Abstract
Purpose:
To study optical coherence tomographic (OCT) results and vision at 6 months after transition (post-Tx) from intravitreal bevacizumab and/or ranibizumab to aflibercept for treatment of neovascular age-related macular degeneration (nAMD). The null hypothesis was the lack of improvements in OCT metrics and vision outcome in study eyes at 6 months after transitioning from bevacizumab or ranibizumab to aflibercept.
Methods:
This retrospective study assessed 6 monthly OCT (Cirrus) data after transitioning to aflibercept for eyes on prior Legacy-ranibizumab, Legacy-bevacizumab, or mixed treatment for nAMD. Outcome measures were subretinal fluid (SRF), cystoid macular edema (CME), pigment epithelial detachment (PED) heights and volumes, central 1- and 3-mm subfield, Macular Volume, and best spectacle and pinhole visual acuity (VA). A single masked investigator performed all OCT measurements.
Results:
One hundred eighty-nine eyes in 172 patients in Legacy-bevacizumab (95 eyes), Legacy-ranibizumab (84 eyes), or Mixed Group(10 eyes) were switched to aflibercept and followed for 6 months. Significant post-Tx reductions were noted in SRF/CME heights and volumes (all P<.001). Similar findings were noted for PED heights (122.8 μm vs 79.4 μm) and PED volumes (all P<.001). Post-Tx VA was better (20/43 vs 20/51, P<.001). There were no differences between Legacy-bevacizumab and Legacy-ranibizumab groups in OCT and VA changes. Post-Tx VA, SRF/CME, and PED heights and volumes were improved for Nonresponders (suboptimal response to bevacizumab/ranibizumab) (P=.001 to <.001), but not Responders (good responses to same). The only adverse event was a retinal pigment epithelial tear in one eye.
Conclusions:
Significant improvements in vision and OCT metrics developed in Nonresponders but not in Responders. Post-Tx VA and OCT measures were similar for eyes on prior bevacizumab or ranibizumab. Post-Tx adverse events were uncommon.
INTRODUCTION
Age-related macular degeneration (AMD) is considered to be a leading cause of severe vision loss in the developed world for individuals above the age of 55.1–5 The neovascular subtype of AMD is known to cause particularly rapid and devastating vision loss for these individuals.5–7 Prior to the introduction of anti–vascular endothelial growth factor (anti-VEGF) therapy for neovascular age-related macular degeneration (nAMD), the standard of care in treatment of this condition was to limit the amount of vision loss, since very few eyes developed vision gain after treatment. In the 1980s and 1990s, the National Eye Institute sponsored a series of laser treatment trials for nAMD under the name of Macular Photocoagulation Study, in which eyes treated with laser were compared with untreated eyes in the number of lines of vision loss.8–23 There was a high recurrence rate of choroidal neovascularization (CNV), and successfully treated eyes were frequently left with dense scotomas despite laser treatment. In addition, only less than 20% of eyes with nAMD were eligible for laser therapy.8–24 Even after the introduction of photodynamic therapy (PDT), whereby intravenously administered verteporfin is activated by low-intensity infrared laser leading to thrombosis of the CNV, a reduction in the number of letter loss by 50% (mean of −8 letters) in comparison to the control eyes was still considered to be therapeutic success.25–44 Similar results in the limitation of vision loss instead of vision gain (mean of −7 letters) were noted to be associated with intravitreal injection of pegaptanib (Macugen; Eyetech/Valeant Pharmaceuticals, Montreal, Quebec),45–56 which was the first anti-VEGF drug developed and approved for treatment of nAMD in the United States, after the successful DNA sequencing of VEGF, the discovery of its key role in tumorigenesis and angiogenesis, and the introduction of targeted VEGF-blockade for treatment of nAMD.24,57–62 This aptamer was specifically designed to block only the VEGF165 isomer.
It was not until the introduction of the pan-VEFG inhibiting drugs bevacizumab and ranibizumab that vision gain was noted to be a frequent occurrence for the treatment of eyes with nAMD and other retinal vascular disorders.24,63–69 In 2005, Rosenfeld and colleagues70,71 discovered the off-label therapeutic benefits of bevacizumab (Avastin; Genentech, South San Francisco, California) for treatment of nAMD and other retinal vascular diseases, a full-length murine-derived, humanized monoclonal antibody that binds all isoforms of VEGF-A, and first approved by the US Food and Drug Administration (FDA) as a first-line treatment for metastatic colorectal cancer. The drug developers at Genentech had believed that full-length antibodies such as bevacizumab (molecular weight of 149 kDa) would not penetrate the inner retina associated with an exclusion limit of 76.5 kDa, based on their animal study.24,72,73 They were also concerned that the Fc segment of bevacizumab would induce an undesirable immunologic response and also systemic adverse events due to sustained systemic VEGF suppression associated with its prolonged systemic half-life.24,72,73 Therefore, they cleaved a Fab-binding fragment from the antibody herceptin-2 (HER2), an anti-VEGF molecule similar to bevacizumab, to create a 48-kDa molecule, rhuFabV2, or ranibizumab (Lucentis; Genentech), which was humanized and affinity enhanced, and subjected to rigorous testing in clinical trials.74 After the completion of two pivotal clinical trials (MARINA and ANCHOR) in 2006,75,76 which demonstrated that monthly injections of ranibizumab resulted in prevention of loss of 15 letters in 94% of treated patients, and the gain of 15 letters in 40% of treated patients, the FDA approved this drug for treatment of nAMD in 2006.
Despite the approval of ranibizumab, intravitreal bevacizumab had gained widespread use in the United States and around the world for treatment of nAMD and other retinal vascular diseases, primarily because of its relatively low cost and its general availability.77 Nevertheless, there was a lack of level 1 data in establishing the efficacy and safety of bevacizumab. Hence the Comparison of Age-related Macular Degeneration Treatment Trials (CATT) were performed.78 The 1-year data for CATT showed comparable visual improvements (+8.0 letters vs +8.5 letters) for patients who received monthly bevacizumab and ranibizumab, respectively, whereas there was somewhat less visual improvements (+5.9 letters vs +6.8 letters) for those who received as-needed injections. The second-year results of CATT showed continued similar benefits of both drugs with little difference in vision gain between them.79 The mean gains in vision for patients receiving monthly injections were more than those treated on an as-needed basis, and patients who were switched from a monthly to the as-needed regimen experienced poorer vision outcome and were less likely to maintain a dry macula. In addition, patients treated with ranibizumab had a greater chance of maintaining a dry macula. Despite the substantial benefit associated with both bevacizumab and ranibizumab in vision gain for treatment of AMD, the multiple clinical trials and common clinical experience have shown that the intravitreal administration of these drugs needs to be continued at a high frequency (ie, 4- to 6-week intervals) on an indefinite basis in order to maintain the same level of sound anatomical and visual outcomes for a large proportion of patients with nAMD. The PIER Study, a randomized, double-mask, sham-controlled trial, showed worse visual outcome for eyes with nAMD treated on a quarterly basis following an initial series of three monthly injections when compared to eyes treated with monthly injections.80 Soon, it became clear that an anti-VEGF drug with a higher affinity to VEGF or more durable therapeutic effects in comparison to ranibizumab and bevacizumab was needed to lengthen the interval between drug administration while maintaining the same level of therapeutic effects.
Aflibercept (VEGF Trap-Eye; Regeneron Pharmaceuticals, Tarrytown, New York) possesses molecular characteristics consistent with such a requirement. This 115-kDa fusion molecule has a high affinity for all isomers of VEGF and neutralizes VEGF-mediated biologic activities, including retinal and CNV, leading to a reduction in retinal edema, subretinal fluid (SRF), and hemorrhage.24,81,82 There are five members of the VEGF family (VEGF-A, VEGF-B, VEGF-C, VEGF-D, and placenta growth factor [PlGF]).83,84 Among them, VEGF-A is considered to be most important for angiogenesis.83,84 There are multiple isoforms of VEGF-A, of which VEGF165 is known to be most critical for angiogenesis.85 Active VEGF binds to three different receptors (VEGFR-1, VEGFR-2, and VEGFR-3).83,85 VEGFR-1 binds to VEGF with the highest affinity, whereas VEGFR-2 plays a major role in angiogenesis. With this knowledge, the drug developers at Regeneron Pharmaceuticals created a protein molecule with recombinant technology by fusing the second immunoglobulin domain (Ig2) of humanVEGFR-1 and the third immunoglobulin domain (Ig3) of VEGFR-2 with the Fc domain of human IgG1. Thus portions of the two receptors are fused together to form a soluble decoy receptor molecule that possesses a high binding affinity for VEGF ligands, more so than the individual receptor binding affinity alone and more consistently than ranibizumab or bevacizumab,81,82,86,87 although Yu and coworkers88 showed similar potencies for ranibizumab and aflibercept for inhibiting VEGF-stimulated proliferation of bovine retinal microvascular endothelial cells, chemotaxis of human umbilical vein endothelial cells, and MPA kinase activation. In fact, aflibercept binds to all isoforms of VEGF-A more tightly than the native receptors of VEGF. Unlike bevacizumab and ranibizumab, it also binds PlGF. Aflibercept is known to bind VEGF dimers in a 1:1 ratio in a “two-fisted” grip without forming multimeric complexes. In contrast, ranibizumab, with one binding site, binds VEGF in a 1:1 or 2:2 ratio, and bevacizumab, with two binding sites, binds VEGF in a 1:2 ratio and tends to form long chains of multimeric complexes termed “daisy chains.”24 Aflibercept has low potential for immunogenicity due to its entire composition of human amino acid sequences. However, it binds VEGF of all species, given the native receptor sequences of its ligand-binding domains. Aflibercept is specially formulated for intraocular injections. In November 2011, the FDA approved aflibercept (Eylea; Regeneron Pharmaceuticals) for treating nAMD,89,90 following the completion of Vascular Endothelial Growth Factor (VEGF) Trap-Eye: Investigation of Efficacy and Safety in Wet Age-Related Macular Degeneration (VIEW 1 and VIEW 2) clinical trials sponsored by Regeneron Pharmaceuticals.91 These two parallel phase 3 pivotal clinical trials established the noninferiority of monthly or bimonthly dosing of aflibercept to monthly dosing of ranibizumab in treatment-naïve eyes with nAMD. Subsequently, multiple favorable reports of aflibercept in treatment of nAMD and other retinal vascular disorders have appeared in the literature.92–101.
Meanwhile, clinicians have been encountering increasing numbers of eyes with partial or suboptimal responses to bevacizumab and ranibizumab in treatment of nAMD, manifesting as persistent SRF and exudative changes due to development of resistance or other causes. For these patients, the switch of their anti-VEGF drugs to aflibercept would be an attractive option, based on the knowledge of aflibercept’s high binding affinity to VEGF. However, it is unknown to what extent the bimonthly dosing of aflibercept after three monthly loading doses, as recommended by the drug label of aflibercept based on the results of the VIEW 1 and 2 trials, is applicable to the heterogeneous population of patients with nAMD in a clinical practice, particularly concerning patients who have developed resistance to anti-VEGF therapy prior to conversion to aflibercept treatment.
The purpose of this retrospective study was to assess the spectral domain–optical coherence tomography (SD-OCT) volumetrics and vision outcomes at 6 months after transition (post-Tx) from bevacizumab, ranibizumab, or bevacizumab/ranibizumab (mixed drug group) to aflibercept for treatment of nAMD. Our study included a large cohort of 189 eyes. The majority of these eyes (82.0%) were refractory to anti-VEGF therapy during the time of transition to aflibercept. Besides assessing the frequency of injections, therapeutic effects, and adverse event profile of aflibercept for the entire cohort of patients, we performed subgroup analyses to compare the same features between different drug groups (Legacy-bevacizumab, Legacy-ranibizumab, or Mixed Group [see the “Methods” section for precise definitions]), Responders vs Nonresponders (eyes refractory to specific ant-VEGF drug [see the “Methods” section for precise definitions]), diabetics vs nondiabetics, phakic vs pseudophakic eyes, and eyes with pigment epithelial detachment (PED) vs eyes without PED at 6 months after transitioning to aflibercept. Thus, the goal of this study was to investigate the 6-month outcomes of eyes with nAMD that were transitioned from bevacizumab, ranibizumab, or both drugs to aflibercept. The null hypothesis was the lack of improvements in OCT metrics and vision outcome in study eyes at 6 months after transitioning from bevacizumab or ranibizumab to aflibercept.
METHODS
This was a retrospective study involving two centers (San Diego Retina Associates and Southern California Desert Retina Consultants). Serial SD-OCT scans were performed before and after transition from (1) Legacy-bevacizumab (L-bevacizumab), (2) Legacy-ranibizumab (L-ranibizumab), or (3) Mixed Group to aflibercept for eyes with nAMD. The Cirrus HD SD-OCT machines manufactured by Carl Zeiss Meditec (Dublin, California) were used for all scans of all eyes throughout the study. For an eye to be considered as a part of a specific “Legacy” anti-VEGF group, it must have received at least three consecutive injections of the same medication immediately prior to transition to aflibercept. Thus, an eye of a specified Legacy anti-VEGF group had either received only a specific anti-VEGF medication or received predominantly a specific anti-VEGF medication and also another anti-VEGF medication in the past during the course of treatment prior to transition to aflibercept. Otherwise, it was considered to be a part of the Mixed Group. The period of drug transition consisted of the interval from November 2011 through February 2013.
INSTITUTIONAL REVIEW BOARD
Institutional review board exemption was prospectively obtained for this retrospective study through the Western Institutional Review Board (WIRB, Puyallup, Washington) in December 2012 before the start of the study. All components of research in this study herein have adhered to the tenets set forth in the Declaration of Helsinki as well as all state and local laws. The WIRB determined and granted an exemption status for this study due to its retrospective nature and the lack of revelation of any patient identifiers and the maintenance of confidentiality on all privacy information related to all study subjects. Because it was a retrospective study, registration for clinical trials was not needed. This study has complied with all regulations stipulated by the Health Insurance Portability and Accountability Act (HIPAA).
EXAMINATION TASKS AND MASKED MEASUREMENTS
The best spectacle-corrected visual acuity (BSCVA) and pinhole visual acuity (PHVA) measurements were obtained at baseline and at each study visit. Serial SD-OCT images were obtained at baseline, month 1, month 2, and month 6 for all study eyes. Additional SDOCT images were acquired between month 2 and month 6 whenever it was deemed to be necessary.
The same investigator (A.J.), masked to the identities of the OCT images corresponding to specific pretransitioned and posttransitioned status as well as the drug treatment groups, performed measurements of all OCT variables in the same fashion throughout the study. Following the data collection, intragroup comparison (baseline vs 6-month posttransition) and intergroup comparison of OCT and vision variables were analyzed.
INCLUSION AND EXCLUSION CRITERIA
Strict inclusion and exclusion criteria were followed for this study. Inclusion criteria were as follows: (1) Patient must be 50 years or older with the diagnosis of exudative or neovascular AMD. (2) Patient must have received at least three prior injections of bevacizumab, ranibizumab, or both drugs before transition to aflibercept. (3) Patient must have undergone recent transition to aflibercept with three intravitreal loading doses (month 0, month 1, and month 2) and subsequent additional aflibercept treatment based on the treat-and-observe strategy, similar to the PRospective Optical cohereNce TOmography imaging of patients with intraocular ranibizumab (PrONTO) strategy.102,103 (4) Patient must have had 6 months of follow-up after transition to aflibercept. Exclusion criteria were as follows: (1) any prior major ocular surgery other than uncomplicated cataract extraction, intraocular implant, and YAG-laser posterior capsulotomy; (2) more than one session of PDT within 6 months prior to transitioning, or history of more than two sessions of PDT prior to transition, or any PDT after transition to aflibercept; (3) any periocular, intraocular, or systemic corticosteroid therapy within 6 months prior to transition; (4) any treatment for AMD besides aflibercept other than oral supplements (multivitamins, beta-carotene, zinc, copper, lutein/zeaxanthin, long-chain polyunsaturated fatty acids); (5) more than mild background diabetes mellitus; and (6) history of any ocular or systemic condition, diagnosis, or treatment that could confound the results of the study.
DEFINITION OF RESPONDERS AND NONRESPONDERS
For the purpose of this study, Responders were defined as eyes that responded well to bevacizumab or ranibizumab or both medications during the time of the drug transition to aflibercept (continued decrease in SRF, cystoid macular edema [CME], macular thickness, and/or PED height and volume or maintenance of macular dryness), whereas Nonresponders were defined as eyes with suboptimal responses to the same drugs during the time of drug transition to aflibercept (persistent or recurrent macular edema, SRF, hemorrhage, exudates, and/or PED).
OUTCOME MEASURES
The primary outcome measures were mean changes in SD-OCT variables, including (1) SRF height and volume, (2) CME height and volume, (3) PED height and volume, (4) central 1- and 3-mm subfield thickness, and (5) Macular Volume (an automated reading directly taken off the Cirrus SD-OCT machine).
The secondary outcome measures were (1) mean changes in BSCVA, (2) mean changes in PHVA, (3) mean number of aflibercept injections per eye, and (4) ocular and systemic adverse events. The BSCVA was defined as visual acuity obtained with the patient’s spectacles consisting of his or her last manifest refraction. In addition, PHVA was obtained on all study eyes to enhance the reliability of the visual data in this study.
MEASUREMENTS OF OCT VOLUMETRICS
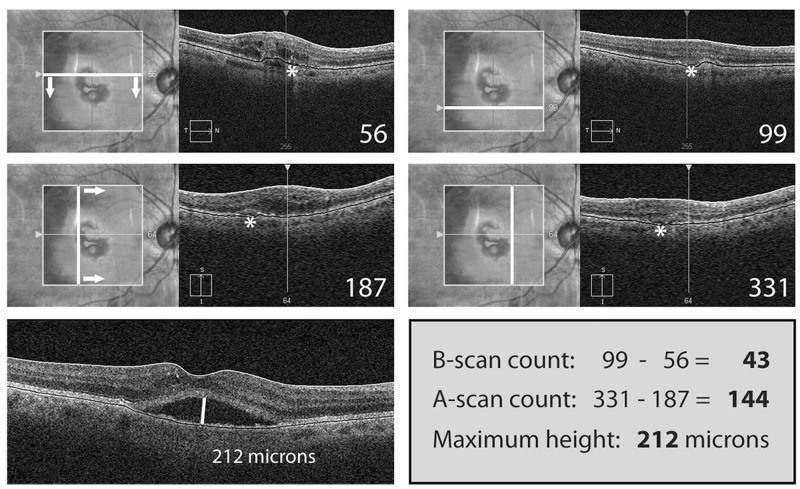
The method of simple estimation of clinically relevant lesion volumes using SD-OCT in nAMD per Heussen and coworkers104 (Simplified Method) was utilized for the measurements and tracking of the changes on all serial OCT variables of the study eyes. In this study, the Simplified Method was applied to the SD-OCT images acquired with the macular cube (512 A-scans × 128 B-scans over a 6 × 6-mm area centered on the fovea) volume SD-OCT scans obtained by the Cirrus HD SD-OCT device (Carl Zeiss Meditec) that was first properly calibrated. Following this Simplified Method, the OCT measurements were obtained from the OCT device (Cirrus HD-OCT, version 5.0.0.326, Carl Zeiss Meditec). B-scan counts and A-scan counts were determined by identifying the first and last scans containing the measured feature. The last scan was determined by moving forward through the volume scan until the specific feature was no longer visible (Figures 1 and 2). The difference between the numbers of the first and last scans constituted the number of scans involved in the measurement (Figures 1 and 2). Thus, difference in A-scan line equals the change in horizontal dimension of lesion and difference in B-scan line equals the change in vertical dimension of lesion. SRF volume estimation equals difference in A-scan line × difference in B-scan line × maximum SRF height; CME volume estimation equals difference in A-scan line × difference in B-scan line × maximum CME height; and PED volume estimation equals difference in A-scan line × difference in B-scan line × maximum PED height. The Simplified Method was utilized to calculate all of the OCT-measured values, except the Macular Volume.
FIGURE 1.
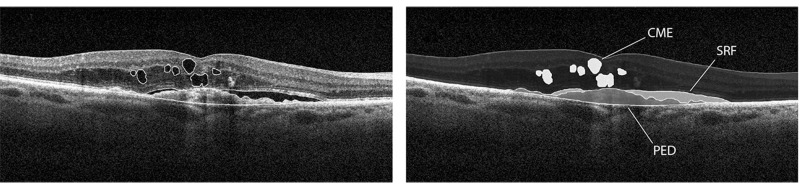
Simplified Method for OCT-measured variables. B-scan images demonstrate measurements of subretinal fluid (SRF), cystoid macular edema (CME), and pigment epithelial detachment (PED) volumetrics with the Simplified Method previously validated by the “gold standard” manual grading with the reading center tool (3D-OCTOR) (Reprinted with permission from the Association for Research in Vision and Ophthalmology.104 Labels of the original published figure have been removed.)
FIGURE 2.
Case example of Simplified Method for OCT measurement. Top left, A sample case on the simplified grading of the subretinal fluid (SRF) lesion that shows the upper boundary of the B-scan. The location of SRF lesion is marked by the asterisk. Top left and right, A progression through the lesion (arrows) until reaching the lower boundary of the B-scan is shown. Middle left, The simplified grading is applied for measurement of the horizontal dimension on the A-scan, and the lateral boundary of the lesion is shown. Middle left and right, A progression through the lesion (arrows) until reaching the medial boundary of the lesion is shown on the A-scan. Bottom left, The maximum lesion height is measured with a caliper on the OCT axial scan. Lower right, Thus, the volumetric estimation of the lesion is obtained by multiplying the difference of the extreme boundaries of the B-scan with the difference of the extreme boundaries of the A-scan and the maximum lesion height. (Reprinted with permission from the Association for Research in Vision and Ophthalmology.104 Labels of the original published figure have been removed.)
DATA COLLECTION
For the purpose of this study, OCT variables collected for all study eyes via the Simplified Method and collated in a master Excel spreadsheet included SRF height and volume, CME height and volume, and PED height and volume at baseline, month 1, month 2, and month 6. In addition, the central 1-mm subfield and mean central 3-mm subfield thickness, and the Macular Volume readings acquired through the automated output of the Cirrus HD SD-OCT device were recorded in the same Excel spreadsheet. The same data were also collected for most study eyes at months 3, 4, and 5.
Demographic data collected included patient’s age, patient’s gender, right eye vs left eye, phakia vs pseudophakia, and diabetes mellitus vs no diabetes mellitus. Visual acuity data collected included BSCVA and PHVA logarithm of the minimum angle of resolution (logMAR) at baseline and at month 1, month 2, and month 6 after transition. The same visual acuity data were obtained for most study eyes at months 3, 4, and 5.
The injection data included the numbers of bevacizumab and/or ranibizumab injections before transition and the numbers of aflibercept injections after transition at month 1 to month 6.
ADVERSE EVENTS
Adverse events were recorded and tabulated for all study eyes, paying special attention to uveitis, retinal pigment epithelial (RPE) tears, endophthalmitis, and systemic complications.
STATISTICAL ANALYSIS
For the visual acuity data, all Snellen visual acuities were converted to logMAR equivalents for statistical calculations. In reporting the changes in visual acuities, the numbers of letters lost or gained (rounded off to one decimal place) corresponding to the changes in logMAR were also given.105
Statistical analyses included both parametric tests (paired and independent t tests; one- and two-way analysis of variance [ANOVA]) and nonparametric tests (chi-square test, Wilcoxon signed-rank test, Friedman test, Mann-Whitney test, and Kruskal-Wallis test). All statistical analyses were performed with Statistical Product and Service Solutions (SPSS) version 22 (IBM SPSS, Armonk, New York). Stepwise logistical regression was performed to evaluate for any baseline factors predictive for being a Responder vs a Nonresponder. Pearson correlation coefficient calculations were performed for vision outcome vs OCT-measured variables. Due to the multiple statistical tests among treatment groups, the Bonferroni adjustments were incorporated into the statistical calculations, so that only a P value of ≤.001 was considered to be significant.
Statistical Power Calculations
Statistical power calculations for α of .05 and power of 80% involving the entire study cohort showed the following: (1) SRF change: effect size of .24 corresponding to a sample size of 140, (2) PED change: effect size of .3 corresponding to a sample size of 91, (3) CME change: effect size of .25 corresponding to a sample size of 140, and (4) vision change: effect size of .31 corresponding to a sample size of 155. The actual sample size of 189 eyes in 172 patients included in the data set exceeded the minimal sample size requirements for this study.
In addition, the sample size of 189 eyes is sufficient to detect ≥10% difference in SRF, CME, and PED and an equivalent of 3- to 5-letter difference (0.06 to 0.1 log units) in visual changes from baseline to posttransitioned visits and between subgroups (α = .05, power = 80%).
