual tumor. Each type of cancer presents its own unique challenges for effective targeting(154). These challenges are influenced further by variations in the anatomic, physiologic, microenvironmental, cellular, and molecular features of the involved tissue site(155). The stage of progression is also critical(156). Advanced-stage cancers that have metastasized can originate in a totally different organ from the one in which they are discovered. In contrast, premalignancies are confined to the site of origin.
Complex multistep biology combined with long progression time frames provide multiple potential points for early detection or intervention using nanotechnology-based approaches. Nanotheranostic tools that simultaneously identify and selectively target premalignant lesions would be extremely useful. This is likely to require modification of probes that selectively identify specific premalignant lesions. Ideally, we will be able personalize theranostic approaches and nanotarget premalignancies by implementing “-omics” approaches for profiling as a first step and selective targeting as a second step (157-159). Some recent advances in theranostics incorporate magnetic resonance imaging for early detection along with targeting (160, 161). A molecular imaging strategy using a new triple-modality MRI photoacoustic-Raman nanoparticle is a unique theranostic approach directed at brain tumors (162).
Premalignancies are generally considered the primary targets for cancer prevention(163). Several characteristics are used to distinguish between premalignant and malignant lesions. These characteristics can potentially impact the effectiveness of nanotechnology-based targeting (see Table 2). In addition to being the most common form of cancer, carcinomas arising from epithelial origins are the easiest to identify on the basis of the morphologic distinctions between precancers and cancers. They are often referred to as carcinoma in situ or intraepithelial neoplasias (IENs). Epithelial precancers are by nature organ-confined and generally remain restricted to ducts or the epithelial strata of tissues(164). Cancers, by contrast, are often identified as having breached or invaded through a fine fibrous complex that forms a sheet-like barrier known as the basement membrane(165). The basement membrane can undergo extensive remodeling or thickening during inflammatory responses (166, 167) or become disorganized in tumor vasculature(168) and in various cancers(169). How early this occurs is not clear, particularly with respect to precancerous lesions. Since the basement membrane serves as a structural base for normal epithelia/endothelia, but can potentially accrue abnormalities during carcinogenesis, its status demands adequate consideration when devising nanotargeting approaches.
Table 2.
Acquired characteristics of premalignant and malignant lesions
| Characteristic | Premalignant | Malignant |
|---|---|---|
| Genetic abnormalities | Few | Numerous |
| Suppressor genes | Semiactive growth suppression | Shut off or fully autonomous |
| Growth signals | Homeostatic, semidependent | Paracrine, autocrine, or fully autonomous |
| Stem and progenitor cells | Semi-expanded population | Expanded population, enhanced chemoresistance |
| Apoptosis | Semifunctional | Dysfunctional, ineffective |
| Invasion status | Non-invasive lesion | Enhanced motility, invasion, and metastatic potential |
| Basement membrane | Intact | Breached |
| Cell morphology | Hyperplasia, dysplasia | Anaplasia |
| Angiogenesis | Semiactive | Sustained dysfunction |
Premalignant IEN lesions are often accompanied by hyperplasia, or the physiological proliferation of cells to a greater extent than normal(153). These precancerous lesions may also exhibit dysplasia or the abnormal maturation of cells. Along the continuum of progression from precancerous to cancerous lesions, the cells may begin to exhibit anaplasia. Anaplastic lesions contain cells that have lost their functional tissue identity and reverted to a more primitive or undifferentiated form. IEN lesions also can display pleomorphism that includes large, darkly stained nuclei, and the ratio of nuclear material to cytoplasmic material increases. These precancers are sometimes widely distributed and/or very small in extent and size and, thus, hard to identify until they grow larger(164). If IENs arise in a ductal structure, they can begin to fill in the vacant space or lumen cavity of the duct. Unless this proliferation is accompanied by the stimulation of angiogenesis, these lesions may begin to show signs of apoptosis(170). These IEN lesion characteristics are likely to influence the delivery of nanotechnology to the target site and should be considered during the design and analytic phases of any proposed prevention study(171).
The Angiogenic Switch and Premalignancy
Although angiogenesis may contribute to premalignancy(172), exactly how early it occurs and to what extent it plays a role in the genesis and progression of precancerous lesions is not fully understood (Figure 5). The biomarkers used to immunochemically identify increased microvascular density include factor VIII, CD31, and CD34(172). Another biomarker, CD105, is also used to identify newly formed vessels(173-177). More advanced tumor-mediated angiogenesis is highly deregulated and leads to disorganized, poorly formed and leaky or highly permeable blood vessels(178).
Figure 5. The angiogenic switch.
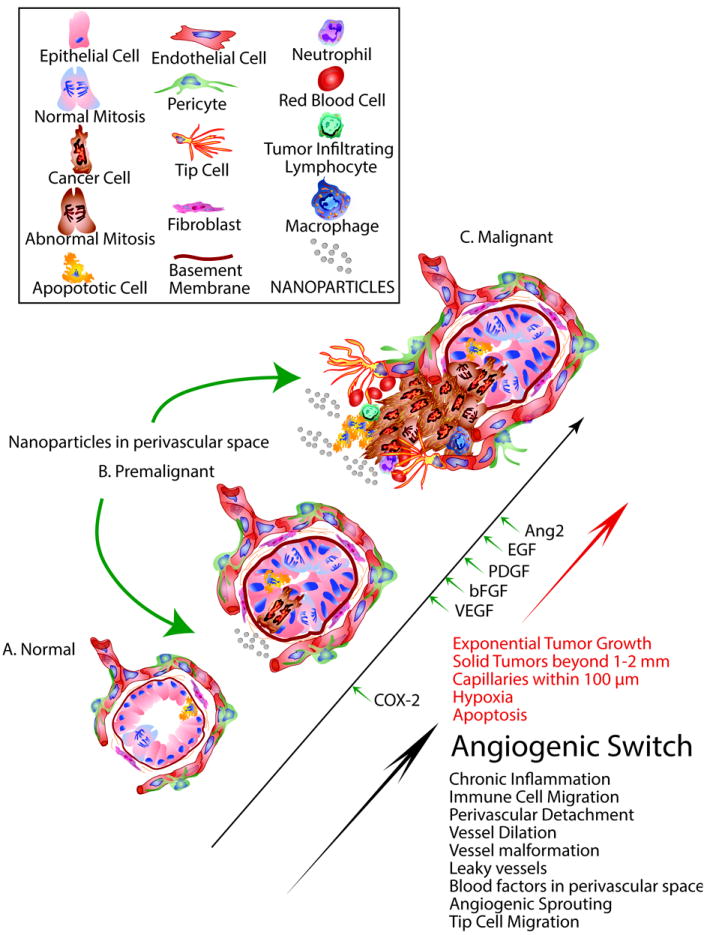
The angiogenic switch can occur at different stages of carcinogenesis. A. normal tissue growth achieves a balance between cell growth and apoptosis that helps maintain normal homeostasis. B. Premalignant disease may involve the earliest stages of angiogenesis. Chronic inflammation involving the accumulation of immune cells, cytokines and prostaglandins during premalignancy helps to promote vessel dilation and the detachment of pericytes from blood vessels. During the early stage conversion of premalignant to malignant tissues progresses blood factors accumulate in the perivascular space. The upregulation of cyclooxygenase-2 and the accumulation of vascular endothelial cell growth facto (VGEF), basic fibroblast growth factor (bFGF), platelet derived growth factor (PDGF) epithelial growth factor (EGF) and angiopoietin 2 (Ang2) trigger the angiogenic switch. C. The onset of malignancy, heightened hypoxia and apoptosis further increases vessel leakiness. The onset of angiogenic sprouting and tip cell mediated migration into the growing tumor leads to further recruitment of immune cells and peri-vascular cells as part of the angiogenic switch.
The “angiogenic switch” that initiates angiogenesis was shown by Folkman(179) to occur before cells achieve the invasive state(180). This switch was recently shown to involve microRNA, among other factors, (181) and may serve as a useful target for cancer prevention(182). Recent evidence indicates that angiogenesis coincides with the onset of dysplasia during adenoma formation in colorectal cancer progression(183). In the breast, angiogenesis appears to begin with the onset of hyperplasia in the mammary duct and progresses through ductal carcinoma in situ and invasive disease(184). Whether angiogenesis starts as a normal inflammatory or tissue repair process during premalignancy and then progresses toward an abnormal state during tumorigenesis or begins as an abnormal process is not known.
Abnormal or leaky blood vessels that arise during tumor-initiated angiogenesis are likely to be attractive targets for nanotechnology-based interventions using vascular delivery approaches(185). However, further work is needed to determine whether vascularbased targeting will be a useful approach for cancer prevention.
Various nano-tools could potentially help identify any connections that may exist between the angiogenic switch and premalignancy. Perhaps nano-tools might help identify early changes in endothelial cells and/or tumor cells that signify the beginning of neoangiognenesis. Optimally, early stage biomarker recognition epitopes or ligands involved in neoangiognenesis will be incorporated as surface molecules on nano-tools that to recognize premalignant changes. The involvement of inter-endothelial transport of modified nano-tools may facilitate identifying not only the early changes in these lesions but also the interconnections between endothelial cells and tumor cells(179). These processes may also be identified by biological changes in the pericytes (186, 187) or vascular endothelial tip cells (188, 189) that are heavily linked to angiogenesis and vascular sprouting. The ability to image and target early-stage changes in the microvasculature of premalignant lesions will help to significantly advance this emerging field.
Risk versus Reward in Cancer Prevention
Determining the usefulness of preventive interventions relies heavily on the therapeutic index of a given treatment(190). This index can be broken down into a riskbenefit ratio that assesses its effectiveness(191, 192). In the case of prevention, the risk to a given population is typically minimized to the greatest extent possible. Any risk assessment needs to be carefully examined, not only for the legal ramifications but also for cost-effectiveness(193). It is also important to identify a reasonable balance between safety, efficacy and affordability, particularly because nanoscale devices are often very complex and can add substantial production costs. Ultimately, a risk assessment also encompasses identifying a given agent’s intended and unintended effects based on an individual’s/cohort’s susceptibilities to both disease development and the agent’s effects. If a subset of patients is more susceptible to the adverse effects of a nanopreventive agent than the general population, these patients must be identified and excluded from any study. Moreover, any nanoscale preventive treatment with severe side-effects would generally not be suitable for use. This type of intervention must also entail tolerable risk that is suitable for long-term use. However, if a nanopreventive approach heightens sustained local release at the target site, substantial benefit may be realized. In the final analysis, any intervention can exhibit risk, but the risk must be worth the reward.
Chemoprevention Subject Populations
Chemoprevention is a term describing the use of agents to reverse, suppress or prevent carcinogenesis and malignancy(194). Subjects for chemoprevention trials are stratified into multiple levels of risk (195). The lowest level of risk encompasses subjects in the general population who are seemingly healthy but may contract sporadic cancers. The allowable risk increases for subjects who present with precancerous lesions, such as IENs(195). The more advanced the premalignancy, the higher the accompanying risk(195). Subjects at high risk, such as those with a genetic predisposition or familial inheritance, are more likely to tolerate more severe side-effects. This class of subject can also include cancer survivors or those at high risk for second cancers, in which case a higher risk of adverse effects becomes more tolerable(196). The same principles that apply to identifying and stratifying subjects for chemoprevention studies should apply to nanopreventive studies. The unique nature of any given nanopreventive intervention is likely to dictate how it is used. This is probably most applicable to nanodevices that might require special monitoring procedures to ensure clearance from the subject’s system.
Barriers to the Implementation of Cancer Nanoprevention
There is a growing number of “at-risk” individuals due to demographics, the aging of the general population and the adoption of unhealthy lifestyles(196, 197). In addition, enhanced screening can lead to identification of more individuals with precancers. And the number of cancer survivors continues to grow with enhanced early detection and treatment(196). The public tends to have a limited understanding of cancer development, risk and prevention, let alone nanotechnology(198). Educating the public should help improve the acceptance and adoption of both prevention and nanotechnology in fighting cancer. Once implemented, nanoprevention strategies may increase the need for insurance coverage/reimbursement for prevention, necessitating the passage of legislation.