RESULTS
DEMOGRAPHICS
There was a total of 189 eyes in 172 patients enrolled in this study. The mean age was 83.4 years. There were 123 women and 66 men. Ninety-eight (51.9 %) were right eyes and 91 (48.1%) were left eyes. One hundred and two eyes (54.0%) had a vascularized PED, and 87 eyes (46.0%) were without a PED. Ninety-five eyes (50.3%) were in the L-bevacizumab group, 84 eyes (44.4%) eyes were in the L-ranibizumab group, and 10 eyes (5.3%) were in the Mixed Group. Of the 189 eyes, 155 (82.0%) were refractory to a specific Legacy anti-VEGF drug (Nonresponders), whereas 34 (18.0%) were Responders. The transitioned period from one of the anti-VEGF treatment groups to aflibercept included the interval from November 2011 through February 2013. All study patients underwent a follow-up of 6 months. Detailed records were available in both centers to keep track of all patients transitioning from bevacizumab and/or ranibizumab to aflibercept during the study period. There were only 11 patients monitored by the HD-Cirrus OCT machine who transitioned from bevacizumab and/or ranibizumab to aflibercept in both centers but were not included in the study during the study period. One of the 11 patients developed Hodgkin’s lymphoma and died, whereas 2 of the 11 patients moved out of the area before the end of the 6-month follow-up period. The other 8 patients had less than 6 months of follow-up. Statistical comparisons of the OCT-measured variables and vision at baseline for these 11 patients with those of the 172 study patients showed no differences in all categories. Considering the small number of excluded patients and the lack of differences in baseline features between them and the patients included in the study, they would not be expected to induce major changes on the results of this study had they been included in the analyses.
BASELINE COMPARISON
Comparison of the three drug groups (L-bevacizumab, L-ranibizumab, and Mixed Group) showed comparable baseline characteristics, including age, gender distribution, right eye vs left eye, SRF height and volume, CME height and volume, PED height and volume, central 1-mm and central-3 mm subfields, Macular Volume, BSCVA, and PHVA (Table 1).
TABLE 1.
BASELINE CHARACTERISTICS (MEAN VALUES) OF THREE DRUG GROUPS (L-BEVACIZUMAB, L-RANIBIZUMAB, MIXED GROUP)*
| VARIABLE |
BEVA VALUE (SD) |
RANI VALUE (SD) |
MIXED VALUE (SD) |
P VALUE |
|---|---|---|---|---|
| Age | 84.2 (6.8) | 83.0 (8.8) | 79.4 (6.5) | .16 |
| Sex | 62F/33M | 54F/30M | 7F/3M | .94† |
| 65.3%F/34.7%M | 64.3%F/35.7%M | 70%F/30%M | ||
| RE vs LE | 48RE/47LE | 44RE/40LE | 6RE/4LE | .84† |
| 50.5%RE/49.5LE | 52.4%RE/47.6%LE | 60%RE/40%LE | ||
| SRF Ht‡ | 43.6 (54.5) | 43.7 (67.7) | 17.2 (37.4) | .40 |
| SRF Vol | 379743.2 (866714.0) | 449165.4 (1071317.5) | 87166.0 (255599.2) | .51 |
| CME Ht‡ | 51.0 (84.9) | 54.6 (91.6) | 100.1 (92.3) | .25 |
| CME Vol | 243319.2 (955729.9) | 389043.4 (1279420.6) | 411046.1 (735187.8) | .65 |
| PED Ht‡ | 121.5 (151.5) | 113.3 (131.0) | 202.5 (213.8) | .19 |
| PED Vol | 1996489.7 (3544238.6) | 1563449.5 (2975769.0) | 3383260.8 (4641326.6) | .24 |
| Central 1-mm‡ | 249.8 (52.6) | 260.9 (63.8) | 257.8 (59.5) | .45 |
| Central 3-mm‡ | 281.8 (43.5) | 287.9 (41.1) | 296.1 (57.3) | .47 |
| Mac Vol§ | 9.1 (1.4) | 9.3 (0.89) | 9.9 (1.9) | .17 |
| BSCVA¶ | 0.41 (0.31) 20/51 | 0.52 (0.31) 20/66 | 0.54 (0.42) 20/69 | .05 |
| PHVA¶ | 0.35 (0.28) 20/45 | 0.46 (0.32) 20/58 | 0.41 (0.30) 20/51 | .06 |
Beva, Legacy-bevacizumab; BSCVA, best spectacle-corrected visual acuity; CME, cystoid macular edema; F, female, Ht, height; LE, left eye; M, male; Mac Vol, Macular Volume; PED, pigment epithelial detachment; PHVA, pinhole visual acuity; Rani, Legacy-ranibizumab; RE, right eye; SD, one standard deviation; SRF, subretinal fluid; Vol, volume.
Mean values for SRF, CME, PED, central 1-and 3-mm subfield, Mac Vol, BSCVA, and PHVA. One-Way ANOVA for all comparisons except Sex and RE vs LE.
Chi-square.
μm.
mm3.
logMAR, log10 of reciprocal of Snellen visual acuity.
MEAN INJECTIONS PER EYE
The mean number of anti-VEGF injections over 6 months prior to transition was 6.5. There were significant differences in the mean numbers of pre-Tx injections among the three drug groups (8.6 for ranibizumab, 4.9 for mixed, and 4.8 for bevacizumab; P<.001, Kruskal-Wallis test). The mean pre-Tx follow-up time was 12.0 months. The mean number of post-Tx aflibercept injections was 5.4 over 6 months. There were no differences in the mean number of aflibercept injections after transition among the three drug groups over 6 months (5.4 for L-bevacizumab, 5.5 for L-ranibizumab, and 5.5 for Mixed Group).
RESULTS OF ENTIRE COHORT
For the entire cohort, pre-Tx and post-Tx OCT metrics and vision data are outlined in detail in Table 2.
TABLE 2.
COMPARISON OF PRE-AFLIBERCEPT AND POST-AFLIBERCEPT TRANSITIONED OUTCOME (SIX MONTHS) FOR ENTIRE GROUP (PAIRED t TEST) (N=189)
| VARIABLES |
MEAN BASELINE VALUE (SD) |
MEAN POST-TX VALUE (SD) |
MEAN CHANGE |
95% CI |
% CHANGE |
P VALUE |
|---|---|---|---|---|---|---|
| SRF Ht* | 42.5 (59.9) | 13.8 (33.1) | 28.6 | 20.3, 36.9 | 69.4 | <.001 |
| SRF Vol | 395117.2 (944404.6) | 65145.1 (327040.3) | NA | NA | 62.0 | <.001 |
| CME Ht* | 55.5 (88.7) | 17.5 (48.2) | 38.0 | 26.8, 49.3 | 70.9 | <.001 |
| CME Vol | 316959.9 (1100363.2) | 56632.4 (281097.9) | NA | NA | 65.5 | .001 |
| PED Ht* | 122.8 (147.2) | 79.4 (107.6) | 43.4 | 30.8, 56.0 | 13.6 | <.001 |
| PED Vol | 1877401.5 (3375407.6) | 1033879.5 (2217905.4) | NA | NA | 31.9 | <.001 |
| Central 1-mm* | 256.5 (55.2) | 231.5 (47.0) | 24.9 | 16.6, 33.3 | 7.6 | <.001 |
| Central 3-mm* | 286.8 (37.9) | 270.2 (29.0) | 16.5 | 11.2, 21.9 | 4.9 | <.001 |
| Mac Vol† | 9.3 (1.1) | 10.0 (9.8) | −0.73 | −2.1, 0.67 | −10.9 | .31 |
| BSCVA‡ | 0.46 (0.32) 20/58 | 0.38 (0.29) 20/48 | 0.081 | 0.053, 0.108 | 15.9 | <.001 |
| PH VA‡ | 0.41 (0.30) 20/51 | 0.33 (0.27) 20/43 | 0.077 | 0.053, 0.102 | 15.2 | <.001 |
BSCVA, best spectacle-corrected visual acuity; CI, confidence interval; CME, cystoid macular edema; Ht, height; Mac Vol, Macular Volume; NA, not applicable; PED, pigment epithelial detachment, PHVA, pinhole visual acuity; post-Tx, post-transitioned; SD, one standard deviation; SRF, subretinal fluid; Vol, volume.
μm.
mm3.
logMAR, log10 of reciprocal of Snellen visual acuity.
There were significant improvements in all OCT and vision results except for Macular Volume at 6 months in comparison to baseline. Mean changes were as follows: SRF height, 28.6 µm (69.4%) reduction, 95% CI (20.3, 36.9 µm), P<.001; CME height, 38.0 µm (70.9%) reduction, 95% CI (26.8, 49.3 µm), P<.001; PED height, 43.4 µm (13.6%) reduction, 95% CI (30.8, 56.0 µm), P<.001; SRF volume, 62% reduction, and PED volume, 31.9% reduction, P<.001 for both; CME volume, 65.5% reduction, P=.001; central 1-mm subfield, 24.9 µm (7.6%) reduction, 95% CI (16.6, 33.3 µm), P<.001; central 3-mm subfield, 16.5 µm (4.9%) reduction, 95% CI (11.2, 21.9 µm), P<.001; BSCVA, 0.08 logMAR or 4.0 letters (15.9%) improvement, 95% CI (0.05, 0.1 logMAR), P<.001; PHVA, 0.08 logMAR or 4.0 letters (15.2%) improvement, 95% CI (0.05, 0.1 logMAR), P<.001.
The only OCT-measured variable that showed the lack of significant change between baseline and 6 months was Macular Volume: −0.7 mm3 (10.9%) increase, 95% CI (−2.1, 0.7 mm3), P=.31 (Table 2), an automated reading taken directly from the Cirrus OCT machine.
COMPARISONS OF STUDY GROUPS
Bevacizumab Group
Pre-Tx and post-Tx OCT metrics and vision data of the bevacizumab group are outlined in detail in Table 3.
TABLE 3.
COMPARISON OF PRE-AFLIBERCEPT AND POST-AFLIBERCEPT TRANSITIONED OUTCOME (SIX MONTHS) FOR L-BEVACIZUMAB GROUP AND L-RANIBIZUMAB GROUP (2-WAY ANOVA)
| L-bevacizumab GROUP (N=95) | L-ranibizumab GROUP (N=84) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VARIABLE(S) |
MEAN BASELINE VALUE (SD) |
MEAN POST-TX VALUE(SD) |
MEAN CHANGE |
95% CI |
% CHANGE |
P VALUE |
MEAN BASELINE VALUE (SD) |
MEAN POST-TX VALUE (SD) |
MEAN CHANGE |
95% CI |
% CHANGE |
P VALUE |
| SRF Ht* | 43.6 (54.5) | 9.8 (29.1) | 33.9 | 22.8, 44.9 | 75.2 | <.001 | 44.3 (67.7) | 19.5 (38.2) | 24.8 | 10.9, 38.6 | 59.7 | <.001 |
| SRF Vol | 379743.2 (866714.0) | 47014.5 (319871.6) | NA | NA | 57.1 | <.001 | 449165.4 (1071317.5) | 91585.1 (353369.2) | NA | NA | 66.9 | <.001 |
| CME Ht* | 51.0 (84.9) | 10.4 (30.8) | 40.7 | 24.4, 56.9 | 76.1 | <.001 | 55.2 (92.0) | 27.6 (63.4) | 27.7 | 12.0, 43.3 | 57.0 | <.001 |
| CME Vol | 243319.2 (955729.9) | 8480.8 (39609.7) | NA | NA | 88.2 | .001 | 389043.4 (1279420.6) | 117831.5 (412757.2) | NA | NA | 27.6 | .001 |
| PED Ht* | 121.5 (151.5) | 82.2 (117.8) | 39.3 | 24.8, 53.8 | 12.2 | <.001 | 114.6 (131.2) | 75.5 (94.4) | 39.1 | 23.2, 55.0 | 11.6 | <.001 |
| PED Vol | 1996489.7 (3544238.6) | 117346.5 (2488310.4) | NA | NA | 29.9 | <.001 | 1563449.5 (2975769.0) | 856035.0 (1905148.5) | NA | NA | 32.9 | <.001 |
| Central 1-mm* | 252.5 (46.0) | 226.7 (39.0) | 25.8 | 16.3, 35.2 | 8.6 | <.001 | 260.9 (63.8) | 237.6 (55.4) | 23.3 | 8.0, 38.6 | 6.3 | <.001 |
| Central 3-mm* | 284.8 (32.4) | 267.3(26.1) | 17.5 | 11.1, 24.0 | 5.5 | <.001 | 287.9 (41.1) | 273.1 (32.8) | 14.7 | 5.6, 23.9 | 4.1 | <.001 |
| Mac Vol† | 9.2 (1.1) | 10.9 (13.8) | −1.71 | −4.5, 1.11 | −23.8 | .32 | 9.3 (0.89) | 9.1(0.73) | 0.211 | 0.044, 0.378 | 1.8 | .32 |
| BSCVA‡ | 0.41 (0.31) 20/51 | 0.34 (0.26) 20/44 | 0.068 | 0.033, 0.102 | 12.2 | <.001 | 0.52 (0.31) 20/66 | 0.45 (0.33) 20/56 | 0.073 | 0.033, 0.114 | 18.7 | <.001 |
| PH VA‡ | 0.35 (0.28) 20/45 | 0.29 (0.24) 20/39 | 0.061 | 0.031, 0.092 | 11.6 | <.001 | 0.46 (0.32) 20/58 | 0.38 (0.31) 20/48 | 0.085 | 0.044, 0.126 | 17.5 | <.001 |
ANOVA, analysis of variance; BSCVA, bestspectacle-corrected visual acuity; CI, confidence interval; CME, cystoid macular edema; Ht, height; L, Legacy; Mac Vol, Macular Volume ; NA, not applicable; PED, pigment epithelial detachment; PHVA, pinholevisual acuity; post-Tx, post-transitioned; SD, one standard deviation; SRF, subretinal fluid; Vol, volume.
μm.
mm3.
logMAR, log10 of reciprocal of Snellen visual acuity.
There were significant improvements in all OCT and vision results except for Macular Volume at 6 months in comparison to baseline. Mean changes were as follows: SRF height, 33.9 µm (75.2%) reduction, 95% CI (22.8, 44.9 µm), P<.001; CME height, 40.7 µm (76.1%) reduction, 95% CI (24.4, 56.9 µm), P<.001; PED height, 39.3 µm (12.2%) reduction, 95% CI (24.8, 53.8 µm), P<.001; SRF volume, 57.1%, reduction, and PED volume, 29.9% reduction, P<.001 for both; CME volume, 88.2% reduction, P=.001; central 1-mm subfield, 25.8 µm (8.6%) reduction, 95% CI (16.3, 35.2 µm), P<.001; central 3-mm subfield, 17.5 µm (5.5%) reduction, 95% CI (11.1, 24.0 µm), P<.001; BSCVA, 0.07 logMAR or 3.5 letters (12.2%) improvement, 95% CI (0.03, 0.1 logMAR), P<.001; PHVA, 0.06 logMAR or 3.0 letters (11.6%) improvement, 95% CI (0.03, 0.09 logMAR), P<.001.
The only OCT-measured variable that showed the lack of significant change between baseline and 6 months was Macular Volume as outlined in Table 3.
Ranibizumab Group
Pre-Tx and post-Tx OCT metrics and vision data of the ranibizumab group are outlined in detail in Table 3.
There were significant improvements in all OCT and vision results except for Macular Volume at 6 months in comparison to baseline. Mean changes were are follows: SRF height, 24.8 µm (59.7%) reduction, 95% CI (10.9, 38.6 µm), P<.001; CME height, 27.7 µm (57%) reduction, 95% CI (12.0, 43.3 µm), P<.001; PED height, 39.1 µm (11.6%) reduction, 95% CI (23.2, 55.0 µm), P<.001; SRF volume, 66.9% reduction and PED volume, 32.9% reduction, P<.001 for both; CME volume, 27.6 % reduction, P=.001; central 1-mm subfield, 23.3 µm (6.3%) reduction, 95% CI (8.0, 38.6 µm), P<.001; central 3-mm subfield, 14.7 µm (4.1%) reduction, 95% CI (5.6, 23.9 µm), P<.001; BSCVA, 0.07 logMAR or 3.5 letters (18.7%) improvement, 95% CI (0.03, 0.1 logMAR), P<.001; PHVA, 0.08 logMAR or 4.0 letters (17.5%) improvement, 95% CI (0.04, 0.1 logMAR), P<.001.
The only OCT-measured variable that showed the lack of significant change between baseline and 6 months was Macular Volume, as shown in Table 3.
Mixed Group
The mean baseline values for SRF height, CME height, PED height, and central 1-mm and central 3-mm subfield thickness were 17.2 ± 37.4 µm, 100.1 ± 92.3 µm, 202.5 ± 213.8 µm, 257.8 ± 59.5 µm, and 296.1 ± 57.3 µm, respectively. The mean values for these variables at 6 months were 7.4 ± 16.0 µm, 0 ± 0 µm, 83.7 ± 117.8 µm, 227.1 ± 35.5 µm, and 274.0 ± 20.7 µm, respectively. The mean baseline BSCVA and PHVA logMAR values were 0.55 ± 0.42 and 0.41 ± 0.30, respectively, and the mean BSCVA and PHVA logMAR values at 6 months were 0.28 ± 0.18 and 0.24 ± 0.16, respectively.
Mean changes for the Mixed Group were as follows: SRF height, 9.8 µm (85.7%) reduction, 95% CI (−15.3, 34.9 µm), P=.40; CME height, 100.1 µm (100%) reduction, 95% CI (34.0, 166.2 µm), P=.01; PED height, 118.8 µm (40.2%) reduction, 95% CI (−43.2, 280.8 µm), P=.13; SRF volume, 93.8% reduction and PED volume, 40.3% reduction, P=.34, P=.09, respectively; CME volume, 100% reduction, P=.11; central 1-mm subfield, 30.7 µm (9.3%) reduction, 95% CI (−9.8, 71.2 µm), P=.12; central 3-mm subfield, 22.1 µm (5.5 %) reduction, 95% CI (−10.8, 55.0 µm), P=.16; Macular Volume, 0.5 mm3 (3.0 %) reduction, 95% CI (−0.5, 1.5 mm3), P=.30; BSCVA, 0.27 logMAR or 13.5 letters (33.2%) improvement, 95% CI (0.04, 0.50 logMAR), P=.03; PHVA, 0.17 logMAR or 8.5 letters (26.2%) improvement, 95% CI (0.03, 0.30 logMAR), P=.02.
Thus, there were significant improvements in all OCT and visual outcomes with the exception of Macular Volume at 6 months after transition in comparison to baseline for both L-bevacizumab and L-ranibizumab groups. Intergroup comparisons were performed on OCT and vision variables for the three drug groups. Figure 3 shows the lack of differences on the plots comparing the course of SRF, CME, PED heights and volumes, and BSCVA and PHVA from baseline to 6 months for the three drug groups (P=.07 and P>.46 for SRF height and volume comparisons, respectively; P≥.08 for both CME height and volume comparisons; P≥.42 for both PED height and volume comparisons; P=.03 at baseline and P=.10 at 6 months, respectively, for BSCVA comparisons; P=.05 for PHVA comparison, between groups. One-way ANOVA was used for comparison within groups, whereas Kruskal-Wallis test was used for comparison between groups for CME height and BSCVA due to group interaction and inequality of variances. Kruskal-Wallis test was also used for comparison between groups for SRF height, CME volume, and PHVA due to inequality of variances. In addition, Kruskal-Wallis test was used for comparison between groups for PED height due to group interaction. It should be noted that for all plots in Figure 3, the P values listed for comparing the 6- month post-Tx results with baseline were derived from 1-way ANOVA for the separate drug groups with smaller sample sizes; therefore, they may differ from the P values derived from 2-way ANOVA associated with larger sample sizes in Table 3.
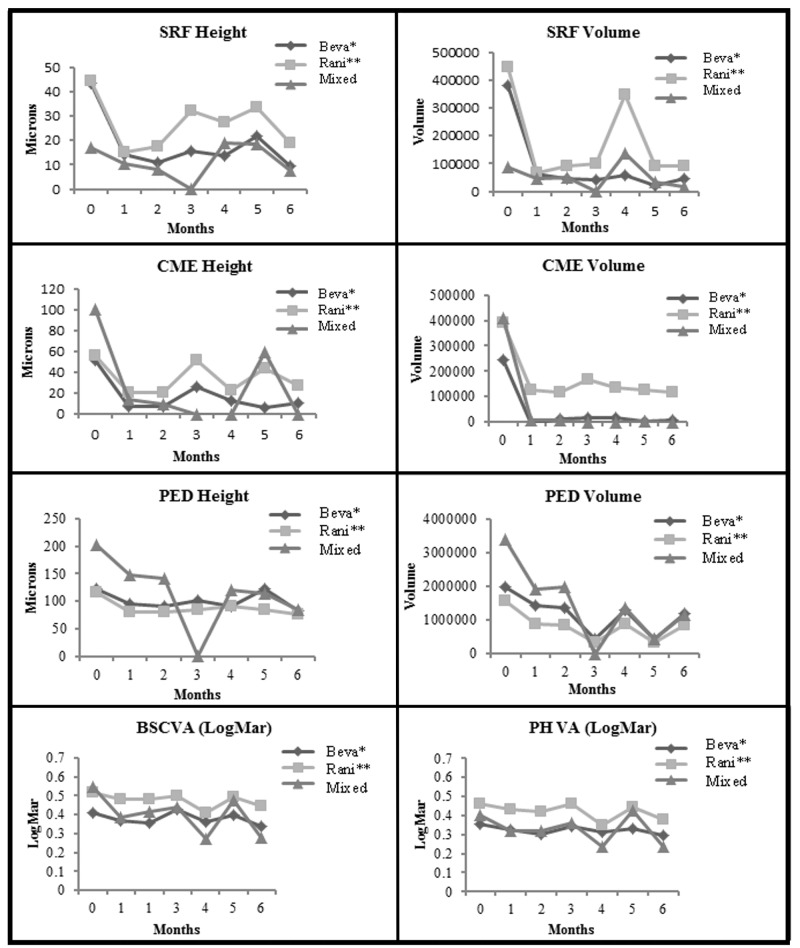
FIGURE 3.
Comparisons of the three drug groups from baseline to month 6. Top row left, For subretinal fluid (SRF) height (P<.001 for beva,* P<.001 for rani,** P=.44 for Mixed Group) (P=.07 for comparison between groups). Top row right, For SRF volume (P<.001 for beva,* P=.001 for rani,** P=.34 for Mixed Group) (P>.46 for comparison between groups). Second row left, For cystoid macular edema (CME) height (P<.001 for beva*, P=.001 for rani**, P=.009 for Mixed Group) (P=.08 for comparison between groups). Second row right, For CME volume (P=.02 for beva*, P=.03 for rani**, P=.11 for Mixed Group) (P>.08 for comparison between groups). Third row left, For pigment epithelial detachment (PED) height (P<.001 for beva*, P<.001 for rani**, P=.13 for Mixed Group) (P>.42 for comparison between groups). Third row right, For PED volume (P<.001 for beva*, P<.001 for rani**, P=.09 for Mixed Group) (P=.42 for comparison between groups). Bottom row left, For best spectacle-corrected visual acuity (BSCVA) in logMAR (P<.001 for beva*, P=.002 for rani**, P=.04 for Mixed Group) (P=.03 at baseline and P=.10 at 6 months for comparison between groups). Bottom row right, For pinhole visual acuity (PHVA) in logMAR (P<.001 for beva*, P<.002 for rani**, P=.006 for Mixed Group) (P>.05 for comparison between groups). (beva*, Legacy-bevacizumab; rani**, Legacy-ranibizumab; logMAR, logarithm in base 10 of the reciprocal of the Snellen visual acuity)
Responders vs Nonresponders
There were 155 Nonresponders and 34 Responders. For the Nonresponders in this study, the reason for transitioning to aflibercept was their refractory response to bevacizumab, ranibizumab, or both drugs. For the Responders, the reasons for transitioning were related to patients’ requests, ie, preference for receiving a new drug or the latest treatment option, or the possibility of extending the interval between drug injections after transitioning to aflibercept.
Nonresponders
Pre-Tx and post-Tx OCT metrics and vision data of the Nonresponders are outlined in detail in Table 4.
TABLE 4.
COMPARISON OF PRE- AFLIBERCEPT AND POST-AFLIBERCEPTTRANSITIONED OUTCOME (SIX MONTHS) FOR NONRESPONDERS AND RESPONDERS (PAIRED t TEST)
| NONRESPONDERS (N=155) | RESPONDERS (N=34) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VARIABLE(S) |
MEAN BASELINE VALUE (SD) |
MEAN POST-TX VALUE(SD) |
MEAN CHANGE |
95% CI |
% CHANGE |
P VALUE |
MEAN BASELINE VALUE (SD) |
MEAN POST-TX VALUE(SD) |
MEAN CHANGE |
95% CI |
% CHANGE |
P VALUE |
| SRF Ht* | 51.7 (62.4) | 16.9 (35.9) | 34.8 | 25.0, 44.7 | 69.4 | <.001 | 0 (0) | 0(0) | NA | NA | 0 | NA |
| SRF Vol | 481788.1 (1023124.9) | 79435.0 (359758.8) | NA | NA | 62.0 | <.001 | 0 (0) | 0 (0) | NA | NA | 0 | NA |
| CME Ht* | 67.3 (93.5) | 21.1 (52.4) | 46.2 | 32.9, 59.5 | 70.9 | <.001 | 0 (0) | 0 (0) | NA | NA | 0 | NA |
| CME Vol | 386486.6 (1204604.9) | 69054.9 (309188.8) | NA | NA | 65.5 | .001 | 0 (0) | 0 (0) | NA | NA | 0 | NA |
| PED Ht* | 148.9 (149.6) | 96.3 (111.4) | 52.7 | 37.8, 67.6 | 13.6 | <.001 | 0 (0) | 0 (0) | NA | NA | 0 | NA |
| PED Vol | 2289218.6 (3599991.2) | 1260665.9 (2391104.8) | NA | NA | 31.9 | <.001 | 0 (0) | 0 (0) | NA | NA | 0 | NA |
| Central 1-mm* | 263.1 (57.4) | 233.8 (49.7) | 29.3 | 19.3, 39.3 | 8.8 | <.001 | 225.4 (27.0) | 220.8 (29.6) | 4.6 | −0.46, 9.6 | 2.0 | .07 |
| Central 3-mm* | 291.0 (39.1) | 271.4 (29.9) | 19.6 | 13.2, 26.0 | 5.7 | <.001 | 267.2 (24.0) | 264.9 (24.6) | 2.3 | −0.54, 5.1 | .84 | .11 |
| Mac Vol† | 9.4 (0.87) | 10.2 (10.8) | −0.831 | −2.54, 0.878 | −7.9 | .34 | 8.7 (1.6) | 9.0 (0.62) | −0.285 | −0.85, 0.279 | −24.7 | .31 |
| BSCVA‡ | 0.47 (0.33) 20/59 | 0.38 (0.29) 20/48 | 0.091 | 0.061, 0.122 | 11.3 | <.001 | 0.42 (0.26) 20/53 | 0.38 (0.30) 20/48 | 0.033 | −0.028, 0.095 | −0.54 | .28 |
| PHVA‡ | 0.41 (0.31) 0/51 | 0.33 (0.27) 0/43 | 0.083 | 0.056, 0.111 | 10.9 | <.001 | 0.39 (0.26) 20/49 | 0.34 (0.30) 20/44 | 0.051 | −0.0056, 0.107 | 4.7 | .08 |
BSCVA, best spectacle-corrected visual acuity; CI, confidence interval; CME, cystoid macular edema; Ht, height; Mac Vol, Macular Volume ; NA, not applicable; PED, pigment epithelial detachment, PHVA, pinhole visual acuity; post-Tx, post-transitioned; SD, one standard deviation; SRF, subretinal fluid; Vol, volume.
μm.
mm3.
logMAR, log10 of reciprocal of Snellen visual acuity.
At 6 months after transition to aflibercept in comparison to baseline, all OCT and visual outcomes with the exception of Macular Volume were significantly improved for the Nonresponders. Mean changes were as follows: SRF height, 34.8 µm (69.4%) reduction, 95% CI (25.0, 44.7 µm), P<.001; CME height, 46.2 µm (70.9%) reduction, CI (32.9, 59.5 µm), P<.001; PED height, 52.7 µm (13.6%) reduction, CI (37.8, 67.6 µm), P<.001; SRF volume, 62.0% reduction and PED volume, 31.9% reduction, P<.001; CME volume, 65.5 % reduction, P=.001; central 1-mm subfield height, 29.3 µm (8.8 %) reduction, CI (19.3, 39.3 µm); 3-mm subfield height, 19.6 µm (5.7%) reduction, CI (13.2, 26.0 µm); BSCVA, 0.09 logMAR or 4.5 letters (11.3%) improvement, 95% CI (0.06, 0.12 logMAR), P<.001; PHVA, 0.08 logMAR or 4.0 letters (10.9%) improvement, 95% CI (0.06, 0.11 logMAR), P<.001; Macular Volume, −0.8 mm3 (7.9%) worsening, P=.34 (Table 4).
Responders
Pre-Tx and post-Tx OCT metrics and vision data of the Responders are outlined in detail in Table 4.
The Responders showed a lack of significant improvements after the transition for all of the OCT and vision results (all P>.05). For these eyes, the OCT-measured variables, BSCVA, and PHVA were maintained after transition in general, although there was a mean of 24.7% increase in the Macular Volume after transition. As mentioned above, the accuracy of the automated readings of Macular Volume is uncertain due to frequent technical errors.
Figure 4 depicts the plots of comparisons between the Responders and Nonresponders from baseline to 6 months for SRF, CME, and PED heights and volumes, and BSCVA and PHVA, respectively. For the SRF height comparison between the two groups, there were differences at baseline (P<.001) but not at 6 months (P=.002). For SRF volume comparison between the two groups, there were differences at baseline (P<.001) and at 6 months (P=.001). For the CME height comparison between the two groups, there was a difference at baseline (P<.001) but no difference at 6 months (P=.009), since only a P value of ≤.001 was considered to be significant due to the Bonferroni adjustment. For the CME volume comparison between the two groups, there was difference at baseline (P<.001) but no difference at 6 months (P=.08) for the same reason. For the PED height comparison between the two groups, there was a difference at baseline (P<.001) and also at 6 months (P<.001). For the PED volume comparison between the two groups, there was a difference at baseline (P<.001) and also at 6 months (P<.001). For the BSCVA comparison between the two groups, there were no differences at baseline and 6 months (P=.45). For the PHVA comparison between the two groups, there was no difference at baseline (P=.94) and also no difference at 6 months (P=.96). It should be pointed out that for the responders, the “flat-line” shape of the SRF and CME heights and volume plots along the x-axis is due to the lack of any SRF and CME at baseline, which remained unchanged after transition to aflibercept throughout the 6-month course. One-way ANOVA was used for all comparisons within groups, whereas the Mann-Whitney test was used for all comparisons between groups due to inequality of variances except for BSCVA. Two-way ANOVA was used for comparison between groups for BSCVA. It should be noted that for all plots in Figure 4, the P values listed for comparing the 6- month post-Tx results with baseline for the individual groups (Responders and Nonresponders) were derived from the paired t test associated with smaller sample sizes; nevertheless, they turned out to be the same as the P values derived from the 2-way ANOVA associated with larger sample sizes in Tables 4.
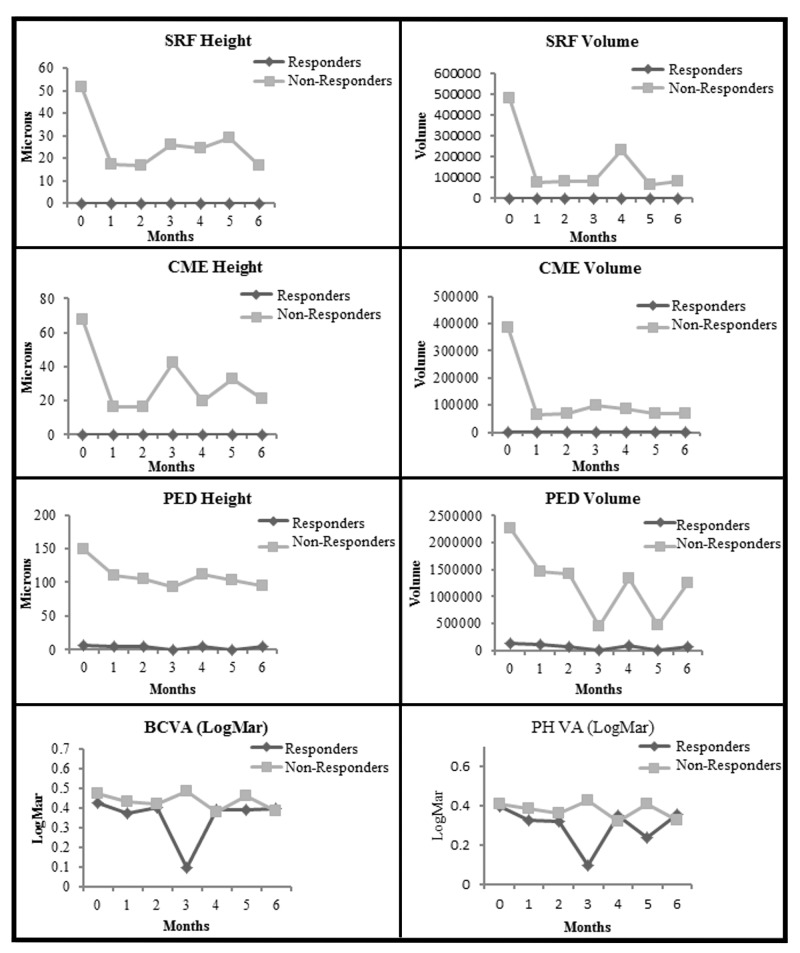
FIGURE 4.
Comparison of Responders with Nonresponders from baseline to month 6. Top row left, For subretinal fluid (SRF) height (P<.001 for Nonresponders), (P<.001 at baseline and P=.002 at 6 months for comparison between Nonresponders and Responders). Top row right, For SRF volume (P<.001 for Nonresponders), (P<.001 at baseline and P=.001 at 6 months for comparison between Nonresponders and Responders). Second row left, For cystoid macular edema (CME) height (P<.001 for Nonresponders), (P<.001 at baseline and P=.009 at 6 months for comparison between Nonresponders and Responders). Second row right, For CME volume (P<.001 for Nonresponders), (P<.001 at baseline and P=.008 at 6 months for comparison between Nonresponders and Responders). Third row left, Pigment epithelial detachment (PED) height (P<.001 for Nonresponders), (P<.001 at baseline and 6 months for comparison between Nonresponders and Responders). Third row right, For PED volume (P<.001 for Nonresponders), (P<.001 at baseline and 6 months for comparison between Nonresponders and Responders). Bottom row left, For best spectacle-corrected visual acuity (BSCVA) in logMAR (P=.13 for Responders and P<.001 for Nonresponders), (P=.45 between groups indicating no difference between Responders and Nonresponders). Bottom row right, For pinhole visual acuity (PHVA) in logMAR (P=.03 for Responders and P<.001 for Nonresponders), (P>.94 between groups at baseline and 6 months, indicating no significant difference between Responders and Nonresponders). (logMAR, logarithm in base 10 of the reciprocal of the Snellen visual acuity; Nonresponders, eyes with suboptimal response to specific anti-VEGF drug at the time of the transition; Responders, eyes with good response to specific anti-VEGF drug at the time of the transition. For the Responders, the “flat-line” shape of the SRF and CME heights and volume plots along the x-axis is due to the lack of any SRF and CME at baseline, which remained unchanged after transition throughout the 6-month course.)
Diabetic vs Nondiabetic Eyes
In this study, only diabetic patients with no diabetic retinopathy or mild background diabetic retinopathy were allowed for inclusion, in case of a history of diabetes mellitus. Patients with more serious diabetic retinopathy were excluded from the study. (See the “Discussion” section on the rationale for assessing diabetic vs nondiabetic eyes.) There were 172 nondiabetic eyes and 17 diabetic eyes.
Diabetic eyes. Pre-Tx and post-Tx OCT metrics and vision data of the diabetic eyes are outlined in detail in Table 5. The mean changes were as follows: SRF height, 24.5 µm (70.2%) reduction, 95% CI (−4.6, 53.6 µm), P<.001; CME height, 33.2 µm (49.5%) reduction, 95% CI (−13.1, 79.4 µm), P<.001; PED height, 35.2 µm (27.0%) reduction, 95% CI (−8.5, 78.8 µm), P<.001; SRF, 55.2% reduction, and CME volume, 67.3% reduction, P=.02 and P=.08, respectively; PED volume, 31.5% reduction, P<.001; central 1-mm subfield, 10.2 µm (3.9%) reduction, 95% CI (−17.3, 37.8 µm), P=.01; central 3-mm subfield, 9.9 µm (3.0%) reduction, 95% CI (−9.3, 29.2 µm), P=.005; BSCVA, 0.097 logMAR or 4.9 letters (13.0%) improvement, 95% CI (0.0028, 0.19 logMAR), P<.001; PHVA, 0.087 logMAR or 4.4 letters (13.8%) improvement, 95% CI (0.014, 0.16 logMAR), P=.001; Macular Volume, 0.65 mm3 (6.7%) reduction, P=.01.
TABLE 5.
COMPARISON OF PRE-AFLIBERCEPT AND POST-AFLIBERCEPT TRANSITIONED OUTCOME (SIX MONTHS) FOR DIABETICS AND NONDIABETICS (2-WAY ANOVA)
| DIABETICS (N=17) | NONDIABETICS (N=172) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VARIABLE(S) |
MEAN BASELINE VALUE (SD) |
MEAN POST-TX VALUE(SD) |
MEAN CHANGE |
95% CI |
% CHANGE |
P VALUE |
MEAN BASELINE VALUE (SD) |
MEAN POST-TX VALUE(SD) |
MEAN CHANGE |
95% CI |
% CHANGE |
P VALUE |
| SRF Ht* | 38.0 (58.9) | 13.5 (48.6) | 24.5 | −4.6, 53.6 | 70.2 | <.001 | 42.9 (60.2) | 13.9 (31.4) | 29.1 | 20.3, 37.8 | 69.4 | <.001 |
| SRF Vol | 385923.3 (837102.9) | 149691.2 (591237.8) | NA | NA | 55.2 | .02 | 396025.9 (956552.4) | 56788.8 (289999.8) | NA | NA | 62.5 | .02 |
| CME Ht* | 54.9 (98.3) | 21.8 (51.5) | 33.2 | −13.1,79.4 | 49.5 | <.001 | 55.6 (88.0) | 17.0 (48.0) | 38.6 | 26.9, 50.2 | 72.9 | <.001 |
| CME Vol | 238913.4 (706324.4) | 39486.5 (109605.2) | NA | NA | 67.3 | .08 | 324673.9 (1133060.2) | 58327.0 (292771.7) | NA | NA | 65.3 | .08 |
| PED Ht* | 126.8 (154.3) | 91.7 (103.4) | 35.2 | −8.5, 78.8 | 27.0 | <.001 | 122.4(146.9) | 78.1 (108.2) | 44.2 | 31.0, 57.5 | 12.5 | <.001 |
| PED Vol | 1751689.0 (3339163.3) | 960009.1 (1918171.7) | NA | NA | 31.5 | <.001 | 1889826.6 (3388369.7) | 1041180.6 (2250171.6) | NA | NA | 31.9 | <.001 |
| Central 1-mm* | 257.5(33.8) | 247.2 (62.9) | 10.2 | −17.3, 37.8 | 3.9 | .01 | 256.4 (56.9) | 230.0 (45.0) | 26.4 | 17.6, 35.3 | 8.0 | .01 |
| Central 3-mm* | 290.1(29.6) | 280.1(35.1) | 9.9 | −9.3, 29.2 | 3.0 | .005 | 286.5 (38.7) | 269.3 (28.3) | 17.2 | 11.6, 22.8 | 5.1 | .005 |
| Mac Vol† | 9.6 (0.59) | 8.9 (0.96) | 0.65 | 0.176, 1.13 | 6.7 | .01 | 9.3 (1.09) | 10.1 (10.3) | −.87 | −2.4, 0.67 | −12.7 | .93 |
| BSCVA‡ | 0.31 (0.22) 20/41 | 0.21 (0.19) 20/32 | 0.097 | 0.0028, 0.191 | 13.0 | <.001 | 0.48 (0.32) 20/60 | 0.40 (0.30) 20/50 | 0.079 | 0.05, 0.108 | 8.8 | <.001 |
| PH VA‡ | 0.25 (0.20) 20/36 | 0.17 (0.16) 20/30 | 0.087 | 0.014, 0.16 | 13.8 | .001 | 0.42 (0.31) 20/53 | 0.34 (0.28) 20/44 | 0.076 | 0.05, 0.103 | 9.4 | <.001 |
ANOVA, analysis of variance; BSCVA, best-spectacle corrected visual acuity; CI, confidence interval; CME, cystoid macular edema; Ht, height; Mac Vol, Macular Volume; μm, microns; NA, not applicable; PED, pigment epithelial detachment, PHVA, pinhole visual acuity; post-Tx, post-transitioned; SD, one standard deviation; SRF, subretinal fluid; Vol, volume.
μm.
mm3.
log Mar, log10 of reciprocal of Snellen visual acuity.
Nondiabetic eyes. Pre-Tx and post-Tx OCT metrics and vision data of the nondiabetic eyes are outlined in detail in Table 5. The mean changes were as follows: SRF height, 29.1 µm (69.4%) reduction, 95% CI (20.3, 37.8 µm), P<.001; CME height, 38.6 µm (72.9%) reduction, 95% CI (26.9, 50.2 µm), P<.001; PED height, 44.2 µm (12.5%) reduction, 95% CI (31.0, 57.5 µm), P<.001; SRF, 62.5% reduction, and CME volume, 65.3 % reduction, P=.02 and P=.08, respectively; PED volume, 31.9 % reduction, P<.001; central 1-mm subfield, 26.4 µm (8.0%) reduction, 95% CI (17.6, 35.3 µm), P=.01; central 3-mm subfield, 17.2 µm (5.1%) reduction, 95% CI (11.6, 22.8 µm), P=.005; BSCVA, 0.079 logMAR or 4.0 letters (8.8%) improvement, 95% CI (0.05, 0.11 logMAR), P<.001; PHVA, 0.076 logMAR or 3.8 letters (9.4%) improvement, 95% CI (0.05, 0.10 logMAR), P<.001; Macular Volume, −.87 mm3 (12.7%) increase, P=.93.
Thus, at 6 months after transition in comparison to baseline, many of the OCT and vision results showed improvements for both diabetic and nondiabetic eyes (Table 5).
Figure 5 depicts the plots of comparisons between the diabetic and nondiabetic eyes from baseline to 6 months for SRF, CME, and PED heights and volumes, and BSCVA and PHVA, respectively. There were no differences between the two groups for all eight plots (P=.92 for SRF height; P=.88 at baseline and P=.56 at 6 months for SRF volume; P=.64 for CME height; P=.85 for CME volume; P=.99 for PED height; P=.99 for PED volume; P=.04 for BSCVA; P=.04 for PHVA). One-way ANOVA was used for all comparisons within groups, whereas 2-way ANOVA was used for all comparisons between groups except for SRF volume. Mann-Whitney test was used for between-group comparison for SRF volume due to inequality of variances. It should also be noted that for all plots in Figure 5, the P values listed for comparing the 6-month Post-Tx results with baseline for the individual groups (diabetics vs nondiabetics) were derived from the paired t test associated with smaller sample sizes; therefore, they may differ from the P values derived from 2-way ANOVA in Table 5 associated with larger sample sizes.
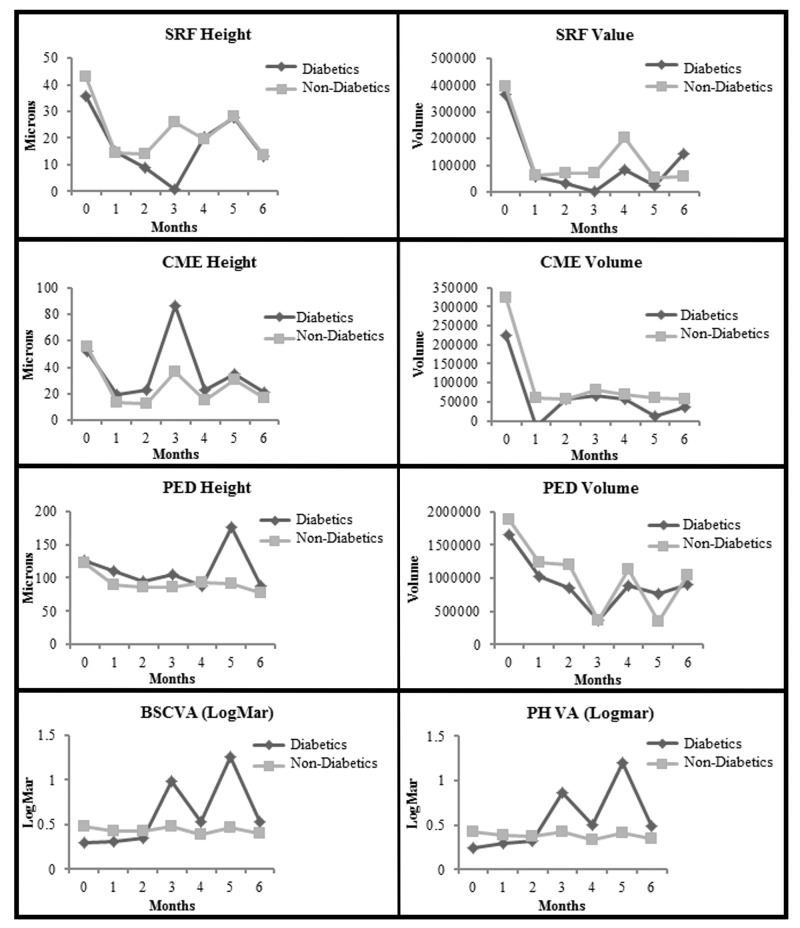
FIGURE 5.
Comparison of diabetic with nondiabetic eyes from baseline to month 6. Top row left, For subretinal fluid (SRF) height (P=.09 for diabetics and P<.001 for nondiabetics), (P=.92 between groups, indicating lack of significant difference between diabetic and nondiabetics). Top row right, For SRF volume (P=.31 for diabetics and P<.001 for nondiabetics), (P=.88 at baseline and P=.56 at 6 months between groups, indicating lack of significant difference between diabetics and nondiabetics). Second row left, For cystoid macular edema (CME) height (P=.15 for diabetics and P<.001 for nondiabetics), (P=.64 between groups, indicating lack of significant difference between diabetic and nondiabetics). Second row right, For CME volume (P=.26 for diabetics and P=.001 for nondiabetics), (P=.85 between groups, indicating lack of significant difference between diabetics and nondiabetics). Third row left, For pigment epithelial detachment (PED) height (P=.12 for diabetics and P<.001 for nondiabetics), (P=.99 between groups, indicating lack of significant difference between diabetics and nondiabetics). Third row right, For PED volume (P=.07 for diabetics and P<.001 for nondiabetics), (P=.99 between groups, indicating lack of significant difference between diabetics and nondiabetics). Bottom row left, For best spectacle-corrected visual acuity (BSCVA) in logMAR (P=.15 for diabetics and P<.001 for nondiabetics), (P=.04 between groups, indicating lack of significant difference between diabetics and nondiabetics). Bottom row right, For pinhole visual acuity (PHVA) in logMAR (P=.07 for diabetics and P<.001 for nondiabetics), (P=.04 between groups, indicating lack of significant difference between diabetics and nondiabetics). (diabetics, eyes with no diabetic retinopathy or only mild background diabetic retinopathy; logMAR, logarithm in base 10 of the reciprocal of the Snellen visual acuity)
Phakia vs Pseudophakia
There were 74 phakic eyes, 114 pseudophakic eyes, and 1 aphakic eye in this study. All except one pseudophakic eye had posterior chamber implants (PCIOLs). The one eye with an anterior chamber implant and the one aphakic eye were eliminated from the statistical analysis, in order to maintain consistency and reduce confounding effects.
Pseudophakic Eyes. Pre-Tx and post-Tx OCT metrics and vision data of the pseudophakic eyes are outlined in detail in Table 6. The mean changes were as follows: SRF height, 27.6 µm (67.3%) reduction, 95% CI (16.5, 38.8 µm), P<.001; CME height, 38.1 µm (71.9 %) reduction, 95% CI (23.9, 52.2 µm), P<.001; PED height, 38.6 µm (5.7%) reduction, 95% CI (22.1, 55.1 µm), P<.001; SRF volume, 49.3% reduction and CME volume, 86.9 % reduction, P<.001 and P=.001, respectively; PED volume, 28.0 % reduction, P<.001; central 1-mm subfield, 17.7 µm (5.4%) reduction, 95% CI (8.8, 26.6 µm), P<.001; central 3-mm subfield, 13.5 µm (4.0%) reduction, 95% CI (7.4, 19.6 µm), P<.001; BSCVA, 0.084 logMAR or 4.2 letters (10.0%) improvement, 95% CI (0.048, 0.12 logMAR), P<.001; PHVA, 0.081 logMAR or 4.1 letters (10.7%) improvement, 95% CI (0.048, 0.11 logMAR), P<.001; Macular Volume, −1.4 mm3 (19.9%) increase, P=.44 (Table 6).
TABLE 6.