New research venues and funding opportunities are needed to improve the insights and options for the nano-based prevention of cancer. Advances in genomics, proteomics, lipidomics, metabolomics, and biospecimen-based risk assessment and prevention have had limited impact to date, perhaps because of concerns about the risk:benefit balance(199). A clear demonstration of enhanced benefit with minimized risk is needed to move this area forward at both the basic and translational levels.
Cancer prevention involves overcoming more complex biology in comparison to successes in the cardiovascular prevention area. In cancer prevention, multiple disease sites, each with their own unique challenges, must be taken into account, along with the difficulty of identifying premalignant lesions. Nanotechnology-based preventive interventions may help solve some of these issues, if they improve the early detection and targetability of premalignancies and cancers as well as the delivery and efficacy of preventive agents.
The US Food and Drug Administration approved various agents for treatment of precancerous lesions or cancer risk reduction (Table 3). These vary in the type of agent and mechanism of action, depending upon the target site. There may be opportunities to make these treatments more effective by applying nanotechnology to achieve more targeted release or uptake of some of the smaller molecules or to enhance the immune response to some of the more complex molecules.
Table 3.
Approved Agents for Treatment of Precancerous Lesions or Cancer Risk Reduction - 2012
| Agent | Targeted Cohort | Indication |
|---|---|---|
| Tamoxifen |
|
Reduce the risk of invasive breast cancer |
|
Reduce the incidence of breast cancer | |
| Raloxifene |
|
Reduction in risk of invasive breast cancer |
| Cervarix |
|
Prevention of the following, caused by HPV types 16 and 18:
|
| Gardasil | Girls and women 9 through 26 years of age | Prevention of cervical, vulvar, vaginal, and anal cancer caused by HPV types 16 and 18; and the following precancerous or dysplastic lesions caused by HPV types 6,11, 16, and 18:
|
| Boys and men 9 through 26 years of age | Prevention of anal cancer caused by HPV types 16 and 18; and anal intraepithelial neoplasia (AIN) grades 1, 2, and 3 caused by HPV types 6, 11, 16, and 18: | |
| Photodynamic Therapy (PDT) with Photofrin | Males and females with high-grade dysplasia in Barrett’s esophagus. | Ablation of high-grade dysplasia (HGD) in Barrett’s esophagus (BE) patients who do not undergo esophagectomy |
| Celecoxib* | Males and females ≥18 years old with familial adenomatous polyposis (FAP) | Reduction in the number of adenomatous colorectal polyps in FAP, as an adjunct o usual care (e.g., endoscopic surveillance, surgery) |
| Bacillus-Calmette-Guerin(BCG) | Males and females with carcinoma in situ (CIS) of the urinary bladder | Intravesical use in the treatment and prophylaxis of carcinoma in situ (CIS) of the urinary bladder and for the prophylaxis of primary or recurrent stage Ta and /or T1 papillary tumors following transurethral resection (TUR) |
| Valrubicin | Males and females with Bacillus-Calmette-Guerin(BCG)-refractory carcinoma in situ (CIS) | Intravesical therapy of BCG-refractory carcinoma in situ (CIS) of the urinary bladder in patients for whom immediate cystectomy would be associated with unacceptable morbidity or mortality. |
| Fluorouracil | Males and females with multiple actinic or solar keratoses | Topical treatment of multiple actinic or solar keratoses |
| Diclofenac sodium | Males and females with actinic keratoses | Topical treatment of actinic keratoses |
| Photodynamic Therapy (PDT) with 5-aminolevulinic acid | Males and females with actinic keratoses of the face or scalp | Topical treatment of minimally to moderately thick actinic keratoses of the face or scalp. |
| Masoprocol** | Males and females with actinic (solar) keratoses | Topical treatment of actinic keratoses |
| Ingenol mebutate | Those with actinic keratoses on the face, scalp, trunk and extremities | Topical treatment of actinic keratoses |
FDA labeling voluntarily withdrawn by Pfizer, February 2011
Withdrawn from US Market June 1996
Nanotechnology for Early Detection
Multiple areas of cancer prevention can benefit from the application of nanotechnology. The easiest application to implement and the most useful for helping to collect scientific data is probably early detection. Nanoscale devices that effectively identify premalignancies or cancer signatures hold great promise.
Early forays into device development have targeted blood analysis. A prime example is the barcode chip(200). Devices such as these that integrate microfluidics and a barcodeed protein-biomarker capture mechanism on a single chip are ideal for point-of-care detection using a finger-prick sample. Other systems employ single-walled carbon nanotubes as multicolor Raman labels to achieve highly sensitive, multiplexed protein detection(201). Still other systems have coupled nanoporous silica chips that selectively enrich and stabilize low-molecular-weight peptides with mass spectrometry for highly sensitive biomarker detection(202). Coupling these nanotechnologies with stabile isotope mass spectrometry-based quantitation methods and/or specific gene subsets to plasma analyses exhibiting predictive or preventive value may significantly advance this field(203, 204). One example of this approach was used to monitor the progression of metastatic breast cancer in mouse xenografts(205). Analysis of urine also is under intensive development. Evaluation of urine biomarkers has been accomplished using near-infrared fluorescent core-shell silica-based nanoparticles (C dots)(206). Similar approaches have been applied to feces(207) and saliva(208). Obtaining these samples is minimally invasive and their use will afford effective monitoring, but are, for the most part, indirect. More direct measures include isolating circulating cells or DNA from blood coupled with next-generation sequencing to identify inherent risk.
Nanotechnology may also be directly applied to the analysis of tissue biopsies or exfoliated cells for early detection. The key advantage to having cellular material is the presence of a primary source of DNA, RNA and proteins. However, obtaining tissue biopsies is generally invasive and involves substantial discomfort and risk to the patient. Thus, obtaining biopsies is usually reserved for high-risk subjects. Another source of cellular material is exfoliated cells. The early detection of oral cancer using exfoliated cells is one example. A nano-biochip sensor technique was applied to exfoliative cytology specimens; these studies illustrate the advantage of targeting both biochemical and morphologic changes associated with early oral tumorigenesis(209). Other sources of exfoliated cells include the colon(210-212), lung(213, 214), bladder(215), cervix(216, 217), and breast(218). Ideally, the source of cells themselves or factors they produce would be suited to point-of-care monitoring(219).
Nanoscale Imaging
Nanotechnology-based procedures are expected to significantly improve the imaging of cancer(220), including early precancerous lesions. In addition to imaging uses already described (Table 1), other examples include magnetic nanoparticles (221), Qdots(222), gold nanoparticles(223), nanoshells(224), and nanotubes(225). When these technologies are coupled with the modification of cell-surface chemistry, adding epitope recognition or antibody/ligand binding sites can enable targeted homing. Coupling homing with unique magnetic signatures, tunable absorption and emission spectral properties, and advanced synthesis and physical characteristics can enhance the usefulness of nanoparticles as probes. A particularly exciting breakthrough involves hyperpolarized magnetic resonance imaging of select molecules and/or nanoparticles at up to 20,000-fold greater signal level for early detection of metabolic changes that occur in both premalignancies and cancer. (226, 227).
Targeted Delivery of Chemoprevention Agents using Nanoparticles
Achieving targeted imaging using nanoparticles in many cases affords the advantage of also delivering a site-specific chemopreventive payload. The selectiveness of these targeting systems can be driven on two levels. The first level includes the surface ligands involved in homing to the lesion site, and the second level relies on the specificity of released molecules that act directly on the target. In some cases, optimized delivery of novel nanoparticles to the tumor microenvironment may take advantage of unique or abnormal vasculature(228). In other cases, the presence of fibrotic stroma may enhance uptake and delivery(229). Furthermore, other cells such as mesenchymal stem cells may be harnessed to act as a targeted delivery system(230).
Another potential approach involves multistage nanovector delivery(179, 231). The rationale for the use of these more complex delivery systems involves many factors. They are expected to overcome endothelial and epithelial barriers or to be taken up by the reticulo-endothelial system(232), are biodegradable and exhibit favorable hemorheology characteristics in circulation(201). The largest component of these nanovectors acts as a porous shuttle that bears the payload to a particular site. This porous shuttle is loaded with smaller nanoliposomes that contain the targeted payload, such as siRNA(233). This type of delivery enables the sustained release of an agent at a specific target site.
Nanomedicine and Immune Modulation
The immune system is capable of recognizing, homing to, and killing premalignant and malignant cells. Harnessing the immune system and enhancing the immune response is another important application of nanotechnology. Nanotechnology can enhance the antibody response(234) and the response of specific immune cells, such as T-helper-17 cells, cytokines(235) and dendritic cells (236). Enhancing the effectiveness of vaccines is another objective(237). These efforts include nanoencapsulation to achieve transcutaneous delivery(238) as well as mucosal immunization(239). Targeted immune system recruitment may also enhance the antitumor immune response(240). These and other strategies may involve personalized regimens to eliminate tumors.
Vaccines can be very effective preventive measures. The prevention of cervical cancer through vaccine development against human papilloma virus (HPV) and early immunization is a good example. However, those individuals already infected with HPV may need other options for controlling or eliminating disease. The delivery of siRNA against NFkappaB through “phototermal transfection” was used to successfully treat nude mice bearing HeLa cervical cancer xenografts(241). This involved using hollow gold nanospheres, to deliver siRNA in conjunction with near-infrared light irradiation to elicit a photothermal effect along with micro-positron emission tomography/computed tomography imaging(241). Engineering viral nanoparticles may also be effective in treating cervical cancer. The use of RNAi/RGD-based mimoretrovirus to target the Zbtb7 gene is one example (242). This mimoretrovirus exhibited excellent anti-tumor capacity in vivo in a nude mouse model of cervical cancer(242). The utility of using nano-tools to target human HPV infected tissues for preventive management or virus eradication remains to be fully tested.
Nanopreventive Success Stories
Nanoparticle-mediated delivery is expected to limit the toxicity of chemopreventive agents while simultaneously enhancing bioavailability and sustained release. Various success stories continue to emerge involving the use of nanodelivery approaches. The application of nano-epigallocatechin-3-gallate (EGCG) is one example(243). EGCG encapsulated in polylactic acid-polyethylene glycol nanoparticles enhanced the biological effectiveness of EGCG at inducing apoptosis and reducing angiogenesis by 10-fold. The use of EGCG in a sustained food release setting has proven useful in a cancer nanochemoprevention setting(244).
Chemoprevention using nonsteriodal anti-inflammatory drugs (NSAIDs) has shown proven efficacy in treating high-risk colorectal cancer patients(245, 246). Cyclooxygenase-2 (COX-2) selective inhibitors, such as Celecoxib, are associated with increased cardiovascular risk(247). Various attempts have been made to incorporate celecoxib into nanoparticles to control release and minimize GI and cardiovascular toxicity(150, 248-252). When applied to arthritis, the compartmentalized injection of a nanolipid-celecoxib formulation directly into joints enabled a localized and gradual sustained release of celecoxib, without any significant increase in cardiovascular drug levels(252). These nanoized formulations of celecoxib also show promise for treating cancer(251). Other investigators used an ibuprofen-conjugated phosphatidylcholine formulation to enhance gastrointestinal safety and analgesic efficacy in osteoarthritic patients (253). Combinatorial nanoencapsulation of NSAIDs with other drugs may also be effective. For example, when aspirin, folic acid and calcium (AFAC) were incorporated into nanoparticles, the combination greatly reduced the formation of premalignant aberrant crypt foci in the colon tissues of azoxymethane-treated rats(156). Encapsulation of various NSAIDs or combination treatments continue to progress as promising approaches for the future of nanochemoprevention.