COMPARISON OF PRE-AFLIBERCEPT AND POST-AFLIBERCEPT TRANSITIONED OUTCOME (SIX MONTHS) FOR PSEUDOPHAKIC EYES AND PHAKIC EYES (2-WAY ANOVA)*
| PSEUDOPHAKIC EYES (N=113) | PHAKIC EYES (N=74) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VARIABLE(S) |
MEAN BASELINE VALUE (SD) |
MEAN POST-TX VALUE(SD) |
MEAN CHANGE |
95% CI |
% CHANGE |
P VALUE |
MEAN BASELINE VALUE (SD) |
MEAN POST-TX VALUE(SD) |
MEAN CHANGE |
95% CI |
% CHANGE |
P VALUE |
| SRF Ht† | 42.2 (62.3) | 14.6 (33.2) | 27.6 | 16.5, 38.8 | 67.3 | <.001 | 43.1 (57.1) | 13.1 (33.6) | 29.9 | 17.0, 42.8 | 71.7 | <.001 |
| SRF Vol | 350270.2 (896815.6) | 49377.9 (238753.3) | NA | NA | 49.3 | < 001 | 449278.0 (1012800.6) | 90982.8 (432217.5) | NA | NA | 79.9 | < .001 |
| CME Ht† | 56.5 (90.0) | 18.4 (47.0) | 38.1 | 23.9, 52.2 | 71.9 | <.001 | 53.2 (87.1) | 12.4 (38.8) | 40.8 | 22.0, 59.6 | 74.7 | <.001 |
| CME Vol | 338626.3 (1137893.1) | 63207.4 (302852.4) | NA | NA | 86.9 | .001 | 284405.3 (1061812.3) | 22962.6 (129892.7) | NA | NA | 39.9 | .001 |
| PED Ht† | 113.0 (138.3) | 74.3 (101.8) | 38.6 | 22.1, 55.1 | 5.7 | <.001 | 141.0(159.6) | 89.1 (116.4) | 51.9 | 31.6, 72.1 | 24.5 | <.001 |
| PED Vol | 1562164.7 (2651875.6) | 832248.6 (1714954.1) | NA | NA | 28.0 | <.001 | 2409517.2 (4244399.4) | 1369717.9 (2819486.9) | NA | NA | 37.3 | <.001 |
| Central 1-mm† | 248.7(49.5) | 231.0 (39.4) | 17.7 | 8.8, 26.6 | 5.4 | <.001 | 268.4 (61.8) | 229.5 (51.5) | 38.9 | 23.6, 54.2 | 12.0 | <.001 |
| Central 3-mm† | 284.1 (36.3) | 270.6 (27.1) | 13.5 | 7.4, 19.6 | 4.0 | <.001 | 291.3 (40.3) | 268.8 (31.5) | 22.5 | 12.8, 32.2 | 6.7 | <.001 |
| Mac Vol‡ | 9.3 (1.2) | 10.7 (12.7) | −1.4 | −3.8, 0.95 | −19.9 | .44 | 9.3 (0.75) | 9.1 (0.68) | 0.28 | 0.083, 0.47 | 2.5 | .44 |
| BSCVA§ | 0.45 (0.33) 20/56 | 0.37 (0.30) 20/47 | 0.084 | 0.048, 0.120 | 10.0 | <.001 | 0.47 (0.31) 20/59 | 0.39 (0.26) 20/49 | 0.086 | 0.047, 0.126 | 12.1 | <.001 |
| PHVA§ | 0.40 (0.31) 20/50 | 0.32 (0.27) 20/42 | 0.081 | 0.048, 0.114 | 10.7 | <.001 | 0.40 (0.29) 20/50 | 0.32 (0.25) 20/42 | 0.077 | 0.044, 0.110 | 12.0 | <.001 |
BSCVA, best-spectacle corrected visual acuity; CI, confidence interval; CME, cystoid macular edema; Ht, height; Mac Vol, Macular Volume (mm3); μm, microns; NA, not applicable; PED, pigment epithelial detachment, PHVA, pinhole visual acuity; post-Tx, post-transitioned; SD, one standard deviation; Vol, volume.
2-way ANOVA used for comparisons except for central 1-mm subfield thickness, for which Wilcoxon signed rank test was used for the comparison due to interaction between variables.
μm.
mm3.
logMAR, log10 of reciprocal of Snellen visual acuity.
Phakic Eyes. Pre-Tx and post-Tx OCT metrics and vision data of the phakic eyes are outlined in detail in Table 6. The mean changes were as follows: SRF height, 29.9 µm (71.7%) reduction, 95% CI (17.0, 42.8 µm), P<.001; CME height, 40.8 µm (74.7%) reduction, 95% CI (22.0, 59.6 µm), P<.001; PED height, 51.9 µm (24.5%) reduction, 95% CI (31.6, 72.1 µm), P<.001; SRF volume, 79.9% reduction and CME volume, 39.9 % reduction, P<.001 and P=.001, respectively; PED volume, 37.3 % reduction, P<.001; central 1-mm subfield, 38.9 µm (12.0%) reduction, 95% CI (23.6, 54.2 µm), P<.001; central 3-mm subfield, 22.5 µm (6.7%) reduction, 95% CI (12.8, 32.2 µm), P<.001; BSCVA, 0.086 logMAR or 4.3 letters (12.1%) improvement, 95% CI (0.047, 0.13 logMAR), P<.001; PHVA, 0.077 logMAR or 3.9 letters (12.0%) improvement, 95% CI (0.044, 0.11 logMAR), P<.001; Macular Volume, 0.28mm3 (2.5%) reduction, P=.44 (Table 6).
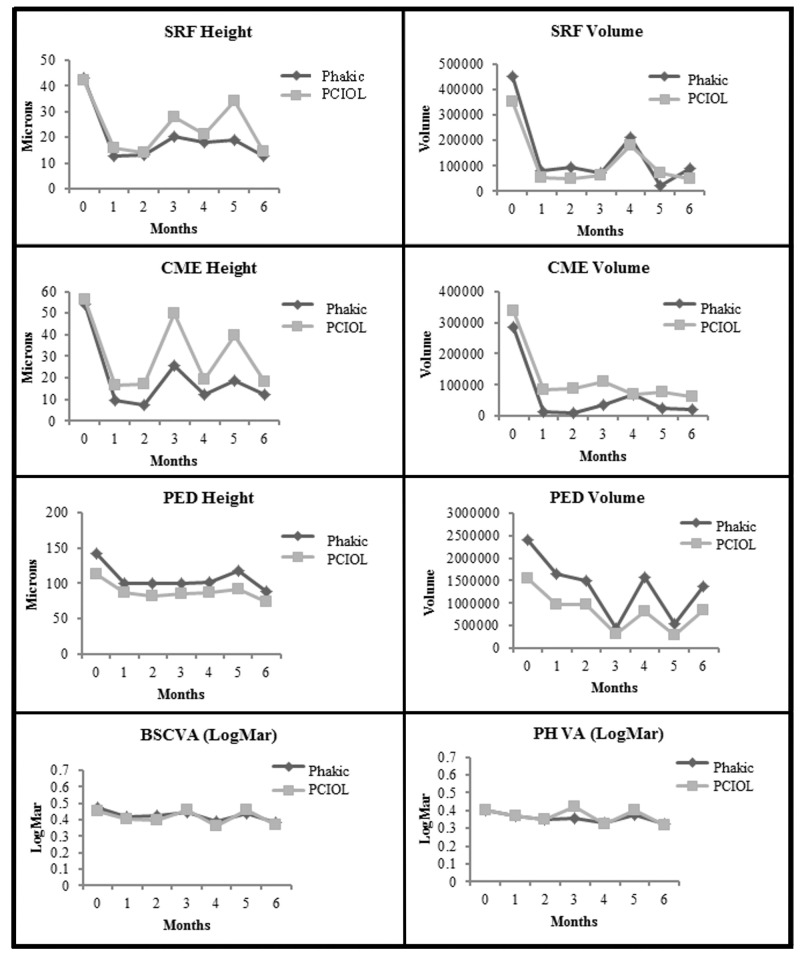
Thus, at 6 months after transition in comparison to baseline, all OCT and visual results with the exception of Macular Volume were improved in both phakic and pseudophakic eyes (Table 6). Two-way ANOVA was used for intragroup comparisons except for central 1-mm subfield thickness, for which the Wilcoxon signed-rank test was used due to interaction between variables.
Figure 6 depicts the plots of comparisons between phakic and pseudophakic eyes from baseline to 6 months for SRF, CME, and PED heights and volumes, and BSCVA and PHVA, respectively. There was no difference between the two groups for all eight plots (P=.96 for SRF height; P=.37 for SRF volume; P=.60 for CME height; P=.79 at baseline and P=.24 at 6 months for CME volume; P=.24 for PED height; P=.36 at baseline and P=.27 at 6 months for PED volume; P= .68 for BSCVA; P=.96 for PHVA. One-way ANOVA was used for all comparisons within groups, whereas 2-way ANOVA was used for all comparisons between groups except for CME volume and PED volume. Mann-Whitney test was used for between-group comparisons for CME volume and PED volume due to inequality of variances. One eye with aphakia and one eye with anterior chamber intraocular implant were eliminated from analysis to avoid confounding the results.
FIGURE 6.
Comparison of phakic eyes with pseudophakic eyes from baseline to month 6. Top row left, For subretinal fluid (SRF) height (P<.001 for phakia and P<.001 for pseudophakia), (P=.96 between groups, indicating a lack of significant difference between phakia and pseudophakia). Top row right, For SRF volume (P=.004 for phakia and P=.001 for pseudophakia), (P=.37 between groups, indicating a lack of significant difference between phakia and pseudophakia). Second row left, For cystoid macular edema (CME) height (P<.001 for phakia and P<.001 for pseudophakia), (P=.60 between groups, indicating a lack of significant difference between phakia and pseudophakia). Second row right, For CME volume (P=.04 for phakia and P=.004 for pseudophakia), (P=.79 at baseline and P=.24 at 6 months between groups, indicating a lack of significant difference between phakia and pseudophakia). Third row left, For pigment epithelial detachment (PED) height (P<.001 for phakia and P<.001 for pseudophakia), (P=.24 between groups, indicating a lack of significant difference between phakia and pseudophakia). Third row right, For PED volume (P<.001 for phakia and P<.001 for pseudophakia), (P=.36 at baseline and P=.27 at 6 months between groups, indicating a lack of significant difference between phakia and pseudophakia). Bottom row left, For best spectacle-corrected visual acuity (BSCVA) in logMAR (P<.001 for phakia and P<.001 for pseudophakia), (P=.68 between groups, indicating a lack of significant difference between phakia and pseudophakia). Bottom row right, for pinhole visual acuity (PHVA) in logMAR (P<.001 for phakia and P<.001 for pseudophakia), (P=.96 between groups, indicating a lack of significant difference between phakia and pseudophakia). (logMAR, logarithm in base 10 of the reciprocal of the Snellen visual acuity; PCIOL, posterior chamber intraocular lens; pseudophakic eyes, eyes with PCIOL;[1 eye with aphakia and 1 eye with anterior chamber intraocular lens were eliminated from analysis])
It should be noted that for all plots in Figure 6, the P values listed for comparing the 6- month post-Tx results with baseline for the individual groups (phakic vs pseudophakic eyes) were derived from the paired t test associated with smaller sample sizes; nevertheless, they turned out to be the same as the P values derived from 2-way ANOVA in Table 6 associated with larger sample sizes.
Eyes With PED vs Eyes Without PED
In this study, there were 102 eyes with a vascularized PED and 87 eyes without any PED.
Ped Eyes. Pre-Tx and post-Tx OCT metrics and vision data of the eyes with PED are outlined in detail in Table 7.
TABLE 7.
COMPARISON OF PRE- AFLIBERCEPT AND POST-AFLIBERCEPTTRANSITIONED OUTCOME (SIX MONTHS) FOR EYES WITH PED AND FOR EYES WITHOUT PED (2-WAY ANOVA)
| EYES WITH PED (N=102) | EYES WITHOUT PED (N=87) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VARIABLE(S) |
MEAN BASELINE VALUE (SD) |
MEAN POST-TX VALUE(SD) |
MEAN CHANGE |
95% CI |
% CHANGE |
P VALUE |
MEAN BASELINE VALUE (SD) |
MEAN POST-TX VALUE(SD) |
MEAN CHANGE |
95% CI |
% CHANGE |
P VALUE |
| SRF Ht* | 60.1 (63.3) | 18.0 (34.0) | 42.1 | 29.7, 54.5 | 72.5 | <.001 | 22.2 (48.7) | 9.0 (31.6) | 13.1 | 3.1, 23.1 | 61.1 | .004 |
| SRF Vol | 589352.6 (1162150.6) | 64304.4 (314192.9) | NA | NA | 81.5 | <.001 | 167393.1 (516104.6) | 66130.8 (343325.4) | NA | NA | 7.0 | .04 |
| CME Ht* | 43.5 (81.4) | 14.0 (45.5) | 29.5 | 16.8, 42.2 | 78.3 | <.001 | 69.7 (95.1) | 21.5 (51.2) | 48.3 | 28.7, 67.8 | 65.5 | <.001 |
| CME Vol | 278602.1 (840421.8) | 61607.1 (316598.8) | NA | NA | 91.2 | .001 | 361931.2 (1346688.6) | 50799.9 (234417.0) | NA | NA | 46.6 | .001 |
| PED Ht* | 226.3 (128.5) | 145.3 (108.3) | 81.0 | 60.4, 101.6 | 13.6 | <.001 | 0 | 0 | NA | NA | 0 | NA |
| PED Vol | 3478714.6 (3947380.0) | 1915250.0 (2729845.5) | NA | NA | 31.9 | <.001 | 0 | 0 | NA | NA | 0 | NA |
| Central 1-mm* | 263.8 (58.6) | 228.9 (42.1) | 34.9 | 23.2, 46.6 | 10.6 | <.001 | 247.9 (49.9) | 234.7 (52.2) | 13.2 | 1.5, 25.0 | 4.0 | .03 |
| Central 3-mm* | 289.4 (38.8) | 268.4 (27.0) | 21.0 | 13.7, 28.3 | 6.3 | <.001 | 283.7 (36.7) | 272.4 (31.3) | 11.3 | 3.3, 19.2 | 3.2 | <.001 |
| Mac Vol† | 9.5 (0.91) | 11.0 (13.3) | −1.5 | −4.1, 1.1 | −14.0 | .35 | 9.1 (1.2) | 8.9 (0.82) | 0.14 | −.15, 0.43 | −7.2 | .35 |
| BSCVA‡ | 0.47 (0.32) 20/59 | 0.38 (0.28) 20/48 | 0.094 | 0.056, 0.133 | 12.3 | <.001 | 0.46 (0.32) 20/58 | 0.39 (0.31) 20/49 | 0.065 | 0.026, 0.104 | 5.5 | <.001 |
| PHVA‡ | 0.39 (0.29) 20/49 | 0.31 (0.26) 20/41 | 0.822 | 0.049, 0.116 | 11.5 | <.001 | 0.42 (0.31) 20/53 | 0.35 (0.29) 20/45 | 0.072 | 0.035, 0.108 | 7.8 | <.001 |
BSCVA, best spectacle-corrected visual acuity; CI, confidence interval; CME, cystoid macular edema; Ht, height; Mac Vol, Macular Volume; NA, not applicable; PED, pigment epithelial detachment; PHVA, pinhole visual acuity; post-Tx, post-transitioned; SD, one standard deviation; SRF, subretinal fluid; Vol, volume.
μm.
mm3.
logMAR, log10 of reciprocal of Snellen visual acuity.
The mean changes were as follows: SRF height, 42.1 µm (72.5%) reduction, 95% CI (29.7, 54.5 µm), P<.001; CME height, 29.5 µm (78.3%) reduction, 95% CI (16.8, 42.2 µm), P<.001; PED height, 81.0 µm (13.6%) reduction, 95% CI (60.4, 101.6 µm), P<.001; SRF volume, 81.5% reduction and CME volume, 91.2% reduction, P<.001 and P=.001, respectively; PED volume, 31.9% reduction, P<.001; central 1-mm subfield, 34.9 µm (10.6%) reduction, 95% CI (23.3, 46.6 µm), P<.001; central 3-mm subfield, 21.0 µm (6.3%) reduction, 95% CI (13.7, 28.3 µm), P<.001; BSCVA, 0.094 logMAR or 4.7 letters (12.3%) improvement, 95% CI (0.056, 0.13 logMAR), P<.001; PHVA, 0.082 logMAR or 4.1 letters (11.5%) improvement, 95% CI (0.049, 0.12 logMAR), P<.001; Macular Volume, −1.5mm3 (14.0%) increase, P=.35 (Table 7).
Non-Ped Eyes. Pre-Tx and post-Tx OCT metrics and vision data of the non-PED eyes are outlined in detail in Table 7.
The mean changes were as follows: SRF height, 13.1 µm (61.1%) reduction, 95% CI (3.1, 23.1 µm), P=.004; CME height, 48.3 µm (65.5%) reduction, 95% CI (28.7, 67.8 µm), P<.001; SRF volume, 7.0% reduction and CME volume, 46.6% reduction, P=.04 and P=.001, respectively; central 1-mm subfield, 13.2 µm (4.0%) reduction, 95% CI (1.5, 25.0), P=.03; central 3-mm subfield, 11.3 µm (3.2%) reduction, 95% CI (3.3, 19.2 µm), P<.001; BSCVA, 0.065 logMAR or 3.3 letters (5.5%) improvement, 95% CI (0.026, 0.10 logMAR), P<.001; PHVA, 0.072 logMAR or 3.6 letters (7.8%) improvement, 95% CI (0.035, 0.11 logMAR), P<.001; Macular Volume, 0.14 mm3 (7.2%) decrease, P=.35 (Table 7).
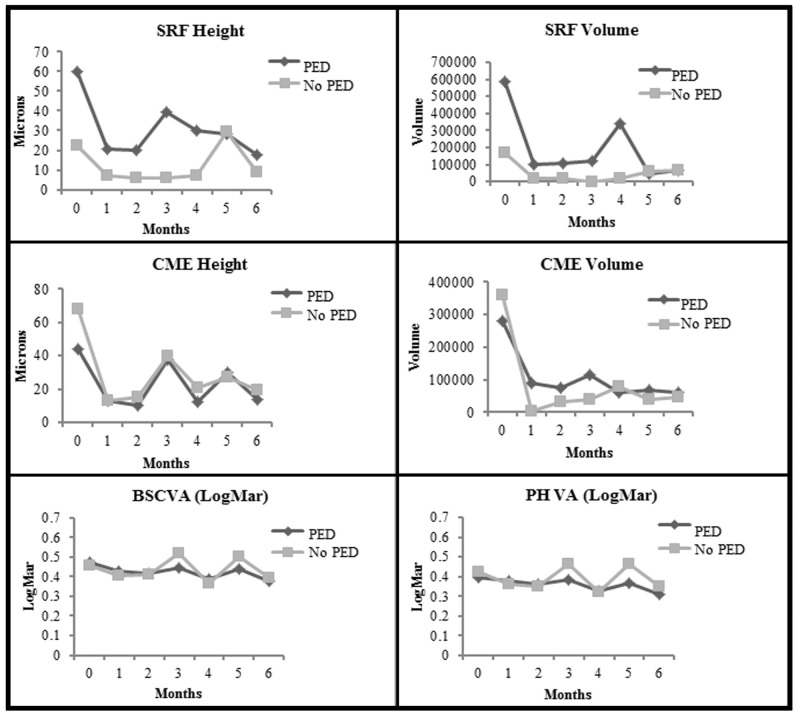
At 6 months after transition in comparison to baseline, there was highly significant improvement in all OCT and visual results for eyes with PED (all P≤.001, paired t test), except for Macular Volume (Table 7). For eyes without a PED, similar but somewhat less significant improvements were noted for all OCT and visual results with the exception of Macular Volume (P values ranged from .04 to .004), paired t test (Table 7).
Figure 7 depicts the plots of comparisons between eyes with a vascularized PED vs eyes without a PED from baseline to 6 months for SRF and CME heights and volumes, and BSCVA and PHVA, respectively. For SRF height comparison, there was a significant difference between the two groups at baseline (P<.001); at 6 months, the P value was .002 between the two groups. For SRF volume comparison, there was a significant difference between the two groups at baseline (P<.001); at 6 months, the P value was .002 between the two groups.. For the CME height comparison, there was no significant difference between the two groups (P=.05). For the CME volume comparison, there was no significant difference between the two groups (P=.69). For the BSCVA comparison, there was no significant difference between the two groups (P=.97). For the PHVA comparison, there was no difference between the two groups (P=.47). One-way ANOVA was used for all comparisons within groups, while 2-way ANOVA was used for all comparisons between groups except for SRF height, SRF volume, PED height, and PED volume comparisons. Mann-Whitney test was used for comparisons between groups for SRF height, SRF volume, PED height, and PED volume due to group interaction and inequality of variances.
FIGURE 7.
Comparison of eyes with pigment epithelial detachment (PED) with eyes without PED from baseline to month 6. Top row left, For subretinal fluid (SRF) height (P<.001 for PED and P=.01 for no PED), (P<.001 at baseline and P=.002 at 6 months between groups, indicating significant difference at baseline and a trend toward difference at 6 months between the two group). Top row right, For SRF volume (P<.001 for PED and P=.08 for no PED), (P<.001 at baseline and P=.002 at 6 months between groups, indicating significant difference at baseline and a trend toward difference at 6 months between the two groups). Middle row left, For cystoid macular edema (CME) height (P<.001 for PED and P<.001 for no PED), (P=.05 between groups, indicating a lack of significant differences between PED and no PED). Middle row right, For CME volume (P=.001 for PED and P=.04 for no PED) (P=.69 between groups, indicating a lack of significant differences between PED and No PED). Bottom row left, For best spectacle-corrected visual acuity (BSCVA) in logMAR (P<.001 for PED and P=.001 for no PED), (P=.97 between groups, indicating a lack of significant differences between PED and no PED). Bottom row right, For pinhole visual acuity (PHVA) in logMAR (P<.001 for PED and P<.001 for No PED), (P=.47 between groups, indicating a lack of significant differences between PED and no PED). (logMAR, logarithm in base 10 of the reciprocal of the Snellen visual acuity)
It should be noted that for all plots in Figure 7, the P values listed for comparing the 6- month post-Tx results with baseline for the individual groups (eyes with PED vs eyes without PED) were derived from the paired t test associated with smaller sample sizes; therefore, they may differ from the P values derived from 2-way ANOVA or Wilcoxon signed-rank test in Table 7.
OCT RESULTS CATEGORIZED AS IMPROVED, UNCHANGED, OR WORSE
For this study, power calculations showed a sufficient sample size to detect a 10% or greater change in OCT-measured values when comparing pretransitioned and posttransitioned results and also between-group results. Therefore, a magnitude of >10% of increase or decrease in OCT-measured value was considered to be a real change from baseline. Table 8 outlines the percentages of eyes with improved, unchanged, or worse SRF height and volume when comparing baseline with 6 months for the entire cohort as well as for the drug groups, Responders and Nonresponders, diabetic and nondiabetics eyes, phakic and pseudophakic eyes, and eyes with PED and eyes without PED. Table 9 outlines the percentages of eyes with improved, unchanged, or worse CME height and volume when comparing baseline with 6 months for the entire cohort as well as for all of the same subgroups outlined in Table 8. Table 10 outlines the percentages of eyes with improved, unchanged, or worse PED height and volume when comparing baseline with 6 months for the entire cohort as well as for all of the same subgroups outlined in Tables 8 and 9. For Tables 8 and 9, eyes with data entry of zero value at baseline and also at 6 months were excluded from the calculation in percentage change, in order to avoid confounding the results. There were 7 eyes with no SRF and 1 eye with no CME at baseline, which subsequently developed increased SRF and CME, respectively, in 6 months after transition. For these eyes, the magnitude of the respective changes was compared to the other eyes in the same cohort, in order to properly categorize them as “unchanged” or “worse” (Tables 8 and 9).
TABLE 8.
CATEGORIZATION OF SUBRETINAL FLUID HEIGHT AND VOLUME AS IMPROVED, NO CHANGE, OR WORSE AT SIX MONTHS AFTER TRANSITION TO AFLIBERCEPT
| SUBRETINAL FLUID HEIGHT (MICRONS) | SUBRETINAL FLUID VOLUME | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| VARIABLE(S) |
TOTAL EYES* |
WORSE (%)† |
NO CHANGE (%)‡ |
IMPROVED (%)§ |
95% CI IMPROVED EYES (%) |
TOTAL EYES* |
WORSE (%)† |
NO CHANGE (%)‡ |
IMPROVED (%)§ |
95% CI IMPROVED EYES (%) |
| Entire cohort | 89 | 14 (15.7) | 2 (2.2) | 73 (82.0) | 74.0, 90.0 | 89 | 13 (14.6) | 1 (1.1) | 75 (84.3) | 76.7, 91.9 |
| Drug groups | ||||||||||
| Beva | 48 | 4 (8.3) | 0 | 44 (91.7) | 83.9, 99.5 | 48 | 3 (6.3) | 1 (2.1) | 44 (91.7) | 83.9, 99.5 |
| Rani | 38 | 9 (23.7) | 2 (5.3) | 27 (71.1) | 56.7, 85.8 | 38 | 9 (23.7) | 0 | 29 (76.3) | 62.8, 89.8 |
| Mixed | 3 | 1 (33.3) | 0 | 2 (66.7) | 13.4, 100 | 3 | 1 (33.3) | 0 | 2 (66.7) | 13.4, 100 |
| Diabetic | 6 | 1 (16.7) | 0 | 5 (83.3) | 53.5, 100 | 6 | 1 (16.7) | 0 | 5 (83.3) | 53.5, 100 |
| Nondiabetic | 83 | 13 (15.7) | 2 (2.4) | 68 (81.9) | 73.4, 90.2 | 83 | 12 (14.5) | 1 (1.2) | 70 (84.3) | 76.5, 92.1 |
| PCIOL | 54 | 9 (16.7) | 2 (3.7) | 43 (79.6) | 68.9, 90.4 | 54 | 8 (14.8) | 1 (1.9) | 45 (83.3) | 73.4, 93.2 |
| No PCIOL | 34 | 5 (14.7) | 0 | 29 (85.3) | 73.4, 97.2 | 34 | 5 (14.7) | 0 | 29 (85.3) | 73.4, 97.2 |
| PED | 66 | 9 (13.6) | 1 (1.5) | 56 (84.8) | 76.1, 93.5 | 66 | 8 (12.1) | 1 (1.5) | 57 (86.4) | 78.1, 94.7 |
| No PED | 23 | 5 (21.7) | 1 (4.3) | 17 (73.9) | 56.0, 91.9 | 23 | 5 (21.7) | 0 | 18 (78.3) | 61.5, 95.2 |
Beva, Legacy-bevacizumab; CI, confidence interval; PCIOL, posterior chamber intraocular lens; PED, pigment epithelial detachment; Rani, Legacy-ranibizumab.
Total number of eyes does not add up to 189, since eyes with absence ofsubretinal fluid at baseline and also throughout the course of study were excluded from analysis to avoid confounding the results.
>10%increase.
−10% to +10% change.
>10% decrease.
TABLE 9.
CATEGORIZATION OF CYSTOID MACULAR EDEMA HEIGHT AND VOLUME AS IMPROVED, NO CHANGE, OR WORSE AT SIX MONTHS AFTER TRANSITION TO AFLIBERCEPT
| CYSTOID MACULAR EDEMA HEIGHT (MICRONS) | CYSTOID MACULAR EDEMA VOLUME | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| VARIABLE(S) |
TOTAL EYES* |
WORSE (%)† |
NO CHANGE (%)‡ |
IMPROVED (%)§ |
95% CI IMPROVED EYES (%) |
TOTAL EYES* |
WORSE (%)† |
NO CHANGE (%)‡ |
IMPROVED (%)§ |
95% CI IMPROVED EYES (%) |
| Entire cohort | 71 | 4 (5.6) | 2 (2.8) | 65 (91.5) | 85.0, 98.0 | 71 | 2 (2.8) | 1 (1.4) | 68 (95.8) | 91.1, 100 |
| Drug groups | ||||||||||
| Beva | 36 | 2 (5.6) | 2 (5.6) | 32 (88.9) | 78.6, 99.2 | 36 | 1 (2.8) | 0 | 35 (97.2) | 91.8, 100 |
| Rani | 28 | 2 (7.1) | 0 | 26 (92.9) | 83.4, 100 | 28 | 2 (7.1) | 0 | 26 (92.9) | 83.4, 100 |
| Mixed | 7 | 0 | 0 | 7 (100.0) | NA | 7 | 0 | 0 | 7 (100.0) | NA |
| Diabetic | 6 | 1 (16.7) | 1 (16.7) | 4 (66.7) | 29.0, 100 | 6 | 0 | 0 | 6 (100.0) | |
| Nondiabetic | 65 | 3 (4.6) | 1 (1.5) | 61 (93.8) | 87.9, 99.7 | 65 | 2 (3.1) | 1 (1.5) | 62 (95.4) | 90.3, 100 |
| PCIOL | 44 | 2 (4.5) | 1 (2.3) | 41 (93.2) | 85.8, 100 | 44 | 0 | 0 | 100 | |
| No PCIOL | 26 | 1 (3.8) | 1 (3.8) | 24 (92.3) | 82.1, 100 | 26 | 1 (3.8) | 1 (3.8) | 24 (92.3) | 82.1, 100 |
| PED | 41 | 4 (9.8) | 2 (4.9) | 35 (85.4) | 74.6, 96.2 | 41 | 2 (4.9) | 1 (2.4) | 38 (92.7) | 84.7, 100 |
| No PED | 30 | 0 | 0 | 100 | NA | 30 | 0 | 0 | 100 | NA |
Beva, Legacy-bevacizumab; CI, confidence interval; PCIOL, posterior chamber intraocular lens; PED, pigment epithelial detachment; Rani, Legacy-ranibizumab.
Total number of eyes does not add up to 189, since eyes with absence of cystoid macular edema at baseline and also throughout the course of study were excluded from analysis to avoid confounding the results.
>10%increase.
−10% to +10% change.
>10% decrease.
TABLE 10.
CATEGORIZATION OF PIGMENT EPITHELIAL DETACHMENT HEIGHTAND VOLUME AS IMPROVED, NO CHANGE, OR WORSE AT SIX MONTHS AFTER TRANSITION TO AFLIBERCEPT
| PIGMENT EPITHELIAL DETACHMENT HEIGHT (microns) | PIGMENT EPITHELIAL DETACHMENT VOLUME | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| VARIABLE(S) |
TOTAL EYES* |
WORSE† (%) |
NO CHANGE‡ (%) |
IMPROVED (%)§ |
95% CI IMPROVED EYES (%) |
TOTAL EYES* |
WORSE (%)† |
NO CHANGE‡ (%) |
IMPROVED (%)§ |
95% CI IMPROVED EYES (%) |
| Entire cohort | 102 | 4 (3.9) | 16 (15.7) | 82 (80.4) | 72.7, 88.1 | 102 | 6 (5.9) | 10 (9.8) | 86 (84.3) | 77.2, 91.4 |
| Drug groups | ||||||||||
| Beva | 49 | 2 (4.1) | 8 (16.3) | 39 (79.6) | 68.3, 90.9 | 49 | 4 (8.2) | 5(10.2) | 40 (81.6) | 70.8, 92.5 |
| Rani | 47 | 1 (2.1) | 7 (14.9) | 39 (83.0) | 72.3, 93.7 | 47 | 1 (2.1) | 4 (8.5) | 42 (89.4) | 80.6, 98.2 |
| Mixed | 6 | 1 (16.7) | 1 (16.7) | 4 (66.7) | 29.0, 100 | 6 | 1 (16.7) | 1 (16.7) | 4 (66.7) | 29.0, 100 |
| Diabetic | 8 | 0 | 3 (37.5) | 5 (62.5) | 29.0, 96.0 | 8 | 1 (12.5) | 3 (37.5) | 4(50.0) | 15.4, 84.6 |
| Nondiabetic | 94 | 4 (4.3) | 13 (13.8) | 77 (81.9) | 74.1, 89.7 | 94 | 5 (5.3) | 7 (7.4) | 82 (87.2) | 80.5, 94.0 |
| PCIOL | 59 | 3 (5.1) | 12 (20.3) | 44 (74.6) | 63.5, 85.7 | 59 | 3 (5.1) | 6 (10.2) | 50 (84.7) | 75.5, 93.9 |
| No PCIOL | 43 | 1 (2.3) | 4 (9.3) | 38 (88.4) | 78.8, 98.0 | 43 | 3 (7.0) | 4 (9.3) | 36 (83.7) | 72.7, 94.7 |
| PED | 102 | 1 (2.3) | 16 (15.7) | 82 (80.4) | 72.7, 88.1 | 102 | 6 (5.9) | 10 (9.8) | 86 (84.3) | 77.2, 91.4 |
| No PED | 87 eyes without PED were excluded | |||||||||
Beva, Legacy-bevacizumab; CI, confidence interval; PCIOL, posterior chamber intraocular lens; PED, pigment epithelial detachment; Rani, Legacy-ranibizumab.
Total number of eyes does not add up to 189, since eyes with absence of pigment epithelial detachment at baseline and also throughout the course of study were excluded from analysis to avoid confounding the results.
>10% increase
−10% to +10% change.
>10% decrease.
For the entire cohort, SRF height and volume were improved in 73 eyes (82.0%) and 75 eyes (84.3%), respectively; unchanged in 2 eyes (2.2%) and 1 eye (1.1%), respectively; and worse in 14 eyes (15.7%) and 13 eyes (14.6%), respectively (Table 8). For the subgroup analyses, SRF height and volume were improved for 66.7% to 91.7% of eyes and 66.7% to 91.7% of eyes, respectively; were unchanged for 0 to 5.3% of eyes and 0 to 2.1% of eyes, respectively; and were worse for 8.3% to 33.3% of eyes and 6.3% to 33.3% of eyes, respectively (Table 8).
Likewise, CME height and volume associated with the entire cohort were improved in 65 eyes (91.5%) and 68 eyes (95.8%), respectively; unchanged in 2 eyes (2.8%) and 1 eye (1.4%), respectively; and worse in 4 eyes (5.6%) and 2 eyes (2.8%), respectively (Table 9). For the subgroup analyses, CME height and volume were improved for 66.7% to 100% of eyes and 92.3% to100% of eyes, respectively; were unchanged for 0 to 16.7% of eyes and 0 to 3.8% of eyes, respectively; and were worse for 0 to 16.7% of eyes and 0 to 7.1% of eyes, respectively (Table 9).
PED height and volume associated with the entire cohort were improved in 82 eyes (80.4%) and 86 eyes (84.3%), respectively; unchanged in 16 eyes (15.7%) and 10 eyes (9.8%), respectively; and worse in 4 eyes (3.9%) and 6 eyes (5.9%), respectively (Table 10). For the subgroup analyses, PED height and volume were improved for 62.5% to 88.4% of eyes and 50.0% to 89.4% of eyes, respectively; were unchanged for 9.3% to 20.3% of eyes and 7.4% to 37.5% of eyes, respectively; and were worse for 0 to 16.7% of eyes and 2.1% to 16.7% of eyes, respectively (Table 10).
VISUAL RESULTS CATEGORIZED AS IMPROVED, NO CHANGE, OR WORSE
In this study, power calculations showed a sufficient sample size to detect a threshold of 3- to 5-letter change (0.06 to 0.1 log unit) from baseline to post-Tx visits and between groups. Table 11 categorizes vision changes based on a 4-letter (0.08 log units) threshold, and Table 12 categorizes vision changes based on a 5-letter (0.1 log unit) threshold.
TABLE 11.
CATEGORIZATION OF VISION CHANGES AS IMPROVED, NO CHANGE, OR WORSE AT SIX MONTHS AFTER TRANSITION TO AFLIBERCEPT (FOUR-LETTER THRESHOLD), EYES (PERCENT)*
| BSCVA | PH VA | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| VARIABLE(S) |
TOTAL EYES* |
WORSE† |
NO CHANGE‡ |
IMPROVED§ |
95% CI IMPROVED EYES (%) |
WORSE† |
NO CHANGE‡ |
IMPROVED§ |
95% CI IMPROVED EYES (%) |
| Entire cohort | 189 | 29 (15.3) | 80 (42.3) | 80 (42.3) | 35, 49 | 25 (13.2) | 87 (46.0) | 77 (40.7) | 34, 48 |
| Drug groups | |||||||||
| Beva | 95 | 17 (17.9) | 41 (43.2) | 37 (38.9) | 29, 49 | 16 (16.8) | 44 (46.3) | 35 (36.8) | 27, 47 |
| Rani | 84 | 11 (13.1) | 36 (42.9) | 37 (44.0) | 33, 55 | 9 (10.7) | 39 (46.4)) | 36 (42.9) | 32, 53 |
| Mixed | 10 | 1 (10.0) | 3 (30.0) | 6 (60.0) | 30, 90 | 0 | 4 (40.0) | 6 (60.0) | 30, 90 |
| Diabetic | 17 | 4 (23.5) | 4 (23.5) | 9 (52.9) | 29, 77 | 3 (17.6) | 4 (23.5) | 10 (58.8) | 35, 82 |
| Nondiabetic | 172 | 25 (14.5) | 76 (44.2) | 71 (41.3) | 34, 49 | 22 (12.8) | 83 (48.3) | 67 (39.0) | 32, 46 |
| PCIOL | 113 | 14 (12.4) | 51 (45.1) | 48 (42.5) | 33, 52 | 13 (11.5) | 55 (48.7) | 45 (39.8) | 31, 49 |
| No PCIOL | 74 | 14 (18.9) | 28 (37.8) | 32 (43.2) | 32, 54 | 11 (14.9) | 32 (43.2) | 31 (41.9) | 31, 53 |
| PED | 102 | 15 (14.7) | 42 (41.2) | 45 (44.1) | 34, 54 | 12 (11.8) | 49 (48.0) | 41 (40.2) | 31, 50 |
| No PED | 87 | 14 (16.1) | 38 (43.7) | 35 (40.2) | 30, 51 | 13 (14.9) | 38 (43.7) | 36 (41.4) | 31, 52 |
Beva, Legacy-bevacizumab; CI, confidence interval; PCIOL, posterior chamber intraocular implant; PED, pigment epithelial detachment; Rani, Legacy-ranibizumab.
Change is defined as Pre logMAR – Post logMAR.
Difference ≥.08 log units increase.
within −.08 to +.08 log units change.
≥.08 log units decrease; .08 log units = 4-letter difference.
TABLE 12.
CATEGORIZATION OF VISIONCHANGES AS IMPROVED, NO CHANGE, OR WORSE AT SIX MONTHS AFTER TRANSITION TO AFLIBERCEPT (FIVE-LETTER THRESHOLD), EYES (PERCENT)*
| BSCVA | PH VA | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| VARIABLE(S) |
TOTAL EYES |
WORSE† |
NO CHANGE‡ |
IMPROVED§ |
95% CI IMPROVED EYES (%) |
WORSE† |
NO CHANGE‡ |
IMPROVED§ |
95% CI IMPROVED EYES (%) |
| Entire cohort | 189 | 20 (10.6) | 106 (56.1) | 63 (33.3) | 26.6, 40.0 | 16 (8.5) | 108 (57.1) | 65 (34.4) | 27.6, 41.2 |
| Drug groups | |||||||||
| Beva | 95 | 11 (11.6) | 54 (54.6) | 30 (31.6) | 22.3, 40.9 | 10 (10.5) | 58 (61.1) | 27 (28.4) | 19.3, 37.5 |
| Rani | 84 | 9 (10.7) | 48 (57.1) | 27 (32.1) | 22.1, 42.1 | 6 (7.1) | 46 (54.8) | 32 (38.1) | 27.7, 48.5 |
| Mixed | 10 | 0 | 4 (40.0) | 6(60.0) | 29.6, 90.4 | 0 | 4 (40.0) | 6 (60.0) | 29.6, 90.4 |
| Diabetic | 17 | 2 (11.8) | 7 (41.2) | 8 (47.1) | 23.4, 70.8 | 2 (11.8) | 6 (35.3) | 9 (52.9) | 29.2, 76.6 |
| Nondiabetic | 172 | 18 (10.5) | 99 (57.6) | 55 (32.0) | 25.0, 39.0 | 14 (8.1) | 102 (59.3) | 56 (32.6) | 25.6, 39.6 |
| PCIOL | 113 | 11 (9.7) | 65 (57.5) | 37 (32.7) | 24.1, 41.3 | 10 (8.8) | 64 (56.6) | 39 (34.5) | 25.7, 43.3 |
| No PCIOL | 74 | 8 (10.8) | 40 (54.1) | 26 (35.1) | 24.4, 46.0 | 5 (6.8) | 44 (59.5) | 25 (33.8) | 23.0, 44.6 |
| PED | 102 | 9 (8.8) | 56 (54.9) | 37 (36.3) | 27.0, 45.6 | 7 (6.9) | 59 (57.8) | 36 (35.3) | 26.0, 44.6 |
| No PED | 87 | 11 (12.6) | 50 (57.5) | 26 (29.9) | 20.3, 39.5 | 9 (10.3) | 49 (56.3) | 29 (33.3) | 23.4, 43.2 |
Beva, Legacy-bevacizumab; CI, confidence interval; PCIOL, posterior chamber intraocular lens; PED, pigment epithelial detachment; Rani, Legacy- ranibizumab.
Change is defined as Pre logMAR – Post logMAR.
Difference≥.10 log units increase.
−.10 to +.10 log units change.
≥.10 log units decrease; .10 log units = 5-letter difference.
For the 4-letter equivalent threshold, BSCVA was improved in 80 eyes (42.3%) and PHVA was improved in 77 eyes (40.7%). BSCVA was unchanged in 80 (42.3%) and PHVA was unchanged in 87 (46.0%), and BSCVA was worse in 29 (15.3%) and PHVA was worse in 16 eyes (8.5%). Regarding the subgroups, BSCVA was improved in 38.9% to 52.9% of eyes, and PHVA was improved in 36.8% to 60.0% of eyes. BSCVA was unchanged in 23.5% to 45.1% of eyes, and PHVA was unchanged in 23.5% to 48.7% of eyes. BSCVA was worse in 10.0% to 23.5% of eyes, and PHVA was worse in 0 to 17.6% of eyes (Table 11).
For the 5-letter equivalent threshold, BSCVA was improved in 63 eyes (33.3%) and PHVA was improved in 65 eyes (34.4%). BSCVA was unchanged in 106 eyes (56.1%) and PHVA was unchanged in 108 eyes (57.1%), and BSCVA was worse in 20 eyes (10.6%) and PHVA was worse in 16 eyes (8.5%). Regarding the subgroups, BSCVA was improved in 29.9% to 60.0% of eyes, and PHVA was improved in 28.4% to 60.0% of eyes. BSCVA was unchanged in 40.0% to 57.6% of eyes, and PHVA was unchanged in 35.3% to 61.1% of eyes. BSCV was worse in 0 to 12.6% of eyes, and PHVA was worse in 0 to 11.8% of eyes (Table 12).
CORRELATION OF OCT-MEASURED VARIABLES WITH VISION OUTCOME
Pearson correlation analysis was performed to assess OCT-measured variables (SRF, CME, and PED heights and volume, and central 1-mm subfield) vs BSCVA and PHVA, respectively, for the entire cohort, as well as for the subgroups (drug groups, diabetic and nondiabetic eyes, Responders and Nonresponders, phakic eyes and pseudophakic eyes, and eyes with PED vs eyes without PED).
Despite the seemingly inverse relationship between OCT-measured variables and vision, correlation analysis has shown highly variable correlation coefficients for the entire cohort as well as for the subgroups. For the entire cohort, the Pearson correlation coefficients ranged from r= −0.01 to 0.15 at baseline and from r= −.11 to .099 at 6 months when correlating the various OCT-measured variables with BSCVA (all P>.05), and they ranged from r=−.035 to .094 at baseline and from r= −.17 to .09 at 6 months when correlating the various OCT-measured variables with PHVA (all P>.05) (Table 13). Similarly variable correlation coefficients were noted for the individual drug groups.
TABLE 13.
PEARSON CORRELATION COEFFICIENTS FOR OPTICAL COHERENCE TOMOGRAPHY–MEASURED VARIABLES VS VISION VARIABLES (ENTIRE COHORT)
| VARIABLE |
BSCVA BASELINE r (P VALUE) |
BSCVA 6 MONTH r (P VALUE) |
PHVA BASELINE r (P VALUE) |
PHVA 6 MONTH r (P VALUE) |
|---|---|---|---|---|
| SRF Ht | .012 (.870) | .057 (.436) | −.002 (.981) | −.024 (.749) |
| SRF Vol | .083 (.257) | .074 (.314) | .094 (.197) | .053 (.469) |
| CME Ht | .126 (.085) | .099 (.177) | .165 (.024) | .090 (.221) |
| CME Vol | .149 (.040) | .011 (.881) | .176 (.015) | −.019 (.790) |
| PED Ht | .050 (.496) | −.021 (.778) | −.005 (.946) | −.069 (.347) |
| PED Vol | .000 (.996) | −.006 (.930) | −.035 (.634) | .059 (.418) |
| Central 1-mm | −.011 (.880) | −.112 (.126) | −.013 (.858) | −.167 (.022) |
BSCVA, best spectacle-corrected visual acuity; CME, cystoid macular edema; Ht, height; PED, pigment epithelial detachment; PHVA, pinhole visual acuity; r, Pearson correlation coefficient; SRF, subretinal fluid; Vol, volume.
PREDICTOR ANALYSIS
Stepwise logistical regression was performed to assess whether any of the baseline variables were significant predictors of being Responders or Nonresponders. The baseline variables examined included age, gender, right eye vs left eye, SRF, CME, and PED heights and volumes, vision metrics (BSCVA, PHVA), central 1- and 3-mm subfield thickness, Macular Volume, drug groups (L-bevacizumab, L-ranibizumab, or Mixed), presence or absence of diabetes mellitus, phakia vs pseudophakia, and PED vs no PED. The results indicated that only PED height (OR=.19, 95% CI [0.0–1.1]) and PED volume (OR=1.0, 95% CI [0.99–1.0]) stayed in the model. However, none of the variables were found to be significant predictors for being Responders or Nonresponders.
DISCUSSION
DEMOGRAPHICS
A large number of the study eyes (102 [54%]) contained a PED. Although the precise reasons for more eyes with a PED than eyes without a PED in this study are unknown, this finding is consistent with prior clinical and natural history studies showing that among eyes with nAMD, type 1 CNV (vascularized PED) predominates over type 2 CNV in frequency.106–109 Furthermore, since eyes with vascularized PED are known to be difficult to treat, it is not surprising that many of these eyes became refractory to treatment with bevacizumab or ranibizumab before transitioning to aflibercept in our study.
SIMPLIFIED METHOD FOR OCT MEASUREMENTS
The Simplified Method as first described by Heussen and associates104 was utilized for tracking the SD-OCT measurements of all study eyes throughout this study. For the sake of consistency and reducing confounding factors in measurements, only eyes measured with the Cirrus OCT machines in both clinics were included in the study. A prior study has validated the Simplified Method for providing reliable and reproducible grading of OCT volumetrics for cross-sectional as well as longitudinal OCT image data in a rapid manner.104 The major advantage of the Simplified Method is that it provides sufficiently accurate and consistent data to be utilized in the setting of a reading center for conducting measurements of OCT volumetrics in clinical trials, and yet it also yields rapid but reliable quantitative results for busy clinicians in tracking serial OCT images for patient management in a clinical setting. By reducing the image grading to only three key components (A-scan count, B-scan count, and maximum lesion height), sufficiently high sensitivity and specificity as well as correlation coefficients are retained with this method when compared with the “gold standard” of manual volumetric measurements.
The sensitivity and specificity analysis has shown favorable results associated with this method of volume estimation (sensitivity/specificity for changes in CME volume, 100%/100%; SRF volume, 91.7% to 100%/66.7 to 85.7%; PED volume, 66.7 to 100%/31.3 to 56.3%).104 Pearson correlation analysis comparing the Simplified Method to the “gold standard” of manual volumetric measurement for longitudinal OCT grading has also shown sufficiently high correlation, being more favorable for SRF volumetrics (R2, 0.88 to 0.99) and CME volumetrics (R2, 0.95 to 0.99) than PED volumetrics (R2, 0.39 to 0.53). The basic premise of the Simplified Method is that fluid-based components have similar morphologies among patients (eg, CME is spherical, and SRF and PEDs are dome-shaped), so that a single dimension of these lesions can be utilized to predict the lesion volume with a high degree of accuracy. Thus, the basically round, oval, regular, and relatively symmetrical contour of CME and SRF lesions is expected to result in higher accuracy, whereas the frequently irregular contour of a vascularized PED may result in lower accuracy. These reasons explain the higher sensitivity, specificity, and correlation coefficients of CME and SRF than the same associated with PED utilizing this method when compared to the “gold standard.” It should be pointed out that the Simplified Method provides a volume estimation that correlates with the actual volume, and the units of this measure are not actual volume units.
In our study, the Simplified Method has allowed consistent tracking of SRF, CME, and PED heights and volumes before and after transition from bevacizumab or ranibizumab to aflibercept. A number of steps were also taken in our study to enhance the consistency and reliability of the quantitative results. For instance, only eyes with SD-OCT measurements performed by the Cirrus-HD OCT machine (Carl Zeiss Meditec) were included in this study. There are two advantages in limiting the measurements to the Cirrus-HD OCT unit. First, the limitation of the OCT instrument associated with a single manufacturer reduces intermachine variability in lesion measurements. Second, although B-scan count and maximum lesion height are measures that are easily obtained with software provided by most OCT manufacturers, A-scan count is much more difficult to acquire. The Cirrus HD-OCT unit is the only OCT instrument that provides a natively supported A-scan display of SD-OCT volume data. For all the other commercially available OCT units, an estimation of the A-scan count is required. By avoiding the utilization of other SD-OCT units for data acquisition for the Simplified Method, shortcomings and potential errors associated with estimating the A-scan counts are circumvented in this study. Finally, all of the OCT measurements were performed by a single investigator (A.J.) in precisely the same manner and in a masked fashion, which further enhanced the integrity and accuracy of the quantitative data in this study.
Superiority of Simplified Method Over Automated OCT Measurements
It is noteworthy to point out the consistent lack of significance in the measure of Macular Volume compared with the consistent presence of significance in SRF, CME, and PED volumes for the overall cohort of patients as well as most of the subgroup analyses in this study. The reason for this contrast is likely related to the technical flaws associated with the automated measurements by the SDOCT instrument for Macular Volume (eg, segmentation errors and other inaccuracies) and the more precise measurements of SRF, CME, and PED OCT volumetrics with the Simplified Method. Another plausible explanation for the frequent lack of significant changes corresponding to the Macular Volume reading is the relatively small area of subfoveal pathological changes surrounded by a much larger area of unaffected macula for many of the study eyes.
FREQUENCY OF ANTI-VEGF DRUG INJECTIONS
Regarding the issue concerning the frequency of anti-VEGF injections for the treatment of eyes with nAMD, there is currently a high level of interest in the ophthalmic community in comparing the numbers of intravitreal injections for bevacizumab and ranibizumab with the number of intravitreal injections for aflibercept within a defined period, due to the potential impact of treatment frequency on the treatment and cost burdens for the patients and the treating clinicians. Preclinical animal studies have shown the intravitreal half-life of aflibercept (molecular weight, 115 kDa) in rabbits to be 4.7 days (Furfine E et al, IOVS 2006;47:ARVO Abstract 1430), which is longer than the half-life of 2.88 days for ranibizumab, but similar to the half-life of bevacizumab (4.32 days).24,110–116 Based on mathematical modeling and preclinical studies, Stewart and colleagues24,83,116 estimated similar findings in the half-lives of ranibizumab, bevacizumab, and aflibercept listed above: 3.2 days, 5.6 days, and 4.8 days, respectively, assuming the molar binding activities of ranibizumab, bevacizumab, and aflibercept to VEGF to be 1.0, 0.05 to 0.2, and 140, respectively. Although there has not been a conclusive study of the half-life of aflibercept in human eyes, the theoretical half-life of aflibercept in the human eye has been estimated to be 7.1 days, considering its molecular size in comparison to the bevacizumab macromolecule associated with a half-life of 8.25 days in the human eye.116 Thus, the preclinical studies suggest the potential for a longer duration of aflibercept in the human eye in comparison to ranibizumab before its clearance once administered.