Another example of a combinatorial nanoparticle approach has also had a major impact on pancreatic cancer prevention. Prabhu et al. generated an aspirin, curcumin, and sulforaphane (ACS) combination in solid liquid nanoparticles (SLNs)(254). These ACS/SLNs were used to perform multimodal targeting of pancreatic cancer. A hamster pancreatic cancer model was generated using N-nitrosobis (2-oxopropyl) amine (BOP)-treatment that developed pancreatic intraepithelial neoplasms (PanIN) and tumors. Nanoencapsulated ACS regimens reduced tumor incidence by as high as 75% at doses 10 times lower than free drug combinations(254).
Other success stories involve nanoencapsulation of proven chemopreventive agents which help solve poor bioavailability problems. Curcumin, the active component of turmeric, is a prime example. Polymeric nanoparticle-encapsulated curcumin (NanoCurc) (255) inhibited tumor growth and systemic metastases in orthotopic pancreatic cancer xenograft models. This approach was also effective in delivering cocktails containing aspirin, curcumin and sulforaphane (ACS) to treat pancreatic cancer(254). Similarly, other forms of curcumin nanoparticles were effective in treating human lung tumor xenografts (256, 257). Enhancing bioavailability and the sustained release of poorly bioavailable compounds may serve as a successful formula for nanoprevention.
Are We There Yet?
We are just beginning our journey into the realm of nanoprevention. A good first step is to clearly identify reasonable goals for this fledgling area of research. High-priority areas where an impact could be made will depend on the type of premalignancy targeted. Finding ways to effectively couple “-omics” into rapid profiling and selective targeting of nano-tools for individualized treatment is an ambitious long-term goal. In the near term, improving theranostics based on more generalized molecular recognition signatures such as neoangiognenesis or viral infection may be a good start. For example, imaging and treatment of HPV as a high-risk factor for cervical or oral dysplasia/cancer by nanoparticle-mediated delivery of RNAi may be achievable. As a measurable outcome, this approach might effectively lead to complete clearance of HPV thereby preventing subsequent disease. As another potential near-term goal, improving methods for nanoencapsulation might enhance the sustained localized release of RNAi or anti-inflammatory agents. For example, one might envision a macro-micro-nanoencapsulation approach that achieves targeted release at the appropriate site in the GI tract. For the field to be successful, it is also critical to not only identify realizable goals but also potential barriers. Ultimately, we must merge the best ideas in prevention with the most effective and least risky nanotechnology.
Acknowledgments
This research was supported in part by the Boone Pickens Distinguished Chair for Early Prevention of Cancer to E. Hawk. Also from CPRIT RP100969 to D. G. Menter. Additional support included 5U54CA151668-03 to D. G. Menter, C. D. Logsdon and A. K. Sood and Cancer Center Support Grant 5P30CA016672-37 from the NIH. The authors are very thankful to Karen L. Colbert for editorial improvements in this manuscript.
Footnotes
The authors declare they have no conflicts of interest.
References
- 1.Feynman R. There’s Plenty of Room at the Bottom. Engineering and Science. 1960;23:5. [Google Scholar]
- 2.Drexler E. Nanosystems: Molecular Machinery, Manufacturing, and Computation. 1992 [Google Scholar]
- 3.Taniguchi N. On the Basic Concept of NanoTechnology. Proc ICPE. 1974 [Google Scholar]
- 4.Binnig B, Rohrer H, Gerber C, Weibel E. Tunneling through a controllable vacuum gap. Appl Phys Lett. 1982;40:178–81. [Google Scholar]
- 5.Kroto H, Heath J, O’Brien S, Curl R, Smalley R. C60: Buckminsterfullerene. Nature. 1985;318:162–3. [Google Scholar]
- 6.Higgins P, Dawson J, Walters M. Nanomedicine: Nanotubes reduce stroke damage. Nat Nanotechnol. 2011;6:83–4. doi: 10.1038/nnano.2011.5. [DOI] [PubMed] [Google Scholar]
- 7.Ohno H, Kobayashi T, Kabata R, Endo K, Iwasa T, Yoshimura SH, et al. Synthetic RNA-protein complex shaped like an equilateral triangle. Nat Nanotechnol. 2011;6:116–20. doi: 10.1038/nnano.2010.268. [DOI] [PubMed] [Google Scholar]
- 8.Guo P. The emerging field of RNA nanotechnology. Nat Nanotechnol. 2010;5:833–42. doi: 10.1038/nnano.2010.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zemp RJ. Nanomedicine: detecting rare cancer cells. Nat Nanotechnol. 2009;4:798–9. doi: 10.1038/nnano.2009.367. [DOI] [PubMed] [Google Scholar]
- 10.Sanhai WR, Sakamoto JH, Canady R, Ferrari M. Seven challenges for nanomedicine. Nat Nanotechnol. 2008;3:242–4. doi: 10.1038/nnano.2008.114. [DOI] [PubMed] [Google Scholar]
- 11.Han HD, Mora EM, Roh JW, Nishimura M, Lee SJ, Stone RL, et al. Chitosan hydrogel for localized gene silencing. Cancer Biol Ther. 2011;11:839–45. doi: 10.4161/cbt.11.9.15185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han HD, Mangala LS, Lee JW, Shahzad MM, Kim HS, Shen D, et al. Targeted gene silencing using RGD-labeled chitosan nanoparticles. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:3910–22. doi: 10.1158/1078-0432.CCR-10-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mann AP, Tanaka T, Somasunderam A, Liu X, Gorenstein DG, Ferrari M. E-selectin-targeted porous silicon particle for nanoparticle delivery to the bone marrow. Adv Mater. 2011;23:H278–82. doi: 10.1002/adma.201101541. [DOI] [PubMed] [Google Scholar]
- 14.Petrenko VA, Jayanna PK. Phage protein-targeted cancer nanomedicines. FEBS letters. 2014;588:341–9. doi: 10.1016/j.febslet.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loi M, Di Paolo D, Soster M, Brignole C, Bartolini A, Emionite L, et al. Novel phage display-derived neuroblastoma-targeting peptides potentiate the effect of drug nanocarriers in preclinical settings. J Control Release. 2013;170:233–41. doi: 10.1016/j.jconrel.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie X, Xia W, Li Z, Kuo HP, Liu Y, Ding Q, et al. Targeted expression of BikDD eradicates pancreatic tumors in noninvasive imaging models. Cancer Cell. 2007;12:52–65. doi: 10.1016/j.ccr.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Li Z, Ding Q, Li Y, Miller SA, Abbruzzese JL, Hung MC. Suppression of pancreatic tumor progression by systemic delivery of a pancreatic-cancer-specific promoter driven Bik mutant. Cancer Lett. 2006;236:58–63. doi: 10.1016/j.canlet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Lopez MV, Rivera AA, Viale DL, Benedetti L, Cuneo N, Kimball KJ, et al. A Tumor-stroma Targeted Oncolytic Adenovirus Replicated in Human Ovary Cancer Samples and Inhibited Growth of Disseminated Solid Tumors in Mice. Mol Ther. 2012 doi: 10.1038/mt.2012.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarkar D, Su ZZ, Park ES, Vozhilla N, Dent P, Curiel DT, et al. A cancer terminator virus eradicates both primary and distant human melanomas. Cancer Gene Ther. 2008;15:293–302. doi: 10.1038/cgt.2008.14. [DOI] [PubMed] [Google Scholar]
- 20.Ulasov IV, Zhu ZB, Tyler MA, Han Y, Rivera AA, Khramtsov A, et al. Survivin-driven and fiber-modified oncolytic adenovirus exhibits potent antitumor activity in established intracranial glioma. Hum Gene Ther. 2007;18:589–602. doi: 10.1089/hum.2007.002. [DOI] [PubMed] [Google Scholar]
- 21.Buzea C, Pacheco II, Robbie K. Nanomaterials and nanoparticles: sources and toxicity. Biointerphases. 2007;2:MR17–71. doi: 10.1116/1.2815690. [DOI] [PubMed] [Google Scholar]
- 22.Chen D, Liu W, Shen Y, Mu H, Zhang Y, Liang R, et al. Effects of a novel pH-sensitive liposome with cleavable esterase-catalyzed and pH-responsive double smart mPEG lipid derivative on ABC phenomenon. Int J Nanomedicine. 2011;6:2053–61. doi: 10.2147/IJN.S24344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riviere JE. Pharmacokinetics of nanomaterials: an overview of carbon nanotubes, fullerenes and quantum dots. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1:26–34. doi: 10.1002/wnan.24. [DOI] [PubMed] [Google Scholar]
- 24.Sarlo K, Blackburn KL, Clark ED, Grothaus J, Chaney J, Neu S, et al. Tissue distribution of 20 nm, 100 nm and 1000 nm fluorescent polystyrene latex nanospheres following acute systemic or acute and repeat airway exposure in the rat. Toxicology. 2009;263:117–26. doi: 10.1016/j.tox.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Toll R, Jacobi U, Richter H, Lademann J, Schaefer H, Blume-Peytavi U. Penetration profile of microspheres in follicular targeting of terminal hair follicles. J Invest Dermatol. 2004;123:168–76. doi: 10.1111/j.0022-202X.2004.22717.x. [DOI] [PubMed] [Google Scholar]
- 26.Tinkle SS, Antonini JM, Rich BA, Roberts JR, Salmen R, DePree K, et al. Skin as a route of exposure and sensitization in chronic beryllium disease. Environ Health Perspect. 2003;111:1202–8. doi: 10.1289/ehp.5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113:823–39. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsuji JS, Maynard AD, Howard PC, James JT, Lam CW, Warheit DB, et al. Research strategies for safety evaluation of nanomaterials, part IV: risk assessment of nanoparticles. Toxicol Sci. 2006;89:42–50. doi: 10.1093/toxsci/kfi339. [DOI] [PubMed] [Google Scholar]
- 29.Bennat C, Muller-Goymann CC. Skin penetration and stabilization of formulations containing microfine titanium dioxide as physical UV filter. Int J Cosmet Sci. 2000;22:271–83. doi: 10.1046/j.1467-2494.2000.00009.x. [DOI] [PubMed] [Google Scholar]
- 30.Jani P, Halbert GW, Langridge J, Florence AT. Nanoparticle uptake by the rat gastrointestinal mucosa: quantitation and particle size dependency. J Pharm Pharmacol. 1990;42:821–6. doi: 10.1111/j.2042-7158.1990.tb07033.x. [DOI] [PubMed] [Google Scholar]
- 31.Hoet PH, Bruske-Hohlfeld I, Salata OV. Nanoparticles - known and unknown health risks. J Nanobiotechnology. 2004;2:12. doi: 10.1186/1477-3155-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lomer MC, Cook WB, Jan-Mohamed HJ, Hutchinson C, Liu DY, Hider RC, et al. Iron requirements based upon iron absorption tests are poorly predicted by haematological indices in patients with inactive inflammatory bowel disease. Br J Nutr. 2012;107:1806–11. doi: 10.1017/S0007114511004971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi HS, Ashitate Y, Lee JH, Kim SH, Matsui A, Insin N, et al. Rapid translocation of nanoparticles from the lung airspaces to the body. Nature biotechnology. 2010;28:1300–3. doi: 10.1038/nbt.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7:771–82. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 35.Adriani G, de Tullio MD, Ferrari M, Hussain F, Pascazio G, Liu X, et al. The preferential targeting of the diseased microvasculature by disk-like particles. Biomaterials. 2012;33:5504–13. doi: 10.1016/j.biomaterials.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boso DP, Lee SY, Ferrari M, Schrefler BA, Decuzzi P. Optimizing particle size for targeting diseased microvasculature: from experiments to artificial neural networks. Int J Nanomedicine. 2011;6:1517–26. doi: 10.2147/IJN.S20283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Batist G, Barton J, Chaikin P, Swenson C, Welles L. Myocet (liposome-encapsulated doxorubicin citrate): a new approach in breast cancer therapy. Expert opinion on pharmacotherapy. 2002;3:1739–51. doi: 10.1517/14656566.3.12.1739. [DOI] [PubMed] [Google Scholar]
- 38.Cabriales S, Bresnahan J, Testa D, Espina BM, Scadden DT, Ross M, et al. Extravasation of liposomal daunorubicin in patients with AIDS-associated Kaposi’s sarcoma: a report of four cases. Oncology nursing forum. 1998;25:67–70. [PubMed] [Google Scholar]
- 39.Gelmon KA, Tolcher A, Diab AR, Bally MB, Embree L, Hudon N, et al. Phase I study of liposomal vincristine. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1999;17:697–705. doi: 10.1200/JCO.1999.17.2.697. [DOI] [PubMed] [Google Scholar]
- 40.Glantz MJ, LaFollette S, Jaeckle KA, Shapiro W, Swinnen L, Rozental JR, et al. Randomized trial of a slow-release versus a standard formulation of cytarabine for the intrathecal treatment of lymphomatous meningitis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1999;17:3110–6. doi: 10.1200/JCO.1999.17.10.3110. [DOI] [PubMed] [Google Scholar]
- 41.Chen KJ, Chaung EY, Wey SP, Lin KJ, Cheng F, Lin CC, et al. Hyperthermia-mediated local drug delivery by a bubble-generating liposomal system for tumor-specific chemotherapy. ACS Nano. 2014;8:5105–15. doi: 10.1021/nn501162x. [DOI] [PubMed] [Google Scholar]
- 42.Hengge UR, Brockmeyer NH, Baumann M, Reimann G, Goos M. Liposomal doxorubicin in AIDS-related Kaposi’s sarcoma. Lancet. 1993;342:497. doi: 10.1016/0140-6736(93)91624-u. [DOI] [PubMed] [Google Scholar]
- 43.Stathopoulos GP, Boulikas T, Vougiouka M, Deliconstantinos G, Rigatos S, Darli E, et al. Pharmacokinetics and adverse reactions of a new liposomal cisplatin (Lipoplatin): phase I study. Oncol Rep. 2005;13:589–95. [PubMed] [Google Scholar]
- 44.Pramanik D, Campbell NR, Das S, Gupta S, Chenna V, Bisht S, et al. A composite polymer nanoparticle overcomes multidrug resistance and ameliorates doxorubicin-associated cardiomyopathy. Oncotarget. 2012;3:640–50. doi: 10.18632/oncotarget.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coelho T, Adams D, Silva A, Lozeron P, Hawkins PN, Mant T, et al. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N Engl J Med. 2013;369:819–29. doi: 10.1056/NEJMoa1208760. [DOI] [PubMed] [Google Scholar]
- 46.Fitzgerald K, Frank-Kamenetsky M, Shulga-Morskaya S, Liebow A, Bettencourt BR, Sutherland JE, et al. Effect of an RNA interference drug on the synthesis of proprotein convertase subtilisin/kexin type 9 (PCSK9) and the concentration of serum LDL cholesterol in healthy volunteers: a randomised, single-blind, placebo-controlled, phase 1 trial. Lancet. 2014;383:60–8. doi: 10.1016/S0140-6736(13)61914-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dahlman JE, Barnes C, Khan O, Thiriot A, Jhunjunwala S, Shaw TE, et al. In vivo endothelial siRNA delivery using polymeric nanoparticles with low molecular weight. Nat Nanotechnol. 2014 doi: 10.1038/nnano.2014.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moreno-Aspitia A, Perez EA. Nanoparticle albumin-bound paclitaxel (ABI-007): a newer taxane alternative in breast cancer. Future oncology. 2005;1:755–62. doi: 10.2217/14796694.1.6.755. [DOI] [PubMed] [Google Scholar]
- 49.Ettinger LJ, Kurtzberg J, Voute PA, Jurgens H, Halpern SL. An open-label, multicenter study of polyethylene glycol-L-asparaginase for the treatment of acute lymphoblastic leukemia. Cancer. 1995;75:1176–81. doi: 10.1002/1097-0142(19950301)75:5<1176::aid-cncr2820750519>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 50.Kim TY, Kim DW, Chung JY, Shin SG, Kim SC, Heo DS, et al. Phase I and pharmacokinetic study of Genexol-PM, a cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies. Clin Cancer Res. 2004;10:3708–16. doi: 10.1158/1078-0432.CCR-03-0655. [DOI] [PubMed] [Google Scholar]
- 51.Nowotnik DP, Cvitkovic E. ProLindac (AP5346): a review of the development of an HPMA DACH platinum Polymer Therapeutic. Adv Drug Deliv Rev. 2009;61:1214–9. doi: 10.1016/j.addr.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 52.Hrkach J, Von Hoff D, Mukkaram Ali M, Andrianova E, Auer J, Campbell T, et al. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Science translational medicine. 2012;4:128ra39. doi: 10.1126/scitranslmed.3003651. [DOI] [PubMed] [Google Scholar]
- 53.Wuolijoki E, Hirvela T, Ylitalo P. Decrease in serum LDL cholesterol with microcrystalline chitosan. Methods and findings in experimental and clinical pharmacology. 1999;21:357–61. doi: 10.1358/mf.1999.21.5.793477. [DOI] [PubMed] [Google Scholar]
- 54.Howard KA, Rahbek UL, Liu X, Damgaard CK, Glud SZ, Andersen MO, et al. RNA interference in vitro and in vivo using a novel chitosan/siRNA nanoparticle system. Mol Ther. 2006;14:476–84. doi: 10.1016/j.ymthe.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 55.Li X, Ferrel GL, Guerra MC, Hode T, Lunn JA, Adalsteinsson O, et al. Preliminary safety and efficacy results of laser immunotherapy for the treatment of metastatic breast cancer patients. Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology. 2011;10:817–21. doi: 10.1039/c0pp00306a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roy K, Mao HQ, Huang SK, Leong KW. Oral gene delivery with chitosan--DNA nanoparticles generates immunologic protection in a murine model of peanut allergy. Nat Med. 1999;5:387–91. doi: 10.1038/7385. [DOI] [PubMed] [Google Scholar]
- 57.Chen C, Zhang H, Hou L, Shi J, Wang L, Zhang C, et al. Single-walled carbon nanotubes mediated neovascularity targeted antitumor drug delivery system. Journal of pharmacy & pharmaceutical sciences : a publication of the Canadian Society for Pharmaceutical Sciences, Societe canadienne des sciences pharmaceutiques. 2013;16:40–51. doi: 10.18433/j3h02c. [DOI] [PubMed] [Google Scholar]
- 58.Injac R, Perse M, Obermajer N, Djordjevic-Milic V, Prijatelj M, Djordjevic A, et al. Potential hepatoprotective effects of fullerenol C60(OH)24 in doxorubicin-induced hepatotoxicity in rats with mammary carcinomas. Biomaterials. 2008;29:3451–60. doi: 10.1016/j.biomaterials.2008.04.048. [DOI] [PubMed] [Google Scholar]
- 59.Kwag DS, Park K, Oh KT, Lee ES. Hyaluronated fullerenes with photoluminescent and antitumoral activity. Chemical communications. 2013;49:282–4. doi: 10.1039/c2cc36596k. [DOI] [PubMed] [Google Scholar]
- 60.Ren J, Shen S, Wang D, Xi Z, Guo L, Pang Z, et al. The targeted delivery of anticancer drugs to brain glioma by PEGylated oxidized multi-walled carbon nanotubes modified with angiopep-2. Biomaterials. 2012;33:3324–33. doi: 10.1016/j.biomaterials.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 61.Sacchetti C, Rapini N, Magrini A, Cirelli E, Bellucci S, Mattei M, et al. In vivo targeting of intratumor regulatory T cells using PEG-modified single-walled carbon nanotubes. Bioconjugate chemistry. 2013;24:852–8. doi: 10.1021/bc400070q. [DOI] [PubMed] [Google Scholar]
- 62.Shao W, Paul A, Zhao B, Lee C, Rodes L, Prakash S. Carbon nanotube lipid drug approach for targeted delivery of a chemotherapy drug in a human breast cancer xenograft animal model. Biomaterials. 2013;34:10109–19. doi: 10.1016/j.biomaterials.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 63.Shi J, Zhang H, Wang L, Li L, Wang H, Wang Z, et al. PEI-derivatized fullerene drug delivery using folate as a homing device targeting to tumor. Biomaterials. 2013;34:251–61. doi: 10.1016/j.biomaterials.2012.09.039. [DOI] [PubMed] [Google Scholar]
- 64.Benezra M, Penate-Medina O, Zanzonico PB, Schaer D, Ow H, Burns A, et al. Multimodal silica nanoparticles are effective cancer-targeted probes in a model of human melanoma. The Journal of clinical investigation. 2011;121:2768–80. doi: 10.1172/JCI45600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shen H, Rodriguez-Aguayo C, Xu R, Gonzalez-Villasana V, Mai J, Huang Y, et al. Enhancing chemotherapy response with sustained EphA2 silencing using multistage vector delivery. Clin Cancer Res. 2013;19:1806–15. doi: 10.1158/1078-0432.CCR-12-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu SH, Lin YS, Hung Y, Chou YH, Hsu YH, Chang C, et al. Multifunctional mesoporous silica nanoparticles for intracellular labeling and animal magnetic resonance imaging studies. Chembiochem : a European journal of chemical biology. 2008;9:53–7. doi: 10.1002/cbic.200700509. [DOI] [PubMed] [Google Scholar]
- 67.Fatehi D, Baral TN, Abulrob A. In vivo imaging of brain cancer using epidermal growth factor single domain antibody bioconjugated to near-infrared quantum dots. J Nanosci Nanotechnol. 2014;14:5355–62. doi: 10.1166/jnn.2014.9076. [DOI] [PubMed] [Google Scholar]
- 68.Kwon H, Lee J, Song R, Hwang SI, Lee J, Kim YH, et al. In vitro and in vivo imaging of prostate cancer angiogenesis using anti-vascular endothelial growth factor receptor 2 antibody-conjugated quantum dot. Korean journal of radiology : official journal of the Korean Radiological Society. 2013;14:30–7. doi: 10.3348/kjr.2013.14.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Savla R, Taratula O, Garbuzenko O, Minko T. Tumor targeted quantum dot-mucin 1 aptamer-doxorubicin conjugate for imaging and treatment of cancer. J Control Release. 2011;153:16–22. doi: 10.1016/j.jconrel.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 70.Arvizo RR, Saha S, Wang E, Robertson JD, Bhattacharya R, Mukherjee P. Inhibition of tumor growth and metastasis by a self-therapeutic nanoparticle. Proc Natl Acad Sci U S A. 2013;110:6700–5. doi: 10.1073/pnas.1214547110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bobyk L, Edouard M, Deman P, Vautrin M, Pernet-Gallay K, Delaroche J, et al. Photoactivation of gold nanoparticles for glioma treatment. Nanomedicine. 2013;9:1089–97. doi: 10.1016/j.nano.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 72.Li FR, Li Q, Zhou HX, Qi H, Deng CY. Detection of circulating tumor cells in breast cancer with a refined immunomagnetic nanoparticle enriched assay and nested-RT-PCR. Nanomedicine. 2013;9:1106–13. doi: 10.1016/j.nano.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 73.Shenoi MM, Iltis I, Choi J, Koonce NA, Metzger GJ, Griffin RJ, et al. Nanoparticle delivered vascular disrupting agents (VDAs): use of TNF-alpha conjugated gold nanoparticles for multimodal cancer therapy. Molecular pharmaceutics. 2013;10:1683–94. doi: 10.1021/mp300505w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jain S, Hirst DG, O’Sullivan JM. Gold nanoparticles as novel agents for cancer therapy. The British journal of radiology. 2012;85:101–13. doi: 10.1259/bjr/59448833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Heath JR, Davis ME. Nanotechnology and cancer. Annu Rev Med. 2008;59:251–65. doi: 10.1146/annurev.med.59.061506.185523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chauhan VP, Jain RK. Strategies for advancing cancer nanomedicine. Nature materials. 2013;12:958–62. doi: 10.1038/nmat3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Otterson GA, Villalona-Calero MA, Hicks W, Pan X, Ellerton JA, Gettinger SN, et al. Phase I/II study of inhaled doxorubicin combined with platinum-based therapy for advanced non-small cell lung cancer. Clin Cancer Res. 2010;16:2466–73. doi: 10.1158/1078-0432.CCR-09-3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lam S, leRiche JC, McWilliams A, Macaulay C, Dyachkova Y, Szabo E, et al. A randomized phase IIb trial of pulmicort turbuhaler (budesonide) in people with dysplasia of the bronchial epithelium. Clin Cancer Res. 2004;10:6502–11. doi: 10.1158/1078-0432.CCR-04-0686. [DOI] [PubMed] [Google Scholar]