Based on the data derived from the preclinical studies and the subsequent pivotal phase 3 VIEW 1 and 2 trials,91 the manufacturer’s package insert for aflibercept approved by the FDA recommends the administration of three monthly doses of intravitreal aflibercept injections, followed by subsequent injections every 8 weeks. VIEW 1 and VIEW 2 showed the noninferiority of bimonthly and monthly aflibercept - in comparison to monthly ranibizumab in preventing vision loss (less than 15-letter loss) and also vision gains and safety.91 If this recommendation were to be adopted for all eyes with nAMD treated with aflibercept, the frequency of injections would be reduced by approximately 50% over the long term in comparison to the monthly injections for ranibizumab as recommended by its manufacturer’s package insert. However, not all eyes receiving ranibizumab require monthly injections indefinitely, and not all eyes receiving aflibercept on a bimonthly basis maintain vision and are free of recurrent submacular leakage associated with nAMD in a clinical setting. The PIER Study, a randomized, double-masked, sham-controlled trial, and the HORIZON study, a long-term safety and efficacy study of multiple intravitreal ranibizumab injections administered at the investigator’s discretion in patients with nAMD enrolled in earlier trials, showed that a dosing regimen less frequent than monthly injections of ranibizumab may result in worse vision outcome.80,117 However, clinicians utilizing the treat-and-extend strategy do encounter certain patients with nAMD who require less than the monthly dosing of ranibizumab without vision loss once vision stability is reached and the macula is devoid of hemorrhage and fluid on subsequent OCT imaging.118,119 In addition, the inclusion of only treatment-naïve patients in VIEW 1 and VIEW 2 raises the question of whether the noninferiority of efficacy associated with the bimonthly injections of aflibercept in comparison to monthly ranibizumab is applicable to nonnaïve patients.91 It is possible that patients who have received prior anti-VEGF treatment might require a more robust regimen of injections for aflibercept after transitioning from other anti-VEGF medications. Other than anecdotal experience, there is a lack of publication of clinical data in the literature relating to a rigorous assessment of the dosing requirements of aflibercept in comparison to bevacizumab and ranibizumab for a large cohort of patients with heterogeneous characteristics of nAMD and variable experiences and responses associated with their treatment.
In a cohort consisting of 189 eyes, our study shows an average of 6.5 injections for eyes receiving either bevacizumab, ranibizumab, or both in 6 months prior to the transition. Statistical comparison shows the highest mean number of injections to be 8.6 for eyes in the L-ranibizumab group, followed by 4.9 injections for eyes in the Mixed Group, and 4.8 for eyes in the L-bevacizumab group (P<.001). The overall mean injection was reduced to 5.4 during the 6 months after transition, showing no differences in the three drug groups. The pretransition and posttransition treatment strategy at both centers was similar to the PrONTO algorithm.102,103 After three monthly loading doses of intravitreal injections of the same anti-VEGF drug, monthly follow-up was carried out and additional injections of the same drug were performed, if indicated, based on OCT and visual findings. If necessary, more frequent than monthly follow-up was performed, depending on the findings. Any persistent or recurrent subretinal/sub-RPE or intraretinal fluid, hemorrhage, or exudates seen on OCT or biomicroscopy deemed to be related to nAMD would trigger another injection of the same medication. A decrease in vision (2 or more lines on the Snellen chart) deemed to be related to nAMD would also trigger another injection. The higher-than-monthly injection frequency for the entire cohort (average of 6.5) and also for the L-ranibizumab subgroup (8.6) reflects the relatively resistant nature of the majority of the eyes in response to their specific anti-VEGF drugs before transition. This finding is consistent with the subgroup analysis showing the majority of the pretreatment eyes (155 [82.0%]) to be Nonresponders (defined as eyes with suboptimal response to the specific anti-VEGF drug) at the time of the transition. Since the mean follow-up time before transition was 12 months, it means that there was sufficient time for the pharmacokinetics and pharmacodynamics to take their courses and the plateau of the drug effects to be established prior to transitioning to aflibercept. In this study, 84 (44.4%) of the study eyes were in the L-ranibizumab group. The higher frequency of injections for L-ranibizumab in comparison to L-bevacizumab and the Mixed Groups is consistent with the shorter half-life of ranibizumab in comparison to bevacizumab shown by prior pharmacokinetic studies. In fact, the mean number of injections for the L-ranibizumab group of 8.6 correlates to an average of 3.0 weeks per injection, a frequency that is higher than the usually recommended monthly injection frequency for ranibizumab. This higher frequency suggests the possible development of a partial resistance of this subgroup of eyes to ranibizumab at the time of the transition.
In a prior publication, Stewart and colleagues116 have shown the theoretical advantage of more frequent dosing of an anti-VEGF drug in managing eyes with nAMD refractory to monthly anti-VEGF injections. They pointed out the greater importance of the trough drug binding level in comparison to the peak binding level. They reported that the key to the success of the increased dosing regimen in overcoming the poor responses to prior anti-VEGF injections on a monthly or longer frequency is the substantially enhanced trough binding level when an anti-VEGF drug is dosed every 2 weeks, since it is not the peak level but rather the amount of residual drug or its trough level that will dictate its efficacy.116 They showed that the 28-day trough binding activity for ranibizumab administered at every 14 days was 21.75 times higher than the same dose given at every 28 days. Regarding bevacizumab, the frequency of 4.8 injections per 6 months for the L-bevacizumab group also points to an increased mean frequency of one injection per 5.4 weeks, in comparison to the often cited frequency of 6 weeks for bevacizumab injections. One previous study showed the therapeutic benefit of bevacizumab of up to 8 weeks for some patients with nAMD in a clinical setting.120
However, the data of our study need to be interpreted with caution, given the inherent limitations associated with a retrospective study. Unknown confounding factors beyond pharmacokinetics and pharmacodynamics could have influenced the frequencies of the drug doses, eg, differences in the patterns of clinicians’ and patients’ personal preferences in drug choices and frequencies of injections, visit frequencies, and insurance issues. In addition, the mean frequency of 5.3 weeks per injection for bevacizumab is still within the commonly accepted range of the 4- to 6- week interval for bevacizumab injections in patients with nAMD in many clinical practices and clinical trials. It should also be pointed out that although predominated by the effects of one drug, many of the Legacy-anti-VEGF eyes in this study frequently had received more than a single anti-VEGF drug during the pretransitioned period, which adds complexities to the interpretation of the injection data. Despite such limitations, there is ample evidence of increased resistance to anti-VEGF therapy associated with the majority of eyes in this study before and after transition to aflibercept. It is interesting to note that after factoring out the three monthly loading doses of aflibercept, there was still an increased frequency above the frequency of bimonthly injections recommended by the package insert for aflibercept following transition. In contrast to pretransition, however, there were no longer any differences in injection frequencies when comparing the three Legacy anti-VEGF groups after transition. These data underscore the disparities in injection frequencies compared to prior clinical trials, likely related to differences in the characteristics of the patient populations in a clinical setting in comparison to patients enrolled in randomized clinical trials, such as the CATT and VIEW 1 and VIEW 2 trials, in which only treatment-naïve patients were included.78,79,91 Such differences were particularly highlighted by the cohort of Nonresponders in this study, which demonstrated resistance to the specific pretransition anti-VEGF drugs. The lower mean posttransition frequency of 5.4 (5.5 for Nonresponders) in comparison to pretransition mean frequency of 6.5 for drug injection provides evidence of longer clinical benefits of aflibercept compared with ranibizumab and bevacizumab, in a cohort of patients demonstrating resistance to anti-VEGF therapy. These results are consistent with the substantially higher peak and trough binding activities of aflibercept in comparison to bevacizumab and ranibizumab predicted by mathematical modeling as well as recent clinical studies of eyes transitioned from bevacizumab or ranibizumab to aflibercept.24,116 Thus, besides a drug’s half-life, the interaction of multiple complex factors associated with its pharmacokinetics and pharmacodynamics may play a role in determining its therapeutic effectiveness and duration, eg, VEGF-binding affinity, molecular size and characteristics, injected dose, lesion type, other growth factors and cytokines, host’s metabolism, and also tachyphylaxis.
COMPARISONS WITH RECENT PUBLICATIONS
There have been a few recent reports in the literature on eyes transitioning to aflibercept after developing refractory responses to bevacizumab, ranibizumab, or both.121–125 Ho and colleagues121 reported short-term outcomes (4 months after first dose) in 96 eyes (85 patients) that were transitioned to aflibercept after demonstrating persistent or recurrent fluid, exudates, or hemorrhage despite prior treatment with bevacizumab, ranibizumab, or both. The investigators reported that 7 (7.0%) of their study eyes developed visual improvement, 82 (85.0%) of their study eyes developed no changes in vision, and 7 (7.0%) of their study eyes developed vision loss after transitioning to aflibercept. In our study, we defined changes in 0.1-log units or more in visual acuity (equivalent to 5 or more letter changes) as increase or decrease in vision; otherwise the vision was considered unchanged.
For the 5-letter change threshold, BSCVA was improved in 63 eyes (33.3)% and PHVA was improved in 65 eyes (34.4%). BSCVA was unchanged in 106 eyes (56.1%) and PHVA was unchanged in 108 eyes (57.1%). BSCVA was worse in 20 eyes (10.6%) and PHVA was worse in 16 eyes (8.5%) in our study (Table 12). The differences in visual outcome between our study and the study by Ho and colleagues are likely due to multiple factors. For instance, there may be differences in the definition of vision changes in the two studies. In addition, disparities in the baseline characteristics of the study groups in the two studies cannot be ruled out.
In a recent study, Bakall and coworkers122 reported the results of 36 eyes of 31patients that converted from either bevacizumab or ranibizumab injections to aflibercept due to recurrent or persistent SRF. They indicated that after receiving three aflibercept injections, 18 (50.0%) of the transitioned eyes showed reduced intraretinal or SRF, 15 (41.7%) of the transitioned eyes showed stable fluid level, and 3 (8.3%) of the transitioned eyes showed worsening of the fluid level. In contrast, our study showed that 75 eyes (84.3%) and 68 eyes (95.8%) of the transitioned eyes developed reduced SRF and CME volumes, respectively; 1 eye (1.1 %) and 1 eye (1.4%) of the transitioned eyes developed unchanged SRF and CME volumes, respectively; and 13 eyes (14.6%) and 2 eyes (2.8%) of the transitioned eyes developed worsening of SRF and CME volumes in 6 months. In our study, we defined only an increase or decrease in more than 10% in the amount of SRF or CME height or volume to constitute real changes in the fluid level. As mentioned in the “Results” section, those eyes with a data entry of zero at baseline and also subsequent visits were excluded from the calculations for changes in SRF and CME heights and volumes, in order to avoid confounding the results (Tables 8 through 10).
MAGNITUDE OF THERAPEUTIC EFFECTS OF POSTTRANSITION INJECTIONS
Regarding the magnitude of therapeutic effects for aflibercept at 6 months after transition, this study provides ample evidence of substantial clinical benefits. The most substantial results were the decrease in SRF heights and volumes (69%, 62% for entire cohort; 75%, 57% for L-bevacizumab; 60%, 67% for L-ranibizumab, respectively) and reduction in CME heights and volumes (71%, 66% for entire cohort; 76%, 88% for L-bevacizumab; 57%, 28% for L-ranibizumab, respectively). The reductions for PED heights and volumes for the entire cohort, as well as the individual drug groups, were also significant but less in magnitude (14%, 32% for entire cohort; 12%, 30% for L-bevacizumab; 12%, 33% for L-ranibizumab, respectively). The reason for the lower magnitude of reductions of the PED is likely the deeper location of the RPE layer in relation to the retina, so that a smaller amount of the anti-VEGF drug likely reached the receptors associated with the RPE layer in comparison to the amount of drug attached to the more superficially located intraretinal and subretinal receptors after each drug administration. The RPE barrier also likely limited the quantity of aflibercept molecules that migrated to the sub-RPE space to resolve the vascular lesions under the PED. This finding of relative difficulty in reducing the PED and the relative ease in resolving the SRF and CME associated with anti-VEGF treatments for eyes with nAMD is consistent with previous publications as well as the clinical experience.106–109 Nevertheless, 12% to 33% reductions in the PED heights and volumes associated with aflibercept treatment are still remarkable, particularly given the history of suboptimal response to other anti-VEGF drugs at the time of the transition, and are consistent with recent anecdotal clinical reports regarding aflibercept on treatment of vPED.98
In this study, statistically significant improvements were noted when comparing the posttransitioned BSCVA and PHVA with their corresponding pretransitioned values for the entire cohort as well as the subgroups, with the exception of the Mixed Group. It is interesting to note that although only a mild to moderate amount of visual acuity improvements were noted for the entire cohort (mean of 4.0 letters gain) and for the subgroups (mean ranging from 3.0 to 4.9 letters gain), statistical significance was reached (P≤.001) due to their sufficiently large sample sizes. In contrast, despite a seemingly large mean gain of 13.5 letters for BSCVA and 8.5 letters for PHVA for the Mixed Group, the results were considered not significant (P=.03 and P=.02, respectively) due to the Bonferroni adjustments and also the small sample size (10 eyes[5.3%]). Had there been a much larger sample size for the Mixed Group, there is a reasonable chance that statistical significance would have been found for the corresponding visual changes.
COMPARISONS OF DRUG GROUPS
An important issue to address in this study is the question of any therapeutic differences of aflibercept for eyes with nAMD that received prior treatment with different anti-VEGF drugs. The lack of differences in responses of the L-bevacizumab and L-ranibizumab groups after transition to aflibercept is clearly shown in this study (Table 3 and Figure 3). Thus, the comparisons of these two drug groups showed consistently similar effects of aflibercept in reducing SRF, CME, and PED metrics and also visual recovery in eyes refractory to prior bevacizumab, ranibizumab, or both. It is noteworthy that the levels of statistical significance of the multiple variables listed in the Tables were frequently more robust in comparison to the levels of significance associated with the Figures. The disparities in the degree of statistical significance between the Tables and the Figures are due to the differences in sample sizes relating to the statistical tests, namely, the use of 2-way ANOVA for the Tables and the use of other statistical tests for the Figures (ie, paired t test, Wilcoxon signed-rank test, Mann-Whitney test). Due to the lack of interaction between groups and the lack of inequalities in variance, 2-way ANOVA was applicable for most of the statistical comparisons in the Tables, whereas other statistical tests were more appropriate for the Figures. The 2-way ANOVA allows a more robust sample size by combining the sample sizes of the comparison groups, whereas the other statistical tests limit the sample sizes to the individual study groups. Thus, the statistical comparisons generated by 2-way ANOVA yielded significant P values more frequently or P values with higher degree of significance when compared to the other statistical tests. There are legitimate statistical reasons associated with the choice of the specific statistical tests for all of the situations in this study.
RESPONDERS VS NONRESPONDERS
Most patients who were responding well to ranibizumab or bevacizumab for treatment of their nAMD were accustomed to continuing the same anti-VEGF drug that was working well for them, and they had no compelling reasons to switch to another drug. On the other hand, those patients who developed a suboptimal response to ranibizumab or bevacizumab were motivated to switch to a new drug that might work better for them. This was likely the reason why Nonresponders predominated over Responders in number in our study cohort. Regarding the substantial differences in responses to aflibercept for Responders vs Nonresponders after transitioning, the results are not surprising. This study shows consistent reductions in SRF, CME, and PED metrics for Nonresponders in response to aflibercept after transitioning, whereas there was a lack of reductions in the same for the Responders. There is a logical explanation for the differential responses to aflibercept after transition associated with these two groups of eyes. The primary reason is likely the ceiling effect associated with the Responders, since little or no reductions in OCT metrics would be expected irrespective of the effectiveness of aflibercept due to the resolution of most of the SRF, CME, and PED prior to transition for the Responders given their continued favorable responses to the respective Legacy-anti-VEGF drugs prior to transition. Despite the lack of further improvements of most of the OCT-measured variables, there was maintenance of the anatomical outcome and visual results after transition for the Responders.
DIABETIC VS NONDIABETIC, PHAKIA VS PSEUDOPHAKIA, AND PED VS NO PED
Subgroup analyses showed similar responses after transitioning to aflibercept when comparing diabetic with nondiabetic eyes, phakic with pseudophakic eyes, and eyes with a PED vs eyes without a PED. Regarding the diabetic eyes included in the study, it should be pointed out that only diabetic patients with no diabetic retinopathy or minimal background diabetic retinopathy were included in the study. Any diabetic patients with serious preproliferative diabetic retinopathy (eg, substantial diabetic maculopathy) or with proliferative diabetic retinopathy were excluded from the study, in order to avoid confounding the results pertaining to the responses associated with the treatment of the nAMD in this study. However, diabetic eyes without or with minimal retinopathy might still be expected to be more prone to vascular leakage due to potentially higher intraocular VEGF level in comparison to nondiabetic eyes.126–129 Therefore, diabetic eyes in the study might be expected to have less favorable responses after transitioning to aflibercept in comparison to nondiabetic eyes. However, this study shows the lack of differences when comparing diabetic patients without diabetic retinopathy or with minimal diabetic retinopathy with patients without diabetes mellitus for all of the OCT-measured variables and vision outcome (ie, SRF, CME, and PED heights and volumes, as well as BSCVA and PHVA [Figure 5]). This means that the anti-VEGF capacity of aflibercept after transitioning is sufficiently robust to suppress any extra VEGF activities associated with diabetes mellitus in addition to the VEGF effects associated with the nAMD in these diabetic patients. The overabundance of anti-VEGF effects associated with an intravitreal dose of aflibercept in inhibiting any extra VEGF activities associated with a diabetic eye may be related to the markedly higher binding activity of aflibercept for VEGF in comparison to bevacizumab and ranibizumab (140 times greater than ranibizumab, and 700 to 2,800 times greater than bevacizumab). Thus, substantially higher relative peak and trough binding activities of aflibercept to VEGF in comparison to bevacizumab and ranibizumab may account for its uniform effectiveness irrespective of the presence or absence of diabetes mellitus.
For instance, Stewart and associates116 have shown through mathematical modeling in a prior study that 2.0 mg of aflibercept administered at every 28 days provides an 838-fold to 3,354-fold higher trough binding activity and 1,486-fold to 5,946-fold higher peak binding activity for VEGF when compared with 1.25 mg of bevacizumab dosed at every 28 days; similarly, 2.0 mg of aflibercept administered at every 28 days results in a 1,829-fold higher trough binding activity and 248-fold higher peak binding activity for VEGF when compared with 0.5 mg of ranibizumab dosed at every 28 days; in addition, 2.0 mg of aflibercept given at every 28 days yields 84-fold higher trough binding activity and 236-fold higher peak binding activity for VEGF when compared with 0.5 mg of ranibizumab dosed at every 14 days. Thus, the high-VEGF binding activity of aflibercept is apparently sufficient to compensate for its expected shorter half-life due to its more rapid clearance in a pseudophakic eye, so that there was a lack of differences in its therapeutic effects when comparing the phakic with pseudophakic eyes for all OCT and vision variables during the posttransition period (ie, SRF, CME, and PED heights and volumes, and BSCVA and PHVA) in our study.
Regarding the assessment of eyes with a PED vs eyes without a PED, aflibercept was effective in improving the majority of the OCT and vision variables for both groups of eyes, although the magnitudes of improvement were somewhat more for the former than the latter eyes. A plausible explanation for the relatively more robust responses of the eyes with a PED in comparison to eyes without a PED to aflibercept after transition is the relative ceiling effect involving certain OCT variables in the eyes without a PED, since there was a substantially smaller amount of SRF height and volume for eyes without a PED in comparison to eyes with a PED (Table 7) at the time of transition given the more resistant nature of the eyes with a PED to treatment.
CORRELATION OF OCT-MEASURED VARIABLES WITH VISION OUTCOME
In this study, the decrease in the magnitudes of the OCT variables including SRF, CME, and PED heights and volumes, as well as the central 1- and 3-mm subfield thicknesses, corresponded with the increase in vision outcome, ie, BSCVA and PHVA, for the entire cohort as well as subgroup analyses (Tables 2 through 7). Such an inverse relationship between OCT results and vision outcome is logical, since the greater the reduction in the intraretinal, subretinal, and sub-RPE fluid, the greater visual improvement would be expected over the course of treatment. However, Pearson correlation analysis shows highly variable correlation coefficients between the OCT-measured variables and visual acuities (ranging from −.002 to .18) (Table 13). One plausible explanation for the inconsistent correlation coefficients is the moderate level of mean visual acuity at baseline for the entire cohort as well as the individual subgroups (ranging from 20/40 to 20/60). The relatively sound baseline vision likely imposed a ceiling effect on visual changes, since only a limited amount of visual improvement was possible irrespective of the extent of the reductions associated with the OCT-measured variables. Furthermore, the variable correlation between the anatomical and visual outcome in our study is consistent with the reports of variable correlation coefficients between OCT-measured center-point thickness and vision results in multiple previous studies.130–143 For instance, the Diabetic Retinopathy Clinical Research Network (DRCR.net) reported a modest correlation coefficient of −0.38 between OCT-measured center-point thickness and visual acuity at 12 months after focal laser treatment for diabetic macular edema in 251 eyes.139 The Standard Care Versus Corticosteroid Treatment for Retinal Vein Occlusion (SCORE) study group reported a low correlation coefficient between center-point thickness and visual acuity of −0.27 for eyes with a central retinal vein occlusion and −0.28 for eyes with a branch retinal vein occlusion.140–143 Our study shows the variable correlation coefficients between OCT results vs vision outcome for not only the OCT-thickness measurements, but also OCT-measured volumetrics (r = −.002 to .18). The variable correlation of OCT-measured results and vision outcome underscores the point that although OCT-measured thickness and volume values constitute an important tool for gauging clinical progress with treatment in clinical trials as well as in a clinical setting, they cannot substitute as surrogates for visual acuity measurements throughout the course of treatment. Other factors besides OCT measurements may influence the visual results at any time point.
ADVERSE EVENTS
Multiple laboratory studies have demonstrated the safety profile of aflibercept. Ammar and coworkers144 performed testing of various concentrations of aflibercept on the viability and metabolism of ocular cells in an in vitro cell assay. They found that the concentrations of aflibercept in the range expected to occur in the human vitreous after an intraocular injection did not induce any harm. Schnichels and colleagues145 also performed comparative toxicity and proliferation testing of aflibercept, bevacizumab, and ranibizumab on different ocular cells. Their conclusion was that aflibercept did not induce any toxic effects on retinal cell lines. Multiple clinical studies conducted by Regeneron Pharmaceuticals (eg, Clear-IT, VIEW 1, VIEW 2) also showed the high safety profile of aflibercept.91,146–148 The VIEW 1 and VIEW 2 clinical trials reported a mean maximum concentration of 0.02 µg/mL (range, 0–0.054 µg/mL) attained in 1 to 3 days after a single intravitreal administration of 2.0 mg of aflibercept.91 The free aflibercept plasma concentrations were undetectable at 2 weeks after dosing in all tested patients. However, Avery and coworkers149 recently reported marked reduced plasma-free VEGF levels for patients treated with intravitreal bevacizumab or aflibercept in contrast to ranibizumab. In VIEW 1 and VIEW 2, eyes treated with 0.5 or 2.0 mg aflibercept had similar low rates of ocular and systemic adverse events in comparison to eyes treated with 0.5 mg ranibizumab.91 There was a lack of dose-related correlation to the adverse events in these trials.
Regarding RPE tears, the natural history of eyes with nAMD includes its development on a spontaneous basis, although multiple reports have documented such an ocular complication in eyes receiving treatment with PDT, intravitreal corticosteroid injections, and intravitreal injections of pegaptanib, bevacizumab, ranibizumab, as well as aflibercept.150–183 The product insert of aflibercept lists the rate of 2.0% for an RPE tear. In our study, a single RPE tear constituted the only adverse event occurring in one of the 189 eyes evaluated closely over 6 months. Recently, there were anecdotal reports of noninfectious uveitis on clusters of eyes treated with aflibercept (Roberts WG, written communication to the FDA from Regeneron Pharmaceuticals, Inc, regarding Post Marketing Reports on postinjection intraocular inflammation, Feb 13, 2012). No such cases of uveitis were observed in any of the eyes in our study. Recent studies, including the year-2 results of CATT, also suggested a potential link between anti-VEGF therapy and the expansion of geographic atrophy for eyes with AMD over the long term.79,184,185 In case of such a complication, its occurrence would be expected over the long term and therefore beyond the time period and scope of this study.
Regarding systemic adverse events, multiple studies have pointed out the risk of arterial thromboembolic and hemorrhagic events as set forth by the Anti-Platelet Trialists’ Collaboration (APTC), including myocardial infarction, bleeding, stroke, and mortality associated with intravitreal anti-VEGF therapy.186–191 However, Curtis and coworkers187 reported that bevacizumab and ranibizumab use was not associated with increased risk of mortality, myocardial infarction, bleeding, or stroke compared with PDT or pegaptanib use in a large retrospective cohort study involving 146,942 Medicare beneficiaries aged 65 years or older with a claim for AMD. In an extensive systemic search of the literature, Van Der Reis and colleagues188 also found a low rate of serious adverse events and the lack of differences in rates of serious adverse events among eyes receiving pegaptanib, bevacizumab, and ranibizumab. Tolentino189 reported no adverse systemic complications for the use of pegaptanib and a low risk of nonocular hemorrhage and stroke associated with ranibizumab. Semeraro and coworkers190 warned against the excessive use of anti-VEGF treatments due to their potential interference with normal vascular physiology. The CATT study reported no differences in ocular and APTC-related thromboembolic events between intravitreal bevacizumab and ranibizumab.78,79 However, serious adverse events related to hospitalization were higher in the bevacizumab group in CATT, the meaning of which is unclear. The CATT study was not designed to provide definite answers to the question of any differences in adverse events among the treatment groups. In our study, there was absence of any systemic complications throughout the entire study period.