- 79.Dahl AR, Grossi IM, Houchens DP, Scovell LJ, Placke ME, Imondi AR, et al. Inhaled isotretinoin (13-cis retinoic acid) is an effective lung cancer chemopreventive agent in A/J mice at low doses: a pilot study. Clin Cancer Res. 2000;6:3015–24. [PubMed] [Google Scholar]
- 80.Mulshine JL, Hirsch FR. Lung cancer chemoprevention: moving from concept to a reality. Lung cancer. 2003;41(Suppl 1):S163–74. doi: 10.1016/s0169-5002(03)00160-0. [DOI] [PubMed] [Google Scholar]
- 81.Kammona O, Kiparissides C. Recent advances in nanocarrier-based mucosal delivery of biomolecules. J Control Release. 2012;161:781–94. doi: 10.1016/j.jconrel.2012.05.040. [DOI] [PubMed] [Google Scholar]
- 82.Manke A, Wang L, Rojanasakul Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. BioMed research international. 2013;2013:942916. doi: 10.1155/2013/942916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Su CK, Sun YC. In vivo monitoring of distributional transport kinetics and extravasation of quantum dots in living rat liver. Nanotechnology. 2013;24:165101. doi: 10.1088/0957-4484/24/16/165101. [DOI] [PubMed] [Google Scholar]
- 84.Menter DG, Tucker SC, Kopetz S, Sood AK, Crissman JD, Honn KV. Platelets and cancer: a casual or causal relationship: revisited. Cancer metastasis reviews. 2014 doi: 10.1007/s10555-014-9498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ilinskaya AN, Dobrovolskaia MA. Nanoparticles and the blood coagulation system. Part I: benefits of nanotechnology. Nanomedicine (Lond) 2013;8:773–84. doi: 10.2217/nnm.13.48. [DOI] [PubMed] [Google Scholar]
- 86.Samuel SP, Jain N, O’Dowd F, Paul T, Kashanin D, Gerard VA, et al. Multifactorial determinants that govern nanoparticle uptake by human endothelial cells under flow. Int J Nanomedicine. 2012;7:2943–56. doi: 10.2147/IJN.S30624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.De Paoli SH, Diduch LL, Tegegn TZ, Orecna M, Strader MB, Karnaukhova E, et al. The effect of protein corona composition on the interaction of carbon nanotubes with human blood platelets. Biomaterials. 2014 doi: 10.1016/j.biomaterials.2014.04.067. [DOI] [PubMed] [Google Scholar]
- 88.Grozovsky R, Hoffmeister KM, Falet H. Novel clearance mechanisms of platelets. Current opinion in hematology. 2010;17:585–9. doi: 10.1097/MOH.0b013e32833e7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fadeel B. Clear and present danger? Engineered nanoparticles and the immune system. Swiss medical weekly. 2012;142:w13609. doi: 10.4414/smw.2012.13609. [DOI] [PubMed] [Google Scholar]
- 90.Peng Q, Zhang S, Yang Q, Zhang T, Wei XQ, Jiang L, et al. Preformed albumin corona, a protective coating for nanoparticles based drug delivery system. Biomaterials. 2013;34:8521–30. doi: 10.1016/j.biomaterials.2013.07.102. [DOI] [PubMed] [Google Scholar]
- 91.Sarkar A, Ghosh M, Sil PC. Nanotoxicity: oxidative stress mediated toxicity of metal and metal oxide nanoparticles. J Nanosci Nanotechnol. 2014;14:730–43. doi: 10.1166/jnn.2014.8752. [DOI] [PubMed] [Google Scholar]
- 92.Fu PP, Xia Q, Hwang HM, Ray PC, Yu H. Mechanisms of nanotoxicity: generation of reactive oxygen species. Journal of food and drug analysis. 2014;22:64–75. doi: 10.1016/j.jfda.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Seabra AB, Paula AJ, de Lima R, Alves OL, Duran N. Nanotoxicity of graphene and graphene oxide. Chemical research in toxicology. 2014;27:159–68. doi: 10.1021/tx400385x. [DOI] [PubMed] [Google Scholar]
- 94.Li J, Chang X, Chen X, Gu Z, Zhao F, Chai Z, et al. Toxicity of inorganic nanomaterials in biomedical imaging. Biotechnology advances. 2014 doi: 10.1016/j.biotechadv.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 95.McCarthy DP, Hunter ZN, Chackerian B, Shea LD, Miller SD. Targeted immunomodulation using antigen-conjugated nanoparticles. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2014;6:298–315. doi: 10.1002/wnan.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fang RH, Hu CM, Luk BT, Gao W, Copp JA, Tai Y, et al. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett. 2014;14:2181–8. doi: 10.1021/nl500618u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Abdelhalim MA. Uptake of gold nanoparticles in several rat organs after intraperitoneal administration in vivo: a fluorescence study. BioMed research international. 2013;2013:353695. doi: 10.1155/2013/353695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hallaj-Nezhadi S, Dass CR, Lotfipour F. Intraperitoneal delivery of nanoparticles for cancer gene therapy. Future oncology. 2013;9:59–68. doi: 10.2217/fon.12.171. [DOI] [PubMed] [Google Scholar]
- 99.Nakamura M, Miyamoto K, Hayashi K, Awaad A, Ochiai M, Ishimura K. Time-lapse fluorescence imaging and quantitative single cell and endosomal analysis of peritoneal macrophages using fluorescent organosilica nanoparticles. Nanomedicine. 2013;9:274–83. doi: 10.1016/j.nano.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 100.Sarhan OM, Hussein RM. Effects of intraperitoneally injected silver nanoparticles on histological structures and blood parameters in the albino rat. Int J Nanomedicine. 2014;9:1505–17. doi: 10.2147/IJN.S56729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Arvizo RR, Miranda OR, Moyano DF, Walden CA, Giri K, Bhattacharya R, et al. Modulating pharmacokinetics, tumor uptake and biodistribution by engineered nanoparticles. PloS one. 2011;6:e24374. doi: 10.1371/journal.pone.0024374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang Y, Bai Y, Jia J, Gao N, Li Y, Zhang R, et al. Perturbation of physiological systems by nanoparticles. Chem Soc Rev. 2014;43:3762–809. doi: 10.1039/c3cs60338e. [DOI] [PubMed] [Google Scholar]
- 103.Yang W, Peters JI, Williams RO., 3rd Inhaled nanoparticles--a current review. Int J Pharm. 2008;356:239–47. doi: 10.1016/j.ijpharm.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 104.Roberts RA, Shen T, Allen IC, Hasan W, DeSimone JM, Ting JP. Analysis of the murine immune response to pulmonary delivery of precisely fabricated nano- and microscale particles. PloS one. 2013;8:e62115. doi: 10.1371/journal.pone.0062115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schleh C, Rothen-Rutishauser B, Kreyling WG. The influence of pulmonary surfactant on nanoparticulate drug delivery systems. Eur J Pharm Biopharm. 2011;77:350–2. doi: 10.1016/j.ejpb.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 106.Naota M, Shimada A, Morita T, Yamamoto Y, Inoue K, Takano H. Caveolae-mediated endocytosis of intratracheally instilled gold colloid nanoparticles at the air-blood barrier in mice. Toxicologic pathology. 2013;41:487–96. doi: 10.1177/0192623312457271. [DOI] [PubMed] [Google Scholar]
- 107.Muhlfeld C, Rothen-Rutishauser B, Blank F, Vanhecke D, Ochs M, Gehr P. Interactions of nanoparticles with pulmonary structures and cellular responses. American journal of physiology Lung cellular and molecular physiology. 2008;294:L817–29. doi: 10.1152/ajplung.00442.2007. [DOI] [PubMed] [Google Scholar]
- 108.Reisetter AC, Stebounova LV, Baltrusaitis J, Powers L, Gupta A, Grassian VH, et al. Induction of inflammasome-dependent pyroptosis by carbon black nanoparticles. J Biol Chem. 2011;286:21844–52. doi: 10.1074/jbc.M111.238519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kolanjiyil AV, Kleinstreuer C. Nanoparticle mass transfer from lung airways to systemic regions--Part I: Whole-lung aerosol dynamics. Journal of biomechanical engineering. 2013;135:121003. doi: 10.1115/1.4025332. [DOI] [PubMed] [Google Scholar]
- 110.Zhu MT, Feng WY, Wang B, Wang TC, Gu YQ, Wang M, et al. Comparative study of pulmonary responses to nano- and submicron-sized ferric oxide in rats. Toxicology. 2008;247:102–11. doi: 10.1016/j.tox.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 111.Jones MC, Jones SA, Riffo-Vasquez Y, Spina D, Hoffman E, Morgan A, et al. Quantitative assessment of nanoparticle surface hydrophobicity and its influence on pulmonary biocompatibility. J Control Release. 2014;183:94–104. doi: 10.1016/j.jconrel.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 112.Zarogoulidis P, Chatzaki E, Porpodis K, Domvri K, Hohenforst-Schmidt W, Goldberg EP, et al. Inhaled chemotherapy in lung cancer: future concept of nanomedicine. Int J Nanomedicine. 2012;7:1551–72. doi: 10.2147/IJN.S29997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Garg T, Rath G, Goyal AK. Comprehensive review on additives of topical dosage forms for drug delivery. Drug Deliv. 2014 doi: 10.3109/10717544.2013.879355. [DOI] [PubMed] [Google Scholar]
- 114.DeLouise LA. Applications of nanotechnology in dermatology. J Invest Dermatol. 2012;132:964–75. doi: 10.1038/jid.2011.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Konieczny P, Goralczyk AG, Szmyd R, Skalniak L, Koziel J, Filon FL, et al. Effects triggered by platinum nanoparticles on primary keratinocytes. Int J Nanomedicine. 2013;8:3963–75. doi: 10.2147/IJN.S49612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kaur J, Tikoo K. Evaluating cell specific cytotoxicity of differentially charged silver nanoparticles. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2013;51:1–14. doi: 10.1016/j.fct.2012.08.044. [DOI] [PubMed] [Google Scholar]
- 117.Hackenberg S, Kleinsasser N. Dermal toxicity of ZnO nanoparticles: a worrying feature of sunscreen? Nanomedicine (Lond) 2012;7:461–3. doi: 10.2217/nnm.12.23. [DOI] [PubMed] [Google Scholar]
- 118.Tang L, Zhang C, Song G, Jin X, Xu Z. In vivo skin penetration and metabolic path of quantum dots. Science China Life sciences. 2013;56:181–8. doi: 10.1007/s11427-012-4404-x. [DOI] [PubMed] [Google Scholar]
- 119.Yu KN, Yoon TJ, Minai-Tehrani A, Kim JE, Park SJ, Jeong MS, et al. Zinc oxide nanoparticle induced autophagic cell death and mitochondrial damage via reactive oxygen species generation. Toxicology in vitro : an international journal published in association with BIBRA. 2013;27:1187–95. doi: 10.1016/j.tiv.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 120.Vandebriel RJ, De Jong WH. A review of mammalian toxicity of ZnO nanoparticles. Nanotechnology, science and applications. 2012;5:61–71. doi: 10.2147/NSA.S23932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gao W, Vecchio D, Li J, Zhu J, Zhang Q, Fu V, et al. Hydrogel containing nanoparticle-stabilized liposomes for topical antimicrobial delivery. ACS Nano. 2014;8:2900–7. doi: 10.1021/nn500110a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Eke G, Kuzmina AM, Goreva AV, Shishatskaya EI, Hasirci N, Hasirci V. In vitro and transdermal penetration of PHBV micro/nanoparticles. Journal of materials science Materials in medicine. 2014 doi: 10.1007/s10856-014-5169-5. [DOI] [PubMed] [Google Scholar]
- 123.Cho HK, Cho JH, Jeong SH, Cho DC, Yeum JH, Cheong IW. Polymeric vehicles for topical delivery and related analytical methods. Archives of pharmacal research. 2014;37:423–34. doi: 10.1007/s12272-014-0342-4. [DOI] [PubMed] [Google Scholar]
- 124.Wadajkar AS, Bhavsar Z, Ko CY, Koppolu B, Cui W, Tang L, et al. Multifunctional particles for melanoma-targeted drug delivery. Acta biomaterialia. 2012;8:2996–3004. doi: 10.1016/j.actbio.2012.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yun Y, Cho YW, Park K. Nanoparticles for oral delivery: targeted nanoparticles with peptidic ligands for oral protein delivery. Adv Drug Deliv Rev. 2013;65:822–32. doi: 10.1016/j.addr.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Landsiedel R, Fabian E, Ma-Hock L, van Ravenzwaay B, Wohlleben W, Wiench K, et al. Toxico-/biokinetics of nanomaterials. Archives of toxicology. 2012;86:1021–60. doi: 10.1007/s00204-012-0858-7. [DOI] [PubMed] [Google Scholar]
- 127.Wang H, Du LJ, Song ZM, Chen XX. Progress in the characterization and safety evaluation of engineered inorganic nanomaterials in food. Nanomedicine (Lond) 2013;8:2007–25. doi: 10.2217/nnm.13.176. [DOI] [PubMed] [Google Scholar]
- 128.Bernkop-Schnurch A. Nanocarrier systems for oral drug delivery: do we really need them? Eur J Pharm Sci. 2013;49:272–7. doi: 10.1016/j.ejps.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 129.Shahbazi MA, Santos HA. Improving oral absorption via drug-loaded nanocarriers: absorption mechanisms, intestinal models and rational fabrication. Current drug metabolism. 2013;14:28–56. [PubMed] [Google Scholar]
- 130.Feng C, Wang Z, Jiang C, Kong M, Zhou X, Li Y, et al. Chitosan/o-carboxymethyl chitosan nanoparticles for efficient and safe oral anticancer drug delivery: in vitro and in vivo evaluation. Int J Pharm. 2013;457:158–67. doi: 10.1016/j.ijpharm.2013.07.079. [DOI] [PubMed] [Google Scholar]
- 131.Kim SH, Lee KY, Jang YS. Mucosal Immune System and M Cell-targeting Strategies for Oral Mucosal Vaccination. Immune Netw. 2012;12:165–75. doi: 10.4110/in.2012.12.5.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Knoop KA, Miller MJ, Newberry RD. Transepithelial antigen delivery in the small intestine: different paths, different outcomes. Curr Opin Gastroenterol. 2013;29:112–8. doi: 10.1097/MOG.0b013e32835cf1cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Menard S, Cerf-Bensussan N, Heyman M. Multiple facets of intestinal permeability and epithelial handling of dietary antigens. Mucosal Immunol. 2010;3:247–59. doi: 10.1038/mi.2010.5. [DOI] [PubMed] [Google Scholar]
- 134.Schulz O, Pabst O. Antigen sampling in the small intestine. Trends Immunol. 2013;34:155–61. doi: 10.1016/j.it.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 135.Crater JS, Carrier RL. Barrier properties of gastrointestinal mucus to nanoparticle transport. Macromolecular bioscience. 2010;10:1473–83. doi: 10.1002/mabi.201000137. [DOI] [PubMed] [Google Scholar]
- 136.Bergin IL, Witzmann FA. Nanoparticle toxicity by the gastrointestinal route: evidence and knowledge gaps. International journal of biomedical nanoscience and nanotechnology. 2013;3 doi: 10.1504/IJBNN.2013.054515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kunisawa J, Kurashima Y, Kiyono H. Gut-associated lymphoid tissues for the development of oral vaccines. Advanced drug delivery reviews. 2012;64:523–30. doi: 10.1016/j.addr.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 138.Mallegol J, Van Niel G, Lebreton C, Lepelletier Y, Candalh C, Dugave C, et al. T84-intestinal epithelial exosomes bear MHC class II/peptide complexes potentiating antigen presentation by dendritic cells. Gastroenterology. 2007;132:1866–76. doi: 10.1053/j.gastro.2007.02.043. [DOI] [PubMed] [Google Scholar]
- 139.Yoshida M, Claypool SM, Wagner JS, Mizoguchi E, Mizoguchi A, Roopenian DC, et al. Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity. 2004;20:769–83. doi: 10.1016/j.immuni.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 140.Faust JJ, Masserano BM, Mielke AH, Abraham A, Capco DG. Engineered nanoparticles induced brush border disruption in a human model of the intestinal epithelium. Adv Exp Med Biol. 2014;811:55–72. doi: 10.1007/978-94-017-8739-0_4. [DOI] [PubMed] [Google Scholar]
- 141.Arques JL, Hautefort I, Ivory K, Bertelli E, Regoli M, Clare S, et al. Salmonella induces flagellin- and MyD88-dependent migration of bacteria-capturing dendritic cells into the gut lumen. Gastroenterology. 2009;137:579–87. 87–e1-2. doi: 10.1053/j.gastro.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 142.McDole JR, Wheeler LW, McDonald KG, Wang B, Konjufca V, Knoop KA, et al. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature. 2012;483:345–9. doi: 10.1038/nature10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–7. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 144.Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–8. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 145.Chieppa M, Rescigno M, Huang AY, Germain RN. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med. 2006;203:2841–52. doi: 10.1084/jem.20061884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Van Niel G, Mallegol J, Bevilacqua C, Candalh C, Brugiere S, Tomaskovic-Crook E, et al. Intestinal epithelial exosomes carry MHC class II/peptides able to inform the immune system in mice. Gut. 2003;52:1690–7. doi: 10.1136/gut.52.12.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mifflin RC, Pinchuk IV, Saada JI, Powell DW. Intestinal myofibroblasts: targets for stem cell therapy. Am J Physiol Gastrointest Liver Physiol. 2011;300:G684–96. doi: 10.1152/ajpgi.00474.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pinchuk IV, Mifflin RC, Saada JI, Powell DW. Intestinal mesenchymal cells. Curr Gastroenterol Rep. 2010;12:310–8. doi: 10.1007/s11894-010-0135-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Powell DW, Pinchuk IV, Saada JI, Chen X, Mifflin RC. Mesenchymal cells of the intestinal lamina propria. Annu Rev Physiol. 2011;73:213–37. doi: 10.1146/annurev.physiol.70.113006.100646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhao P, Jiang H, Jiang T, Zhi Z, Wu C, Sun C, et al. Inclusion of celecoxib into fibrous ordered mesoporous carbon for enhanced oral bioavailability and reduced gastric irritancy. Eur J Pharm Sci. 2012;45:639–47. doi: 10.1016/j.ejps.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 151.Zhu Q, Talton J, Zhang G, Cunningham T, Wang Z, Waters RC, et al. Large intestine-targeted, nanoparticle-releasing oral vaccine to control genitorectal viral infection. Nat Med. 2012;18:1291–6. doi: 10.1038/nm.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Laroui H, Sitaraman SV, Merlin D. Gastrointestinal delivery of anti-inflammatory nanoparticles. Methods in enzymology. 2012;509:101–25. doi: 10.1016/B978-0-12-391858-1.00006-X. [DOI] [PubMed] [Google Scholar]
- 153.Cote R, Suster S, Weiss L, Weidne N. Modern Surgical Pathology. Philadelphia, PA: Saunders; 2009. [Google Scholar]
- 154.Dreaden EC, Austin LA, Mackey MA, El-Sayed MA. Size matters: gold nanoparticles in targeted cancer drug delivery. Therapeutic delivery. 2012;3:457–78. doi: 10.4155/tde.12.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Upreti M, Jyoti A, Sethi P. Tumor microenvironment and nanotherapeutics. Translational cancer research. 2013;2:309–19. doi: 10.3978/j.issn.2218-676X.2013.08.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chaudhary A, Sutaria D, Huang Y, Wang J, Prabhu S. Chemoprevention of colon cancer in a rat carcinogenesis model using a novel nanotechnology-based combined treatment system. Cancer prevention research. 2011;4:1655–64. doi: 10.1158/1940-6207.CAPR-11-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Soundararajan V, Warnock K, Sasisekharan R. Multifunctional nanoscale platforms for targeting of the cancer cell immortality spectrum. Macromol Rapid Commun. 2010;31:202–16. doi: 10.1002/marc.200900596. [DOI] [PubMed] [Google Scholar]
- 158.Daka A, Peer D. RNAi-based nanomedicines for targeted personalized therapy. Advanced drug delivery reviews. 2012;64:1508–21. doi: 10.1016/j.addr.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 159.Maojo V, Fritts M, Martin-Sanchez F, De la Iglesia D, Cachau RE, Garcia-Remesal M, et al. Nanoinformatics: developing new computing applications for nanomedicine. Comput Sci Eng. 2012;94:521–39. doi: 10.1007/s00607-012-0191-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Casciaro S. Theranostic applications: Non-ionizing cellular and molecular imaging through innovative nanosystems for early diagnosis and therapy. World J Radiol. 2011;3:249–55. doi: 10.4329/wjr.v3.i10.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yoo D, Lee JH, Shin TH, Cheon J. Theranostic magnetic nanoparticles. Acc Chem Res. 2011;44:863–74. doi: 10.1021/ar200085c. [DOI] [PubMed] [Google Scholar]
- 162.Kircher MF, de la Zerda A, Jokerst JV, Zavaleta CL, Kempen PJ, Mittra E, et al. A brain tumor molecular imaging strategy using a new triple-modality MRI-photoacoustic-Raman nanoparticle. Nat Med. 2012;18:829–34. doi: 10.1038/nm.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Delahunt B, Scolyer RA, Srigley JR. Premalignancy: the lull before the storm. Pathology. 2013;45:207–8. doi: 10.1097/PAT.0b013e32835f618e. [DOI] [PubMed] [Google Scholar]
- 164.Kelloff GJ, Sullivan DC, Baker H, Clarke LP, Nordstrom R, Tatum JL, et al. Workshop on imaging science development for cancer prevention and preemption. Cancer Biomark. 2007;3:1–33. doi: 10.3233/cbm-2007-3101. [DOI] [PubMed] [Google Scholar]
- 165.Vincent TL, Gatenby RA. An evolutionary model for initiation, promotion, and progression in carcinogenesis. Int J Oncol. 2008;32:729–37. [PubMed] [Google Scholar]
- 166.Westergren-Thorsson G, Larsen K, Nihlberg K, Andersson-Sjoland A, Hallgren O, Marko-Varga G, et al. Pathological airway remodelling in inflammation. Clin Respir J. 2010;4(Suppl 1):1–8. doi: 10.1111/j.1752-699X.2010.00190.x. [DOI] [PubMed] [Google Scholar]
- 167.Sveinsson OA, Orvar KB, Birgisson S, Jonasson JG. Microscopic colitis - review. Laeknabladid. 2008;94:363–70. [PubMed] [Google Scholar]