Since all eyes were monitored closely with biomicroscopy, indirect ophthalmoscopy, and OCT essentially on a monthly basis, the failure to detect an ocular adverse occurrence in this study is unlikely. Despite the use of only SD-OCT imaging (except for certain eyes that also underwent fundus photography and fluorescein angiography at baseline), the careful clinical examination as well as meticulous inspection of multiple A- and B-scans and axial views associated with the Simplified Method in the process of obtaining the OCT measurements likely prevented the failure to spot an adverse ocular event. Theoretically, there is a possibility of the investigators missing a small ocular lesion outside of the central macular region, eg, a small subclinical RPE tear. However, any such lesions would be expected to be of minimal or no clinical relevance. Regarding the tracking of the systemic complications, the investigators kept in close contact with all patients enrolled in the study, their family members, and their general physicians to ensure appropriate capture of all pertinent systemic events.
STRENGTHS AND LIMITATIONS
The strengths of this study include a large cohort of patients monitored closely in a uniform manner in both study centers with ocular examination and high-quality SD-OCT imaging, assessment of multiple OCT-measured variables including not only lesion heights but also lesion volumetrics not available in most other similar studies, and careful and rigorous statistical analyses including extensive subgroup comparisons and plots while incorporating proper sample size consideration and Bonferroni adjustment for multiple statistical comparisons in the study. In addition, reliable and detailed records were available for all patients receiving anti-VEGF treatments in both study centers, allowing accurate data collection for all study patients during the study period. This study also provides pertinent results involving a large cohort of patients transitioning from bevacizumab or ranibizumab to aflibercept in a “real-world” clinical situation instead of the more controlled and restricted conditions of a clinical trial. Finally, a single investigator masked to the identities and pretransitioned and posttransitioned status of the study eyes performed all OCT measurements in the same fashion with a reliable method and on a consistent basis for all of the study eyes. The limitations of this study include shortcomings inherent in a retrospective study, the nonstandardized measurements of the visual acuity, and the lack of data beyond 6 months. However, the goal of this study from the outset was to assess the 6-month results of eyes with nAMD transitioned to aflibercept instead of the long-term findings beyond 6 months, which are beyond the scope of this study. The authors of this study wanted to know how eyes with suboptimal response as well as eyes with good response to bevacizumab or ranibizumab would fare after transitioning to aflibercept in terms of anatomic and visual outcomes. Could aflibercept resolve the recalcitrant leakage or preserve macular dryness while maintaining or improving vision for these eyes without substantial adverse effects over the short and intermediate terms? This study provides clear answers to these questions.
CONCLUSION
To our knowledge, this is currently one of the largest studies on transition from bevacizumab or ranibizumab to aflibercept for nAMD. The results of our study indicate the rejection of the null hypothesis for the Nonresponders (the majority of eyes in the study) but not for the Responders. Thus, the 6-month posttransitioned OCT metrics and vision results were improved for eyes that were suboptimal in response to bevacizumab, ranibizumab, or both medications but not for eyes with good response to the same at the time of the transition; for the latter eyes, the OCT measures and vision results were maintained at 6 months. When comparing drug groups, there were no differences in posttransitioned OCT measures and vision outcomes for eyes treated with prior bevacizumab, ranibizumab, or mixed drug therapy during the same time period. Posttransitioned adverse events were rare, since only one eye developed a RPE tear and there was a lack of other ocular complications and any systemic problems.
Acknowledgments
Funding/Support: A simple grant was provided by Regeneron Pharmaceuticals, Inc, Tarrytown, New York, without any stipulations or preconditions. The authors have maintained complete and independent control of all aspects of the research throughout the entire process, including the design, collection, analysis, and interpretation of the data, and finally the thesis writing. The authors employed an independent statistical service for the analysis of the data. The primary author (C.K.C.) independently made the decision for submission of the thesis to the American Ophthalmological Society, and Regeneron has played no role in this decision.
Financial Disclosures: Research and grant support: Acucela (C.K.C.), Allergan (S.S.), Carl Zeiss-Meditec (S.S.), Genentech (C.K.C., S.S.), National Eye Institute (C.K.C.), Optos (S.S.), Regeneron (C.K.C., A.J.), Sequenom (C.K.C.); advisory board/consultant: Allergan (A.J., C.K.C., S.S.), Applied Biomedical (A.J.), Carl Zeiss-Meditec (S.S.), Genentech (S.S.), Optos (S.S.), Regeneron (C.K.C., A.J.), ThromboGenics (C.K.C.),Valeant (C.K.C.); speakers bureau: Alcon (A.J.), Regeneron (A.J.).
Author Contributions: Conception and design (C.K.C., A.J.); analysis and interpretation of data (C.K.C., A.J); writing the thesis (C.K.C., A.J.); critical revision of thesis (C.K.C., A.J., S.S., N.V.); data collection (C.K.C., A.J.); final approval of the thesis (C.K.C., A.J., S.S., N.V.); provision of materials, patients, or resources (C.K.C., A.J., S.S.); statistical expertise (C.K.C., A.J.); obtaining funding (C.K.C., A.J.); literature search (C.K.C., A.J.); administrative, technical, or logistical support (C.K.C., A.J, S.S., N.V.).
Other Acknowledgments: Grenith Zimmerman, PhD, and Noha Daher, PhD, of the School of Allied Health Professions, Loma Linda University, Loma Linda, California, performed and reviewed the statistical design and analyses for this study.
REFERENCES
- 1.Friedman DS, O’Colmain BJ, Munoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 2.Cong R, Zhou B, Sun Q, Gu H, Tang N, Wang B. Smoking and the risk of age-related macular degeneration: a meta-analysis. Ann Epidemiol. 2008;18(8):647–656. doi: 10.1016/j.annepidem.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Ferris FL, Fine SL, Hyman I. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol. 1984;102(11):1640–1642. doi: 10.1001/archopht.1984.01040031330019. [DOI] [PubMed] [Google Scholar]
- 4.Klein R, Klein BE, Linton KL, De Mets DL. The Beaver Dam Eye Study: the relation of age-related maculopathy to smoking. Am J Epidemiol. 1993;137(2):190–200. doi: 10.1093/oxfordjournals.aje.a116659. [DOI] [PubMed] [Google Scholar]
- 5.Verner-Cole EA, Davis SJ, Lauer AK. Aflibercept for the treatment of neovascular age-related macular degeneration. Drugs Today (Barc) 2012;48(5):317–329. doi: 10.1358/dot.2012.48.5.1805931. [DOI] [PubMed] [Google Scholar]
- 6.Kwak N, Okamoto N, Wood JM, Campochiaro PA. VEGF is major stimulator of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2000;41(10):3158–3164. [PubMed] [Google Scholar]
- 7.Krzystolik MG, Afshari MA, Adamis AP, et al. Prevention of experimental choroidal neovascularization with intravitreal anti-vascular endothelial growth factor antibody fragment. Arch Ophthalmol. 2002;120(3):338–346. doi: 10.1001/archopht.120.3.338. [DOI] [PubMed] [Google Scholar]
- 8.Fine SL, Hawkins B, Maguire M. Macular photocoagulation study. Arch Ophthalmol. 1984;102(11):1583. doi: 10.1001/archopht.1984.01040031283001. [DOI] [PubMed] [Google Scholar]
- 9.Age-related macular degeneration. The Macular Photocoagulation Study Group. Am J Ophthalmol. 1984;98(3):376–377. doi: 10.1016/0002-9394(84)90332-5. [DOI] [PubMed] [Google Scholar]
- 10.Macular Photocoagulation Study Group. Changing the protocol: a case report from the Macular Photocoagulation Study. Control Clin Trials. 1984;5(3):203–216. doi: 10.1016/0197-2456(84)90024-2. [DOI] [PubMed] [Google Scholar]
- 11.Macular Photocoagulation Study Group. Argon laser photocoagulation for neovascular maculopathy. Three year results from randomized clinical trials. Arch Ophthalmol. 1986;104(5):694–701. [PubMed] [Google Scholar]
- 12.Recurrent choroidal neovascularization after argon laser photocoagulation for neovascular maculopathy. Macular Photocoagulation Study Group. Arch Ophthalmol. 1986;104(4):503–512. doi: 10.1001/archopht.1986.01050160059012. [DOI] [PubMed] [Google Scholar]
- 13.Krypton laser photocoagulation for neovascular lesions of ocular histoplasmosis. Results of a randomized clinical trial. Macular Photocoagulation Study Group. Krypton laser photocoagulation for neovascular lesions of ocular histoplasmosis. Results of a randomized clinical trial. Arch Ophthalmol. 1987;105(11):1499–1507. doi: 10.1001/archopht.1987.01060110045029. [DOI] [PubMed] [Google Scholar]
- 14.Blackhurst DW, Maguire MG. Reproducibility of refraction and visual acuity measurement under a standard protocol. The Macular Photocoagulation Study Group. Retina. 1989;9(3):163–169. [PubMed] [Google Scholar]
- 15.Chamberlin JA, Bressler NM, Bressler SB, et al. The use of fundus photographs and fluorescein angiograms in the identification and treatment of choroidal neovascularization in the Macular Photocoagulation Study. The Macular Photocoagulation Study Group. Ophthalmology. 1989;96(10):1526–1534. doi: 10.1016/s0161-6420(89)32707-2. [DOI] [PubMed] [Google Scholar]
- 16.Persistent and recurrent neovascularization after krypton laser photocoagulation for neovascular lesions of ocular histoplasmosis. Macular Photocoagulation Study Group. Arch Ophthalmol. 1989;107(3):344–352. doi: 10.1001/archopht.1989.01070010354023. [DOI] [PubMed] [Google Scholar]
- 17.Argon laser photocoagulation for neovascular maculopathy Five-year results from randomized clinical trials. Macular Photocoagulation Study Group. Arch Ophthalmol. 1991;109(8):1109–1114. [PubMed] [Google Scholar]
- 18.Krypton laser photocoagulation for neovascular lesions of age-related macular degeneration. Results of a randomized clinical trial. Macular Photocoagulation Study Group. Arch Ophthalmol. 1990;108(6):816–824. doi: 10.1001/archopht.1990.01070080058036. [DOI] [PubMed] [Google Scholar]
- 19.Persistent and recurrent neovascularization after krypton laser photocoagulation for neovascular lesions of age-related macular degeneration. Macular Photocoagulation Study Group. Arch Ophthalmol. 1990;108(6):825–831. doi: 10.1001/archopht.1990.01070080067037. [DOI] [PubMed] [Google Scholar]
- 20.Krypton laser photocoagulation for idiopathic neovascular lesions. Results of a randomized clinical trial. Macular Photocoagulation Study Group. Arch Ophthalmol. 1990;108(6):832–837. [PubMed] [Google Scholar]
- 21.Bressler SB, Maguire MG, Bressler NM, Fine SL. Relationship of drusen and abnormalities of the retinal pigment epithelium to the prognosis of neovascular macular degeneration. The Macular Photocoagulation Study Group. Arch Ophthalmol. 1990;108(10):1442–1447. doi: 10.1001/archopht.1990.01070120090035. [DOI] [PubMed] [Google Scholar]
- 22.Laser photocoagulation of subfoveal neovascular lesions of age-related macular degeneration. Updated findings from two clinical trials. Macular Photocoagulation Study Group. Arch Ophthalmol. 1993;111(9):1200–1209. doi: 10.1001/archopht.1993.01090090052019. [DOI] [PubMed] [Google Scholar]
- 23.Laser photocoagulation for juxtafoveal choroidal neovascularization. Five-year results from randomized clinical trials. Macular Photocoagulation Study Group. Arch Ophthalmol. 1994;112(4):500–509. [PubMed] [Google Scholar]
- 24.Stewart M. Aflibercept (VEGF Trap-Eye) for the treatment of exudative age-related macular degeneration. Expert Rev Clin Pharmacol. 2013;6(2):103–113. doi: 10.1586/ecp.12.81. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt-Erfurth U. [Photodynamic therapy-a conservative alternative in treatment of exudative macular degeneration] Klin Monatsbl Augenheilkd. 1998;213(6):A11–15. [PubMed] [Google Scholar]
- 26.Schmidt-Erfurth U. [Photodynamic therapy. Minimally invasive treatment of choroidal neovascularization] Ophthalmologe. 1998;95(10):725–731. doi: 10.1007/s003470050343. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt-Erfurth U, Miller J, Sickenberg M, et al. Photodynamic therapy of subfoveal choroidal neovascularization; clinical and angiographic examples. Graefes Arch Clin Exp Ophthalmol. 1998;236(5):365–374. doi: 10.1007/s004170050092. [DOI] [PubMed] [Google Scholar]
- 28.Fine SL. Photodynamic therapy with verteporfin is effective for selected patients with neovascular age-related macular degeneration. Arch Ophthalmol. 1999;117(10):1400–1402. doi: 10.1001/archopht.117.10.1400. [DOI] [PubMed] [Google Scholar]
- 29.Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials—TAP report. Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Arch Ophthalmol. 1999;117(10):1329–1345. [PubMed] [Google Scholar]
- 30.Miller JW, Schmidt-Erfurth U, Sickenberg M, et al. Photodynamic therapy with verteporfin for choroidal neovascularization caused by age-related macular degeneration: results of retreatments in a phase 1 and 2 study. Arch Ophthalmol. 1999;117(9):1177–1187. doi: 10.1001/archopht.117.9.1177. [DOI] [PubMed] [Google Scholar]
- 31.Miller JW, Schmidt-Erfurth U, Sickenberg M, et al. Photodynamic therapy with verteporfin for choroidal neovascularization caused by age-related macular degeneration: results of a single treatment in a phase 1 and 2 study. Arch Ophthalmol. 2000;118(4):488. doi: 10.1001/archopht.117.9.1161. [DOI] [PubMed] [Google Scholar]
- 32.Sickenberg M, Schmidt-Erfurth U, Miller JW, et al. A preliminary study of photodynamic therapy using verteporfin for choroidal neovascularization in pathologic myopia, ocular histoplasmosis syndrome, angioid streaks, and idiopathic causes. Arch Ophthalmol. 2000;118(2):327–336. doi: 10.1001/archopht.118.3.327. [DOI] [PubMed] [Google Scholar]
- 33.Bressler NM, Bressler SB. Photodynamic therapy with verteporfin (Visudyne): impact on ophthalmology and visual sciences. Invest Ophthalmol Vis Sci. 2000;41(3):624–628. [PubMed] [Google Scholar]
- 34.Schmidt-Erfurth U, Hasan T. Mechanisms of action of photodynamic therapy with verteporfin for the treatment of age-related macular degeneration. Surv Ophthalmol. 2000;45(3):195–214. doi: 10.1016/s0039-6257(00)00158-2. [DOI] [PubMed] [Google Scholar]
- 35.Bressler NM. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: two-year results of 2 randomized clinical trials-TAP report 2. Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Arch Ophthalmol. 2001;119(2):198–207. [PubMed] [Google Scholar]
- 36.Verteporfin in Photodynamic Therapy Study Group Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization—verteporfin in photodynamic therapy report 2. Am J Ophthalmol. 2001;131(5):541–560. doi: 10.1016/s0002-9394(01)00967-9. [DOI] [PubMed] [Google Scholar]
- 37.Bressler NM, Arnold J, Benchaboune M, et al. Verteporfin therapy of subfoveal choroidal neovascularization in patients with age-related macular degeneration: additional information regarding baseline lesion composition’s impact on vision outcomes— TAP report No. 3. Arch Ophthalmol. 2002;120(11):1443–1454. doi: 10.1001/archopht.120.11.1443. [DOI] [PubMed] [Google Scholar]
- 38.Bressler NM. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization—verteporfin in photodynamic therapy report 2. Am J Ophthalmol. 2002;133(1):168–169. doi: 10.1016/s0002-9394(01)01237-5. [DOI] [PubMed] [Google Scholar]
- 39.Verteporfin Roundtable 2000 and 2001 Participants. Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group principal investigators. Verteporfin in Photodynamic Therapy (VIP) Study Group principal investigators Guidelines for using verteporfin (Visudyne) in photodynamic therapy to treat choroidal neovascularization due to age-related macular degeneration and other causes. Retina. 2002;22(1):6–18. doi: 10.1097/00006982-200202000-00003. [DOI] [PubMed] [Google Scholar]
- 40.Pieramici DJ, Bressler SB, Koester JM, Bressler NM. Occult with no classic subfoveal choroidal neovascular lesions in age-related macular degeneration: clinically relevant natural history information in large lesions with good vision from the Verteporfin in Photodynamic Therapy (VIP) Trial: VIP Report No. 4. Arch Ophthalmol. 2006;124(5):660–664. doi: 10.1001/archopht.124.5.660. [DOI] [PubMed] [Google Scholar]
- 41.Gaynes BI, Fiescella RG. Safety of verteporfin for treatment of subfoveal choroidal neovascular membranes associated with age-related macular degeneration. Expert Opin Drug Saf. 2004;3(4):345–361. doi: 10.1517/14740338.3.4.345. [DOI] [PubMed] [Google Scholar]
- 42.Kaiser PK, Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) study Group Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: 5-year results of two randomized clinical trials with an open-label extension: TAP report no. 8. Graefes Arch Clin Exp Ophthalmol. 2006;244(9):1132–1142. doi: 10.1007/s00417-005-0199-9. [DOI] [PubMed] [Google Scholar]
- 43.Reeves BC, Harding SP, Langham J, et al. Verteporfin photodynamic therapy for neovascular age-related macular degeneration: cohort study for the UK. Health Technol Assess. 2012;6(6):1–200. doi: 10.3310/hta16060. [DOI] [PubMed] [Google Scholar]
- 44.Tsuchihashi T, Mori K, Yeyama K, Yoneya S. Five-year results of photodynamic therapy with verteporfin for Japanese patients with neovascular age-related macular degeneration. Clin Ophthalmol. 2013;7:615–620. doi: 10.2147/OPTH.S43566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gragoudas ES, Adamis AP, Cunningham ET, Jr, Feinsod M, Guyer DR, VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351(27):2805–2816. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- 46.Gonzales CR, VEGF Inhibition Study in Ocular Neovascularization (V.I.S.I.O.N.) Clinical Trial Group Enhanced efficacy associated with early treatment of neovascular age-related macular degeneration with pegaptanib sodium: an exploratory analysis. Retina. 2005;25(7):815–827. doi: 10.1097/00006982-200510000-00001. [DOI] [PubMed] [Google Scholar]
- 47.Maberley D. Pegaptanib for neovascular age-related macular degeneration. Issues Emerg Health Technol. 2005;(76):1–4. [PubMed] [Google Scholar]
- 48.Fraunfelder FW. Pegaptanib for wet macular degeneration. Drugs Today. 2005;41(11):703–709. doi: 10.1358/dot.2005.41.11.917340. [DOI] [PubMed] [Google Scholar]
- 49.Siddiqui MA, Keating GM. Pegaptanib: in exudative age-related macular degeneration. Drugs. 2005;65(11):1571–1577. doi: 10.2165/00003495-200565110-00010. discussion 1578–1579. [DOI] [PubMed] [Google Scholar]
- 50.Doggrell SA. Pegaptanib: the first antiangiogenic agent approved for neovascular macular degeneration. Expert Opin Pharmacother. 2005;6(8):1421–1423. doi: 10.1517/14656566.6.8.1421. [DOI] [PubMed] [Google Scholar]
- 51.D’Amico DJ, Masonson HN, Patel M, et al. Pegaptanib sodium for neovascular age-related macular degeneration: two-year safety results of the two prospective, multicenter, controlled clinical trials. Ophthalmology. 2006;113(6):992–1001. doi: 10.1016/j.ophtha.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 52.VEGF Inhibition Study in Ocular Neovascularization (V.I.S.I.O.N) Clinical Trial Group. Chakravarthy U, Adamis AP, et al. Year 2 efficacy results of 2 randomized controlled clinical trials of pegaptanib for neovascular age-related macular degeneration. Ophthalmology. 2006;113(9):1508, e1–25. doi: 10.1016/j.ophtha.2006.02.064. [DOI] [PubMed] [Google Scholar]
- 53.Kourlas H, Schiller DS. Pegaptanib sodium for treatment of neovascular age-related macular degeneration: a review. Clin Ther. 2006;28(1):36–44. doi: 10.1016/j.clinthera.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 54.Zhou B, Wang B. Pegaptanib for treatment of age-related macular degeneration. Exp Eye Res. 2006;83(3):615–619. doi: 10.1016/j.exer.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 55.Leys A, Zlateva G, Shah SN, Patel M. Quality of life in patients with age-related macular degeneration: results from the V.I.S.I.O.N. Study. Eye. 2008;22(6):792–798. doi: 10.1038/sj.eye.6702900. [DOI] [PubMed] [Google Scholar]
- 56.Chakravarthy U, Staurenghi G, Kwok K, et al. Treating early choroidal neovascularization with pegaptanib sodium in patients with neovascular age-related macular degeneration: Findings of the PERSPECTIVES study. Br J Ophthalmol. 2012;96(10):1351–1354. doi: 10.1136/bjophthalmol-2011-301444. [DOI] [PubMed] [Google Scholar]
- 57.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219(4587):983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 58.Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989;161(2):851–858. doi: 10.1016/0006-291x(89)92678-8. [DOI] [PubMed] [Google Scholar]
- 59.Connolly DT, Heuvelman DM, Nelson R, et al. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Invest. 1989;84(5):1470–1478. doi: 10.1172/JCI114322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular specific growth factors and blood vessel formation. Nature. 2000;407(6801):242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 61.Kuo CJ, Farnebo F, Yu EY, et al. Comparative evaluation of the antitumor activity of antiangiogenic proteins delivered by gene transfer. Proc Natl Acad Sci U S A. 2001;98(8):4605–4610. doi: 10.1073/pnas.081615298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stewart MW. The expanding role of vascular endothelial growth factor inhibitors in ophthalmology. Mayo Clin Proc. 2012;87(1):77–88. doi: 10.1016/j.mayocp.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dadgostar H, Waheed N. The evolving role of vascular endothelial growth factor inhibitors in the treatment of neovascular age-related macular degeneration. Eye. 2008;22(6):761–767. doi: 10.1038/eye.2008.86. [DOI] [PubMed] [Google Scholar]
- 64.Bressler SB. Introduction: understanding the role of angiogenesis and antiangiogenic agents in age-related macular degeneration. Ophthalmology. 2009;116(10 Suppl):S1–S7. doi: 10.1016/j.ophtha.2009.06.045. [DOI] [PubMed] [Google Scholar]
- 65.Schmidt-Erfurth U, Pollreisz A, Mitsch C, Bolz M. Antivascular endothelial growth factors in age-related macular degeneration. Dev Ophthalmol. 2010;46:21–38. doi: 10.1159/000320007. [DOI] [PubMed] [Google Scholar]
- 66.Campa C, Harding SP. Anti-VEGF compounds in the treatment of neovascular age-related macular degeneration. Curr Drug Targets. 2011;12(2):173–181. doi: 10.2174/138945011794182674. [DOI] [PubMed] [Google Scholar]
- 67.Neri P, Mariotti C. Antivascular endothelial growth factor sequential therapy for neovascular age-related macular degeneration: Is this the new deal? Curr Med Res Opin. 2012;28(3):395–400. doi: 10.1185/03007995.2012.662153. [DOI] [PubMed] [Google Scholar]
- 68.Veritti D, Sarao V, Lanzetta P. Neovascular age-related macular degeneration. Ophthalmologica. 2012;227(1 Suppl):11–20. doi: 10.1159/000337154. [DOI] [PubMed] [Google Scholar]
- 69.Scott AW, Bressler SB. Long-term follow-up of vascular endothelial growth factor inhibitor therapy for neovascular age-related macular degeneration. Curr Opin. 2013;24(3):190–196. doi: 10.1097/ICU.0b013e32835fefee. [DOI] [PubMed] [Google Scholar]
- 70.Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005;36(4):331–335. [PubMed] [Google Scholar]
- 71.Rosenfeld PJ, Fung AE, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for macular edema from central retinal vein occlusion. Ophthalmic Surg Lasers Imaging. 2005;36(4):336–339. [PubMed] [Google Scholar]
- 72.Jackson TL, Antcliff RJ, Hillenkamp J, Marshall J. Human retinal molecular weight exclusion limit and estimate of species variation. Invest Ophthalmol Vis Sci. 2003;44(5):2141–2146. doi: 10.1167/iovs.02-1027. [DOI] [PubMed] [Google Scholar]