- 168.di Tomaso E, Capen D, Haskell A, Hart J, Logie JJ, Jain RK, et al. Mosaic tumor vessels: cellular basis and ultrastructure of focal regions lacking endothelial cell markers. Cancer Res. 2005;65:5740–9. doi: 10.1158/0008-5472.CAN-04-4552. [DOI] [PubMed] [Google Scholar]
- 169.Eyden B, Tzaphlidou M. Structural variations of collagen in normal and pathological tissues: role of electron microscopy. Micron. 2001;32:287–300. doi: 10.1016/s0968-4328(00)00045-7. [DOI] [PubMed] [Google Scholar]
- 170.Krop I, Marz A, Carlsson H, Li X, Bloushtain-Qimron N, Hu M, et al. A putative role for psoriasin in breast tumor progression. Cancer Res. 2005;65:11326–34. doi: 10.1158/0008-5472.CAN-05-1523. [DOI] [PubMed] [Google Scholar]
- 171.Yu J, Monaco SE, Onisko A, Bhargava R, Dabbs DJ, Cieply KM, et al. A validation study of quantum dot multispectral imaging to evaluate hormone receptor status in ductal carcinoma in situ of the breast. Hum Pathol. 2013;44:394–401. doi: 10.1016/j.humpath.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 172.Menakuru SR, Brown NJ, Staton CA, Reed MW. Angiogenesis in pre-malignant conditions. Br J Cancer. 2008;99:1961–6. doi: 10.1038/sj.bjc.6604733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fox SB, Harris AL. Histological quantitation of tumour angiogenesis. APMIS. 2004;112:413–30. doi: 10.1111/j.1600-0463.2004.apm11207-0803.x. [DOI] [PubMed] [Google Scholar]
- 174.Smith SJ, Tilly H, Ward JH, Macarthur DC, Lowe J, Coyle B, et al. CD105 (Endoglin) exerts prognostic effects via its role in the microvascular niche of paediatric high grade glioma. Acta Neuropathol. 2012;124:99–110. doi: 10.1007/s00401-012-0952-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ratajczak P, Leboeuf C, Wang L, Briere J, Loisel-Ferreira I, Thieblemont C, et al. BCL2 expression in CD105 positive neoangiogenic cells and tumor progression in angioimmunoblastic T-cell lymphoma. Mod Pathol. 2012;25:805–14. doi: 10.1038/modpathol.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Dubinski W, Gabril M, Iakovlev VV, Scorilas A, Youssef YM, Faragalla H, et al. Assessment of the prognostic significance of endoglin (CD105) in clear cell renal cell carcinoma using automated image analysis. Hum Pathol. 2012;43:1037–43. doi: 10.1016/j.humpath.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 177.Marioni G, Staffieri A, Manzato E, Ralli G, Lionello M, Giacomelli L, et al. A higher CD105-assessed microvessel density and worse prognosis in elderly patients with laryngeal carcinoma. Arch Otolaryngol Head Neck Surg. 2011;137:175–80. doi: 10.1001/archoto.2010.244. [DOI] [PubMed] [Google Scholar]
- 178.Fang J, Nakamura H, Maeda H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev. 2011 doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 179.Ferrati S, Shamsudeen S, Summers HD, Rees P, Abbey JV, Schmulen J, et al. Inter-endothelial Transport of Microvectors using Cellular Shuttles and Tunneling Nanotubes. Small. 2012;8:3151–60. doi: 10.1002/smll.201200472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Folkman J, Watson K, Ingber D, Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989;339:58–61. doi: 10.1038/339058a0. [DOI] [PubMed] [Google Scholar]
- 181.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–10. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 182.Grandis JR, Argiris A. Targeting angiogenesis from premalignancy to metastases. Cancer Prev Res (Phila Pa) 2009;2:291–4. doi: 10.1158/1940-6207.CAPR-09-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Staton CA, Chetwood AS, Cameron IC, Cross SS, Brown NJ, Reed MW. The angiogenic switch occurs at the adenoma stage of the adenoma carcinoma sequence in colorectal cancer. Gut. 2007;56:1426–32. doi: 10.1136/gut.2007.125286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bluff JE, Menakuru SR, Cross SS, Higham SE, Balasubramanian SP, Brown NJ, et al. Angiogenesis is associated with the onset of hyperplasia in human ductal breast disease. Br J Cancer. 2009;101:666–72. doi: 10.1038/sj.bjc.6605196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Decuzzi P, Causa F, Ferrari M, Netti PA. The effective dispersion of nanovectors within the tumor microvasculature. Ann Biomed Eng. 2006;34:633–41. doi: 10.1007/s10439-005-9072-6. [DOI] [PubMed] [Google Scholar]
- 186.Cooke VG, LeBleu VS, Keskin D, Khan Z, O’Connell JT, Teng Y, et al. Pericyte depletion results in hypoxia-associated epithelial-to-mesenchymal transition and metastasis mediated by met signaling pathway. Cancer Cell. 2012;21:66–81. doi: 10.1016/j.ccr.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Barlow KD, Sanders AM, Soker S, Ergun S, Metheny-Barlow LJ. Pericytes on the Tumor Vasculature: Jekyll or Hyde? Cancer Microenviron. 2012 doi: 10.1007/s12307-012-0102-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Virgintino D, Rizzi M, Errede M, Strippoli M, Girolamo F, Bertossi M, et al. Plasma membrane-derived microvesicles released from tip endothelial cells during vascular sprouting. Angiogenesis. 2012;15:761–9. doi: 10.1007/s10456-012-9292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ribatti D, Crivellato E. “Sprouting angiogenesis”, a reappraisal. Dev Biol. 2012;372:157–65. doi: 10.1016/j.ydbio.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 190.Lammers T, Hennink WE, Storm G. Tumour-targeted nanomedicines: principles and practice. Br J Cancer. 2008;99:392–7. doi: 10.1038/sj.bjc.6604483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kostarelos K, Bianco A, Prato M. Promises, facts and challenges for carbon nanotubes in imaging and therapeutics. Nat Nanotechnol. 2009;4:627–33. doi: 10.1038/nnano.2009.241. [DOI] [PubMed] [Google Scholar]
- 192.Donaldson K, Seaton A. The Janus faces of nanoparticles. J Nanosci Nanotechnol. 2007;7:4607–11. [PubMed] [Google Scholar]
- 193.Faunce T, Shats K. Researching safety and cost-effectiveness in the life cycle of nanomedicine. J Law Med. 2007;15:128–35. [PubMed] [Google Scholar]
- 194.Lippman SM, Hawk ET. Cancer prevention: from 1727 to milestones of the past 100 years. Cancer Res. 2009;69:5269–84. doi: 10.1158/0008-5472.CAN-09-1750. [DOI] [PubMed] [Google Scholar]
- 195.Kelloff GJ, Lippman SM, Dannenberg AJ, Sigman CC, Pearce HL, Reid BJ, et al. Progress in chemoprevention drug development: the promise of molecular biomarkers for prevention of intraepithelial neoplasia and cancer--a plan to move forward. Clin Cancer Res. 2006;12:3661–97. doi: 10.1158/1078-0432.CCR-06-1104. [DOI] [PubMed] [Google Scholar]
- 196.Ganz PA. Survivorship: adult cancer survivors. Primary care. 2009;36:721–41. doi: 10.1016/j.pop.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 197.Myint PK, Smith RD, Luben RN, Surtees PG, Wainwright NW, Wareham NJ, et al. Lifestyle behaviours and quality-adjusted life years in middle and older age. Age and ageing. 2011;40:589–95. doi: 10.1093/ageing/afr058. [DOI] [PubMed] [Google Scholar]
- 198.Nagahara LA, Lee JS, Molnar LK, Panaro NJ, Farrell D, Ptak K, et al. Strategic workshops on cancer nanotechnology. Cancer Res. 2010;70:4265–8. doi: 10.1158/0008-5472.CAN-09-3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wild CP, Scalbert A, Herceg Z. Measuring the exposome: a powerful basis for evaluating environmental exposures and cancer risk. Environmental and molecular mutagenesis. 2013;54:480–99. doi: 10.1002/em.21777. [DOI] [PubMed] [Google Scholar]
- 200.Fan R, Vermesh O, Srivastava A, Yen BK, Qin L, Ahmad H, et al. Integrated barcode chips for rapid, multiplexed analysis of proteins in microliter quantities of blood. Nat Biotechnol. 2008;26:1373–8. doi: 10.1038/nbt.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Tasciotti E, Liu X, Bhavane R, Plant K, Leonard AD, Price BK, et al. Mesoporous silicon particles as a multistage delivery system for imaging and therapeutic applications. Nat Nanotechnol. 2008;3:151–7. doi: 10.1038/nnano.2008.34. [DOI] [PubMed] [Google Scholar]
- 202.Bouamrani A, Hu Y, Tasciotti E, Li L, Chiappini C, Liu X, et al. Mesoporous silica chips for selective enrichment and stabilization of low molecular weight proteome. Proteomics. 2010;10:496–505. doi: 10.1002/pmic.200900346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wang H, Hanash S. Intact-protein analysis system for discovery of serum-based disease biomarkers. Methods in molecular biology. 2011;728:69–85. doi: 10.1007/978-1-61779-068-3_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lu H, Ladd J, Feng Z, Wu M, Goodell V, Pitteri SJ, et al. Evaluation of known oncoantibodies, HER2, p53, and cyclin B1, in prediagnostic breast cancer sera. Cancer prevention research. 2012;5:1036–43. doi: 10.1158/1940-6207.CAPR-11-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Fan J, Deng X, Gallagher JW, Huang H, Huang Y, Wen J, et al. Monitoring the progression of metastatic breast cancer on nanoporous silica chips. Philos Transact A Math Phys Eng Sci. 2012;370:2433–47. doi: 10.1098/rsta.2011.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Burns AA, Vider J, Ow H, Herz E, Penate-Medina O, Baumgart M, et al. Fluorescent silica nanoparticles with efficient urinary excretion for nanomedicine. Nano Lett. 2009;9:442–8. doi: 10.1021/nl803405h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Li Y, Xu W. Highly sensitive detection of Shigella flexneri using fluorescent silica nanoparticles. New Microbiol. 2009;32:377–83. [PubMed] [Google Scholar]
- 208.Luther T, Carrion CF, Cobb N, Le G, Edwards C, Schwartz S, et al. Methods for analyzing saliva proteins for systemic disease detection. Gen Dent. 2010;58:110–3. [PubMed] [Google Scholar]
- 209.Weigum SE, Floriano PN, Redding SW, Yeh CK, Westbrook SD, McGuff HS, et al. Nano-bio-chip sensor platform for examination of oral exfoliative cytology. Cancer Prev Res (Phila) 2010;3:518–28. doi: 10.1158/1940-6207.CAPR-09-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Nagasaka T, Tanaka N, Cullings HM, Sun DS, Sasamoto H, Uchida T, et al. Analysis of fecal DNA methylation to detect gastrointestinal neoplasia. J Natl Cancer Inst. 2009;101:1244–58. doi: 10.1093/jnci/djp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.White V, Scarpini C, Barbosa-Morais NL, Ikelle E, Carter S, Laskey RA, et al. Isolation of stool-derived mucus provides a high yield of colonocytes suitable for early detection of colorectal carcinoma. Cancer Epidemiol Biomarkers Prev. 2009;18:2006–13. doi: 10.1158/1055-9965.EPI-08-1145. [DOI] [PubMed] [Google Scholar]
- 212.Loktionov A, Bandaletova T, Llewelyn AH, Dion C, Lywood HG, Lywood RC, et al. Colorectal cancer detection by measuring DNA from exfoliated colonocytes obtained by direct contact with rectal mucosa. Int J Oncol. 2009;34:301–11. [PubMed] [Google Scholar]
- 213.Varella-Garcia M, Schulte AP, Wolf HJ, Feser WJ, Zeng C, Braudrick S, et al. The detection of chromosomal aneusomy by fluorescence in situ hybridization in sputum predicts lung cancer incidence. Cancer Prev Res (Phila) 2010;3:447–53. doi: 10.1158/1940-6207.CAPR-09-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Qiu Q, Todd NW, Li R, Peng H, Liu Z, Yfantis HG, et al. Magnetic enrichment of bronchial epithelial cells from sputum for lung cancer diagnosis. Cancer. 2008;114:275–83. doi: 10.1002/cncr.23596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Negraes PD, Favaro FP, Camargo JL, Oliveira ML, Goldberg J, Rainho CA, et al. DNA methylation patterns in bladder cancer and washing cell sediments: a perspective for tumor recurrence detection. BMC Cancer. 2008;8:238. doi: 10.1186/1471-2407-8-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Tsai HT, Tsai YM, Yang SF, Lee CH, Lin LY, Lee S, et al. A notable accessory screening program for detection of cervical intraepithelial neoplasia. Pathol Biol (Paris) 2009;57:477–82. doi: 10.1016/j.patbio.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 217.Wang SS, Smiraglia DJ, Wu YZ, Ghosh S, Rader JS, Cho KR, et al. Identification of novel methylation markers in cervical cancer using restriction landmark genomic scanning. Cancer Res. 2008;68:2489–97. doi: 10.1158/0008-5472.CAN-07-3194. [DOI] [PubMed] [Google Scholar]
- 218.Cazzaniga M, Decensi A, Bonanni B, Luini A, Gentilini O. Biomarkers for risk assessment and prevention of breast cancer. Curr Cancer Drug Targets. 2009;9:482–99. doi: 10.2174/156800909788486768. [DOI] [PubMed] [Google Scholar]
- 219.Rusling JF, Kumar CV, Gutkind JS, Patel V. Measurement of biomarker proteins for point-of-care early detection and monitoring of cancer. Analyst. 2010;135:2496–511. doi: 10.1039/c0an00204f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Nune SK, Gunda P, Thallapally PK, Lin YY, Forrest ML, Berkland CJ. Nanoparticles for biomedical imaging. Expert Opin Drug Deliv. 2009;6:1175–94. doi: 10.1517/17425240903229031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Shubayev VI, Pisanic TR, 2nd, Jin S. Magnetic nanoparticles for theragnostics. Adv Drug Deliv Rev. 2009;61:467–77. doi: 10.1016/j.addr.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Bentolila LA, Ebenstein Y, Weiss S. Quantum dots for in vivo small-animal imaging. J Nucl Med. 2009;50:493–6. doi: 10.2967/jnumed.108.053561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Boisselier E, Astruc D. Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity. Chem Soc Rev. 2009;38:1759–82. doi: 10.1039/b806051g. [DOI] [PubMed] [Google Scholar]
- 224.LaRocque J, Bharali DJ, Mousa SA. Cancer detection and treatment: the role of nanomedicines. Mol Biotechnol. 2009;42:358–66. doi: 10.1007/s12033-009-9161-0. [DOI] [PubMed] [Google Scholar]
- 225.Hartman KB, Wilson LJ. Carbon nanostructures as a new high-performance platform for MR molecular imaging. Adv Exp Med Biol. 2007;620:74–84. doi: 10.1007/978-0-387-76713-0_6. [DOI] [PubMed] [Google Scholar]
- 226.Ross BD, Bhattacharya P, Wagner S, Tran T, Sailasuta N. Hyperpolarized MR imaging: neurologic applications of hyperpolarized metabolism. AJNR Am J Neuroradiol. 2010;31:24–33. doi: 10.3174/ajnr.A1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Zacharias NM, Chan HR, Sailasuta N, Ross BD, Bhattacharya P. Real-time molecular imaging of tricarboxylic acid cycle metabolism in vivo by hyperpolarized 1-(13)C diethyl succinate. J Am Chem Soc. 2012;134:934–43. doi: 10.1021/ja2040865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Fang J, Nakamura H, Maeda H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Advanced Drug Delivery Reviews. 2010 doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 229.Eck W, Craig G, Sigdel A, Ritter G, Old LJ, Tang L, et al. PEGylated gold nanoparticles conjugated to monoclonal F19 antibodies as targeted labeling agents for human pancreatic carcinoma tissue. ACS Nano. 2008;2:2263–72. doi: 10.1021/nn800429d. [DOI] [PubMed] [Google Scholar]
- 230.Roger M, Clavreul A, Venier-Julienne MC, Passirani C, Sindji L, Schiller P, et al. Mesenchymal stem cells as cellular vehicles for delivery of nanoparticles to brain tumors. Biomaterials. 2010;31:8393–401. doi: 10.1016/j.biomaterials.2010.07.048. [DOI] [PubMed] [Google Scholar]
- 231.Serda RE, Godin B, Blanco E, Chiappini C, Ferrari M. Multi-stage delivery nanoparticle systems for therapeutic applications. Biochim Biophys Acta. 2011 doi: 10.1016/j.bbagen.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Serda RE, Ferrati S, Godin B, Tasciotti E, Liu X, Ferrari M. Mitotic trafficking of silicon microparticles. Nanoscale. 2009;1:250–9. doi: 10.1039/b9nr00138g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Tanaka T, Mangala LS, Vivas-Mejia PE, Nieves-Alicea R, Mann AP, Mora E, et al. Sustained small interfering RNA delivery by mesoporous silicon particles. Cancer Res. 2010;70:3687–96. doi: 10.1158/0008-5472.CAN-09-3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Chen YS, Hung YC, Lin WH, Huang GS. Assessment of gold nanoparticles as a size-dependent vaccine carrier for enhancing the antibody response against synthetic foot-and-mouth disease virus peptide. Nanotechnology. 2010;21:195101. doi: 10.1088/0957-4484/21/19/195101. [DOI] [PubMed] [Google Scholar]
- 235.Bielinska AU, Gerber M, Blanco LP, Makidon PE, Janczak KW, Beer M, et al. Induction of Th17 cellular immunity with a novel nanoemulsion adjuvant. Crit Rev Immunol. 2010;30:189–99. doi: 10.1615/critrevimmunol.v30.i2.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Klippstein R, Pozo D. Nanotechnology-based manipulation of dendritic cells for enhanced immunotherapy strategies. Nanomedicine. 2010;6:523–9. doi: 10.1016/j.nano.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 237.Sheng WY, Huang L. Cancer Immunotherapy and Nanomedicine. Pharm Res. 2011 doi: 10.1007/s11095-010-0258-8. [DOI] [PubMed] [Google Scholar]
- 238.Mattheolabakis G, Lagoumintzis G, Panagi Z, Papadimitriou E, Partidos CD, Avgoustakis K. Transcutaneous delivery of a nanoencapsulated antigen: induction of immune responses. Int J Pharm. 2010;385:187–93. doi: 10.1016/j.ijpharm.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 239.Chadwick S, Kriegel C, Amiji M. Nanotechnology solutions for mucosal immunization. Adv Drug Deliv Rev. 2010;62:394–407. doi: 10.1016/j.addr.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 240.Ge W, Hu PZ, Huang Y, Wang XM, Zhang XM, Sun YJ, et al. The antitumor immune responses induced by nanoemulsion-encapsulated MAGE1-HSP70/SEA complex protein vaccine following different administration routes. Oncol Rep. 2009;22:915–20. doi: 10.3892/or_00000517. [DOI] [PubMed] [Google Scholar]
- 241.Lu W, Zhang G, Zhang R, Flores LG, 2nd, Huang Q, Gelovani JG, et al. Tumor site-specific silencing of NF-kappaB p65 by targeted hollow gold nanosphere-mediated photothermal transfection. Cancer research. 2010;70:3177–88. doi: 10.1158/0008-5472.CAN-09-3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Tian Z, Wang H, Jia Z, Shi J, Tang J, Mao L, et al. Tumor-targeted inhibition by a novel strategy - mimoretrovirus expressing siRNA targeting the Pokemon gene. Current cancer drug targets. 2010;10:932–41. doi: 10.2174/156800910793357907. [DOI] [PubMed] [Google Scholar]
- 243.Siddiqui IA, Adhami VM, Bharali DJ, Hafeez BB, Asim M, Khwaja SI, et al. Introducing nanochemoprevention as a novel approach for cancer control: proof of principle with green tea polyphenol epigallocatechin-3-gallate. Cancer Res. 2009;69:1712–6. doi: 10.1158/0008-5472.CAN-08-3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Siddiqui IA, Adhami VM, Ahmad N, Mukhtar H. Nanochemoprevention: sustained release of bioactive food components for cancer prevention. Nutr Cancer. 2010;62:883–90. doi: 10.1080/01635581.2010.509537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–52. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 246.Barton MK. Daily aspirin reduces colorectal cancer incidence in patients with Lynch syndrome. CA Cancer J Clin. 2012;62:143–4. doi: 10.3322/caac.21136. [DOI] [PubMed] [Google Scholar]
- 247.Solomon SD, McMurray JJ, Pfeffer MA, Wittes J, Fowler R, Finn P, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1071–80. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- 248.Morgen M, Bloom C, Beyerinck R, Bello A, Song W, Wilkinson K, et al. Polymeric nanoparticles for increased oral bioavailability and rapid absorption using celecoxib as a model of a low-solubility, high-permeability drug. Pharmaceutical research. 2012;29:427–40. doi: 10.1007/s11095-011-0558-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Margulis-Goshen K, Weitman M, Major DT, Magdassi S. Inhibition of crystallization and growth of celecoxib nanoparticles formed from volatile microemulsions. J Pharm Sci. 2011 doi: 10.1002/jps.22623. [DOI] [PubMed] [Google Scholar]
- 250.Tan A, Davey AK, Prestidge CA. Silica-lipid hybrid (SLH) versus non-lipid formulations for optimising the dose-dependent oral absorption of celecoxib. Pharmaceutical research. 2011;28:2273–87. doi: 10.1007/s11095-011-0458-x. [DOI] [PubMed] [Google Scholar]
- 251.Venkatesan P, Puvvada N, Dash R, Prashanth Kumar BN, Sarkar D, Azab B, et al. The potential of celecoxib-loaded hydroxyapatite-chitosan nanocomposite for the treatment of colon cancer. Biomaterials. 2011;32:3794–806. doi: 10.1016/j.biomaterials.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 252.Thakkar H, Kumar Sharma R, Murthy RS. Enhanced retention of celecoxib-loaded solid lipid nanoparticles after intra-articular administration. Drugs R D. 2007;8:275–85. doi: 10.2165/00126839-200708050-00002. [DOI] [PubMed] [Google Scholar]
- 253.Lanza FL, Marathi UK, Anand BS, Lichtenberger LM. Clinical trial: comparison of ibuprofen-phosphatidylcholine and ibuprofen on the gastrointestinal safety and analgesic efficacy in osteoarthritic patients. Aliment Pharmacol Ther. 2008;28:431–42. doi: 10.1111/j.1365-2036.2008.03765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Grandhi BK, Thakkar A, Wang J, Prabhu S. A novel combinatorial nanotechnology-based oral chemopreventive regimen demonstrates significant suppression of pancreatic cancer neoplastic lesions. Cancer Prev Res (Phila) 2013;6:1015–25. doi: 10.1158/1940-6207.CAPR-13-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Bisht S, Mizuma M, Feldmann G, Ottenhof NA, Hong SM, Pramanik D, et al. Systemic administration of polymeric nanoparticle-encapsulated curcumin (NanoCurc) blocks tumor growth and metastases in preclinical models of pancreatic cancer. Mol Cancer Ther. 2010;9:2255–64. doi: 10.1158/1535-7163.MCT-10-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Aqil F, Jeyabalan J, Kausar H, Bansal SS, Sharma RJ, Singh IP, et al. Multi-layer polymeric implants for sustained release of chemopreventives. Cancer Lett. 2012;326:33–40. doi: 10.1016/j.canlet.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Bansal SS, Goel M, Aqil F, Vadhanam MV, Gupta RC. Advanced drug delivery systems of curcumin for cancer chemoprevention. Cancer prevention research. 2011;4:1158–71. doi: 10.1158/1940-6207.CAPR-10-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]