- 73.Mordenti J, Cuthbertson RA, Ferrara N, et al. Comparisons of the intraocular tissue distribution pharmacokinetics, and safety of 235I-labeled full-length and Fab antibodies in rhesus monkeys following intravitreal administration. Toxicol Pathol. 1999;27(5):536–544. doi: 10.1177/019262339902700507. [DOI] [PubMed] [Google Scholar]
- 74.Ferrara N, Damico I, Shams N, Lowman H, Kim R. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 2006;26(8):859–870. doi: 10.1097/01.iae.0000242842.14624.e7. [DOI] [PubMed] [Google Scholar]
- 75.Rosenfeld PJ, Brown DM, Heier JS, et al. MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 76.Brown DM, Kaiser PK, Michels M, et al. ANCHOR Study Group Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 77.Brechner RJ, Rosenfeld PJ, Babish JD, Caplan S. Pharmacotherapy for neovascular age-related macular degeneration: an analysis of the 100% 2008 medicare fee-for-service part B claims file. Am J Ophthalmol. 2011;151(5):887–895. doi: 10.1016/j.ajo.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 78.Martin DF, Maguire MG, Ying GS, et al. Comparison of Age-related Macular Degeneration Treatment Trials Research Group Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martin DF, Maguire MG, Fine SL, et al. Comparison of Age-related Macular Degeneration Treatment Trials Research Group Ranibizumab and bevacizumab for neovascular age-related macular degeneration. Two-year results. Ophthalmology. 2012;119(7):1388–1398. doi: 10.1016/j.ophtha.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Abraham P, Yue H, Wilson L. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year-2. Am J Ophthalmol. 2010;150(3):315–324. doi: 10.1016/j.ajo.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 81.Holash J, Davis S, Papadopoulos N, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A. 2002;99(17):11393–11398. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Papadopoulos N, Martin J, Ruan Q, et al. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis. 2012;15(2):171–185. doi: 10.1007/s10456-011-9249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Verner-Cole EA, Davis SJ, Lauer AK. Aflibercept for the treatment of neovascular age-related macular degeneration. Drugs Today. 2012;48(5):317–329. doi: 10.1358/dot.2012.48.5.1805931. [DOI] [PubMed] [Google Scholar]
- 84.Ferrara N, Gerber HP, Le Couter J. The biology of VEGF and its receptor. Nat Med. 2003;9(6):669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 85.Houck KA, Leung DW, Rowland AM, Winer J, Ferrara N. Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J Biol Chem. 1992;267(36):26031–26037. [PubMed] [Google Scholar]
- 86.Lowe J, Araujo J, Yang J, et al. Ranibizumab inhibits multiple forms of biologically active vascular endothelial growth factor in vitreo and in vivo. Exp Eye Res. 2007;85(4):425–430. doi: 10.1016/j.exer.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 87.Chen Y, Wiesmann C, Fuh G, et al. Selection and analysis of an optimized anti-VEGF antibody: crystal structure of an affinity-matured Fab in complex with antigen. J Mol Biol. 1999;293(4):865–881. doi: 10.1006/jmbi.1999.3192. [DOI] [PubMed] [Google Scholar]
- 88.Yu L, Liang XH, Ferrara N. Comparing protein VEGF inhibitors: in vitro biological studies. Biochem Biophys Res Commun. 2011;408(2):276–281. doi: 10.1016/j.bbrc.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 89.Traynor K. Aflibercept approved for macular degeneration. Am J Health Syst Pharm. 2012;69(1):6. doi: 10.2146/news120001. [DOI] [PubMed] [Google Scholar]
- 90.Stewart MW. Aflibercept (VEGF Trap-eye): the newest anti-VEGF drug. Br J Ophthalmol. 2012;96(9):1157–1158. doi: 10.1136/bjophthalmol-2011-300654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Heier JS, Brown DM, Chong V, et al. for the VIEW 1 and VIEW 2 Study Groups Intravitreal aflibercept (VEGF Trap-Eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537–2548. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 92.Do DV, Schmidt-Erfurth U, Gonzalez VH, et al. The DA VINCI Study: phhase 2 primary results of VEGF Trap-Eye in patients with diabetic macular edema. Ophthalmology. 2011;118(9):1819–1826. doi: 10.1016/j.ophtha.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 93.Do DV, Nguyen QD, Boyer D, et al. for the DA VINCI Study Group One-year outcomes of the DA VINCI study of VEGF Trap-Eye in eyes with diabetic macular edema. Ophthalmology. 2012;119(8):1658–1665. doi: 10.1016/j.ophtha.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 94.Browning DJ, Kaiser PK, Rosenfeld PJ, Stewart MW. Aflibercept for age-related macular degeneration: A game-changer or quiet addition? Am J Ophthalmol. 2012;154(12):222–226. doi: 10.1016/j.ajo.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 95.Xu D, Kaiser PK. Intravitreal aflibercept for neovascular age-related macular degeneration. Immunotherapy. 2013;5(2):121–130. doi: 10.2217/imt.12.158. [DOI] [PubMed] [Google Scholar]
- 96.Stewart MW, Grippon S, Kirkpatrick P. Aflibercept. Nat Rev. 2012;11(4):269–270. doi: 10.1038/nrd3700. [DOI] [PubMed] [Google Scholar]
- 97.Frampton JE. Aflibercept for intravitreal injection in neovascular age-related macular degeneration. Drugs Aging. 2012;29(10):839–846. doi: 10.1007/s40266-012-0015-2. [DOI] [PubMed] [Google Scholar]
- 98.Chaikitmongkol V, Bressler NM. Dramatic resolution of choroidal neovascular abnormalities after single aflibercept injection following years of ranibizumab use. JAMA Ophthalmol. 2013;131(2):260–262. doi: 10.1001/jamaophthalmol.2013.1733. [DOI] [PubMed] [Google Scholar]
- 99.Heier JS. Neovascular age-related macular degeneration, individualizing therapy in the era of anti-angiogenic treatments. Ophthalmology. 2013;120(5 Suppl):S23–S25. doi: 10.1016/j.ophtha.2013.01.059. [DOI] [PubMed] [Google Scholar]
- 100.Korobelnik JF, Holz FG, Roider J, et al. Intravitreal aflibercept injection for macular edema resulting from central retinal vein occlusion: one-year results of the phase 3 GALILEO Study. Ophthalmology. 2013;(13):S0161–6420. 00730–00736. doi: 10.1016/j.ophtha.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 101.Brown DM, Heier JS, Clark WL, et al. Intravitreal aflibercept injection for macular edema secondary to central retinal vein occlusion: 1-year results from the phase 3 COPERNICUS Study. Am J Ophthalmol. 2013;155(3):429–437. doi: 10.1016/j.ajo.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 102.Fung AE, Lalwani GA, Rosenfeld PJ, et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007;143(10):566–583. doi: 10.1016/j.ajo.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 103.Lalwani GA, Rosenfeld PJ, Fung AE, et al. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO Study. Am J Ophthalmol. 2009;148(1):43–58. doi: 10.1016/j.ajo.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 104.Heussen FM, Ouyang Y, Sadda SR, Walsh AC. Simple estimation of clinically relevant lesion volumes using spectral domain-optical coherence tomography in neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52(10):7792–7798. doi: 10.1167/iovs.11-8023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gregori NZ, Feuer W, Rosenfeld PJ. Novel method for analyzing Snellen visual acuity measurements. Retina. 2010;30(7):1046–1050. doi: 10.1097/IAE.0b013e3181d87e04. [DOI] [PubMed] [Google Scholar]
- 106.Chan CK, Meyer CH, Gross JG, et al. Retinal pigment epithelial tears after intravitreal bevacizumab injection for neovascular age-related macular degeneration. Retina. 2007;27(5):541–551. doi: 10.1097/IAE.0b013e3180cc2612. [DOI] [PubMed] [Google Scholar]
- 107.Chuang EL, Bird AC. The pathogenesis of tears of the retinal pigment epithelium. Am J Ophthalmol. 1988;105(3):285–290. doi: 10.1016/0002-9394(88)90011-6. [DOI] [PubMed] [Google Scholar]
- 108.Pauleikoff D, Löffert D, Spital G, et al. Pigment epithelial detachment in the elderly. Clinical differentiation, natural course and pathogenetic implications. Graefes Arch Clin Exp Ophthalmol. 2002;240(7):533–538. doi: 10.1007/s00417-002-0505-8. [DOI] [PubMed] [Google Scholar]
- 109.Mrejen S, Sarraf D, Mukkamala SK, Freund KB. Multimodal imaging of pigment epithelial detachment: a guide to evaluation. Retina. 2013;33(9):1735–1762. doi: 10.1097/IAE.0b013e3182993f66. [DOI] [PubMed] [Google Scholar]
- 110.Christoforidis JB, Carlton MM, Knopp MV, Hinkle GH. PET/CT imaging of I-124-radiolabeled bevacizumab and ranibizumab after intravitreal injection in a rabbit model. Invest Ophthalmol Vis Sci. 2011;52(8):5899–5903. doi: 10.1167/iovs.10-6862. [DOI] [PubMed] [Google Scholar]
- 111.Bakri SJ, Snyder MR, Reid JM, Pulido JS, Ezzar MK, Singh RJ. Pharmacokinetics of intravitreal ranibizumab (Lucentis) Ophthalmology. 2007;114(12):2179–2182. doi: 10.1016/j.ophtha.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 112.Bakri SJ, Snyder MR, Reid JM, Pulido JS, Singh RJ. Pharmacokinetics of intravitreal bevacizumab (Avastin) Ophthalmology. 2007;114(5):855–859. doi: 10.1016/j.ophtha.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 113.Stewart MW. Predicted biologic activity of intravitreal bevacizumab. Retina. 2007;27(5):1196–1200. doi: 10.1097/IAE.0b013e318158ea28. [DOI] [PubMed] [Google Scholar]
- 114.Stewart MW, Rosenfeld PJ. Predicted biological activity of intravitreal VEGF Trap. Br J Ophthalmol. 2008;92(5):667–668. doi: 10.1136/bjo.2007.134874. [DOI] [PubMed] [Google Scholar]
- 115.Stewart MW. What are the half-lives of ranibizumab and aflibercept (VEGF Trap-Eye) in human eyes? Calculations with a mathematical model. Eye Reports. 2011;1(1):e5. [Google Scholar]
- 116.Stewart MH, Rosenfeld PJ, Penha FM, et al. Pharmacokinetic rationale for dosing every 2 weeks versus 4 weeks with intravitreal ranibizumab, bevacizumab, and aflibercept (vascular endothelial growth factor trap-eye) Retina. 2012;32(3):434–457. doi: 10.1097/IAE.0B013E31822C290F. [DOI] [PubMed] [Google Scholar]
- 117.Singer MA, Awh CC, Sadda S, et al. HORIZON: an open-label extension trial of ranibizumab for choroidal neovascularization secondary to age-related macular degeneration. Ophthalmology. 2012;119(6):1175–1183. doi: 10.1016/j.ophtha.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 118.Engelbert M, Zweifel SA, Freund KB. “Treat and extend” dosing of intravitreal antivascular endothelial growth factor therapy for type 3 neovascularization/retinal angiomatous proliferation. Retina. 2009;29(10):1424–1431. doi: 10.1097/IAE.0b013e3181bfbd46. [DOI] [PubMed] [Google Scholar]
- 119.Engelbert M, Zweifel SA, Freund KB. Long-term follow-up for type 1 (subretinal pigment epithelium) neovascularization suing a modified “treat and extend” dosing regimen of intravitreal antivascular endothelial growth factor therapy. Retina. 2010;30(9):1368–1375. doi: 10.1097/IAE.0b013e3181d50cbf. [DOI] [PubMed] [Google Scholar]
- 120.Conrad PW, Zacks DN, Johnson MW. Intravitreal bevacizumab has initial clinical benefit lasting eight weeks in eyes with neovascular age-related macular degeneration. Clin Ophthalmol. 2008;2(4):727–733. doi: 10.2147/opth.s2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ho VY, Yeh S, Olsen TW, et al. Short-term outcomes of aflibercept for neovascular age-related macular degeneration in eyes previously treated with other vascular endothelial growth factor inhibitors. Am J Ophthalmol. 2013;156(1):23–28. doi: 10.1016/j.ajo.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bakall B, Folk JC, Boldt HC, et al. Aflibercept therapy for exudative age-related macular degeneration resistant to bevacizumab and ranibizumab. Am J Ophthalmol. 2013;156(1):15–22. doi: 10.1016/j.ajo.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 123.Kumar N, Marsiglia M, Mrejen S, et al. Visual and anatomical outcomes of intravitreal aflibercept in eyes with persistent subfoveal fluid despite previous treatments with ranibizumab in patients with neovascular age-related macular degeneration. Retina. 2013;33(8):1605–1612. doi: 10.1097/IAE.0b013e31828e8551. [DOI] [PubMed] [Google Scholar]
- 124.Patel KH, Chow CC, Rathod R, et al. Rapid response of retinal pigment epithelial detachments to intravitreal aflibercept in neovascular age-related macular degeneration refractory to bevacizumab and ranibizumab. Eye (Lond) 2013;27(5):663–667. doi: 10.1038/eye.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yonekawa Y, Andreoli C, Miller JB, et al. Conversion to aflibercept for chronic refractory or recurrent neovascular age-related macular degeneration. Am J Ophthalmol. 2013;156(1):29–35. doi: 10.1016/j.ajo.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 126.Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331(22):1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 127.Adamis AP, Miller JW, Bernal MT, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994;118(4):445–450. doi: 10.1016/s0002-9394(14)75794-0. [DOI] [PubMed] [Google Scholar]
- 128.Forooghian F, Kertes PJ, Eng KT, Agron E, Chew EY. Alterations in the intraocular cytokine milieu after intravitreal bevacizumab. Invest Ophthalmol Vis Sci. 2010;51(5):2388–2392. doi: 10.1167/iovs.09-4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cheung CMG, Vania M, Ang M, Chee SP, Li J. Comparison of aqueous humor cytokine and chemokine levels in diabetic patients with and without retinopathy. Mol Vis. 2012;18:830–837. [PMC free article] [PubMed] [Google Scholar]
- 130.Hee MR, Puliafito CA, Wong C, et al. Quantitative assessment of macular edema with optical coherence tomography. Arch Ophthalmol. 1995;113(5):1019–1029. doi: 10.1001/archopht.1995.01100080071031. [DOI] [PubMed] [Google Scholar]
- 131.Goebel W, Kretzchmar-Gross T. Retinal thickness in diabetic retinopathy: a study using optical coherence tomography (OCT) Retina. 2002;22(6):759–767. doi: 10.1097/00006982-200212000-00012. [DOI] [PubMed] [Google Scholar]
- 132.Bandello F, Polito A, Del Borrello M, et al. “Light” versus “classic” laser treatment for clinically significant diabetic macular edema. Br J Ophthalmol. 2005;89(7):864–870. doi: 10.1136/bjo.2004.051060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Otani T, Kishi S. Tomographic findings of foveal hard exudates in diabetic macular edema. Am J Ophthalmol. 2001;131(1):50–54. doi: 10.1016/s0002-9394(00)00661-9. [DOI] [PubMed] [Google Scholar]
- 134.Martidis A, Duker JS, Greenberg PB, et al. Intravitreal triamcinolone for refractory diabetic macular edema. Ophthalmology. 2002;109(5):920–927. doi: 10.1016/s0161-6420(02)00975-2. [DOI] [PubMed] [Google Scholar]
- 135.Laursen ML, Moeller F, Sander B, Sjoelie AK. Subthreshold micropulse diode laser treatment in diabetic macular edema. Br J Ophthalmol. 2004;88(9):1173–1179. doi: 10.1136/bjo.2003.040949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Catier A, Tadayoni R, Paques M, et al. Characterization of macular edema from various etiologies by optical coherence tomography. Am J Ophthalmol. 2005;140(2):200–206. doi: 10.1016/j.ajo.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 137.Ozdemir H, Karacorlu M, Karacorlu SA. Regression of serous macular detachment after intravitreal triamcinolone acetonide in patients with diabetic macular edema. Am J Ophthalmol. 2005;140(2):251–255. doi: 10.1016/j.ajo.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 138.Massin P, Duguid G, Erginay A, et al. Optical coherence tomography for evaluating diabetic macular edema before and after vitrectomy. Am J Ophthalmol. 2003;135(2):169–177. doi: 10.1016/s0002-9394(02)01837-8. [DOI] [PubMed] [Google Scholar]
- 139.Diabetic Retinopathy Clinical Research Network. Browning DJ, Glassman AR, et al. Relationship between optical coherence tomography-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology. 2007;114(3):525–536. doi: 10.1016/j.ophtha.2006.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Scott IU, Van Veldhuisen PC, Oden NL, et al. SCORE Study report 1: baseline associations between central retinal thickness and visual acuity in patients with retinal vein occlusion. Ophthalmology. 2009;116(3):504–512. doi: 10.1016/j.ophtha.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Scott IU, VanVeldhuisen PC, Oden NL, et al. Baseline predictors of visual acuity and retinal thickness outcomes in patients with retinal vein occlusion: Standard Care Versus COrticosteroid for REtinal Vein Occlusion Study report 10. Ophthalmology. 2011;118(2):345–352. doi: 10.1016/j.ophtha.2010.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ip MS, Scott IU, VanVeldhuisen PC, Oden NL, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: The Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) Study Report 5. Arch Ophthalmol. 2009;127(9):1101–1114. doi: 10.1001/archophthalmol.2009.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Scott IU, Ip MS, VanVeldhuisen PC, Oden NL, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with standard care to treat vision loss associated with macular edema secondary to branch retinal vein occlusion: The Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) Study Report 6. Arch Ophthalmol. 2009;127(9):1115–1128. doi: 10.1001/archophthalmol.2009.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ammar DA, Mandava N, Kahook MY. The effects of aflibercept on the viability and metabolism of ocular cells in vitro. Retina. 2013;33(5):1056–1061. doi: 10.1097/IAE.0b013e31827b646d. [DOI] [PubMed] [Google Scholar]
- 145.Schnichels S, Hagemann U, Januschowski K, et al. Comparative toxicity and proliferation testing of aflibercept, bevacizumab and ranibizumab on different ocular cells. Br J Ophthalmol. 2013;97(7):917–923. doi: 10.1136/bjophthalmol-2013-303130. [DOI] [PubMed] [Google Scholar]
- 146.Nguyen QD, Campochiaro PA, Shah SM, et al. Evaluation of very high- and very low-dose intravitreal aflibercept in patients with neovascular age-related macular degeneration. J Ocul Pharmacol Ther. 2012;28(6):581–588. doi: 10.1089/jop.2011.0261. [DOI] [PubMed] [Google Scholar]
- 147.Heier JS, Boyer D, Nguyen QD, et al. CLEAR-IT-2 Investigators The 1-year results of CLEAR-IT 2, a phase 2 study of vascular endothelial growth factor trap-eye dosed as-needed after 12-week fixed dosing. Ophthalmology. 2011;118(6):1098–1106. doi: 10.1016/j.ophtha.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 148.Brown DM, Heier JS, Ciulla T, et al. CLEAR-IT 2 Investigators Primary endpoint results of a phase II study of vascular endothelial growth factor trap-eye in wet age-related macular degeneration. Ophthalmology. 2011;118(6):1089–1097. doi: 10.1016/j.ophtha.2011.02.039. [DOI] [PubMed] [Google Scholar]
- 149.Avery RL, Castellarin AA, Steinle NC, et al. Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD. Br J Ophthalmol. 2014 Jul 7; doi: 10.1136/bjophthalmol-2014-305252. [epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gelisken F, Inhoffen W, Partsch M, et al. Retinal pigment epithelial tear after photodynamic therapy for choroidal neovascularization. Am J Ophthalmol. 2001;131(4):518–520. doi: 10.1016/s0002-9394(00)00813-8. [DOI] [PubMed] [Google Scholar]
- 151.Pece A, Introini U, Bottoni F, Brancato R. Acute retinal pigment epithelial tear after photodynamic therapy. Retina. 2001;21(6):661–665. doi: 10.1097/00006982-200112000-00017. [DOI] [PubMed] [Google Scholar]
- 152.Srivastava SK, Sternberg P., Jr Retinal pigment epithelial tear weeks following photodynamic therapy with verteporfin for choroidal neovascularization secondary to pathologic myopia. Retina. 2002;22(5):669–671. doi: 10.1097/00006982-200210000-00027. [DOI] [PubMed] [Google Scholar]
- 153.Arnold JJ, Blinder KJ, Bressler NM, et al. Acute severe visual acuity decrease after photodynamic therapy with verteporfin: case reports from randomized clinical trials-TAP and VIP report no. 3. Am J Ophthalmol. 2004;137(4):683–696. doi: 10.1016/j.ajo.2003.11.059. [DOI] [PubMed] [Google Scholar]
- 154.Goldstein M, Heilweil G, Barak A, Loewenstein A. Retinal pigment epithelial tear following photodynamic therapy for choroidal neovascularization secondary to AMD. Eye (Lond) 2005;19(12):1315–1324. doi: 10.1038/sj.eye.6701765. [DOI] [PubMed] [Google Scholar]
- 155.Michels S, Aue A, Simader C, et al. Retinal pigment epithelial tears following verteporfin therapy combined with intravitreal triamcinolone. Am J Ophthalmol. 2006;141(2):396–398. doi: 10.1016/j.ajo.2005.08.046. [DOI] [PubMed] [Google Scholar]
- 156.Sutter FK, Kurz-Levin MM, Scherrer M, Barthelmes D, Fleischhauer JC, Helbig H. Intravitreal triamcinolone acetonide for serous retinal pigment epithelial detachments in exudative age-related macular degeneration. Klin Monatsbl Augenheilkd. 2007;224(4):297–299. doi: 10.1055/s-2007-962949. [DOI] [PubMed] [Google Scholar]
- 157.Dhalla MS, Blinder KJ, Tewari A, et al. Retinal pigment epithelial tear following intravitreal pegaptanib sodium. Am J Ophthalmol. 2006;141(4):752–754. doi: 10.1016/j.ajo.2005.10.053. [DOI] [PubMed] [Google Scholar]
- 158.Singh RP, Sears JE. Retinal pigment epithelial tears after pegaptanib injection for exudative age-related macular degeneration. Am J Ophthalmol. 2006;142(1):160–162. doi: 10.1016/j.ajo.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 159.Chang LK, Flaxel CJ, Lauer AK, Sarraf D. RPE tears after pegaptanib treatment in age-related macular degeneration. Retina. 2007;27(7):857–863. doi: 10.1097/IAE.0b013e3180342c42. [DOI] [PubMed] [Google Scholar]
- 160.Carvounis PE, Kopel AC, Benz MS. Retinal pigment epithelium tears following ranibizumab for exudative age-related macular degeneration. Am J Ophthalmol. 2007;143(3):504–505. doi: 10.1016/j.ajo.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 161.Bakri SJ, Kitzmann AS. Retinal pigment epithelial tear after intravitreal ranibizumab. Am J Ophthalmol. 2007;143(3):505–507. doi: 10.1016/j.ajo.2006.11.047. [DOI] [PubMed] [Google Scholar]
- 162.Chan CK, Lin SG. Retinal pigment epithelial tear after ranibizumab therapy for subfoveal fibrovascular pigment epithelial detachment. Eur J Ophthalmol. 2007;17(4):674–676. doi: 10.1177/112067210701700432. [DOI] [PubMed] [Google Scholar]
- 163.Meyer CH, Mennel S, Schmidt JC, Kroll P. Acute retinal pigment epithelial tear following intravitreal bevacizumab (Avastin) injection for occult choroidal neovascularization secondary to age related macular degeneration. Br J Ophthalmol. 2006;90(9):1207–1208. doi: 10.1136/bjo.2006.093732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fung AE, Rosenfeld PJ, Reichel E. The international intravitreal bevacizumab safety survey: using the internet to assess drug safety worldwide. Br J Ophthalmol. 2006;90(11):1344–1349. doi: 10.1136/bjo.2006.099598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shah CP, Hsu J, Garg S, et al. Retinal pigment epithelial tear after intravitreal bevacizumab injection. Am J Ophthalmol. 2006;142(6):1070–1072. doi: 10.1016/j.ajo.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 166.Spandau UH, Jonas JB. Retinal pigment epithelium tear after intravitreal bevacizumab for exudative age-related macular degeneration. Am J Ophthalmol. 2006;142(6):1068–1070. doi: 10.1016/j.ajo.2006.06.048. [DOI] [PubMed] [Google Scholar]
- 167.Garg S, Brod R, Kim D, Lane RG, Maguire J, Fischer D. Retinal pigment epithelial tears after intravitreal bevacizumab injection for exudative age-related macular degeneration. Clin Experiment Ophthalmol. 2008;36(3):252–256. doi: 10.1111/j.1442-9071.2008.01710.x. [DOI] [PubMed] [Google Scholar]
- 168.Shah CP, Hsu J, Garg SJ, Fischer DH, Kaiser R. Retinal pigment epithelial tear after intravitreal bevacizumab injection. Am J Ophthalmol. 2006;142(6):1070–1072. doi: 10.1016/j.ajo.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 169.Arias L, Caminal JM, Rubio M, Pujol O, Arruga J. Retinal pigment epithelial tears after intravitreal bevacizumab injection for predominantly classic choroidal neovascularization. Eur J Ophthalmol. 2007;17(6):992–995. doi: 10.1177/112067210701700622. [DOI] [PubMed] [Google Scholar]
- 170.Shaikh S, Olson JC, Richmond PP. Retinal pigment epithelial tears after intravitreal bevacizumab injection for exudative age-related macular degeneration. Indian J Ophthalmol. 2007;55(6):470–472. doi: 10.4103/0301-4738.36489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cleary CA, Jungkim S, Ravikumar K, Kelliher C, Acheson RW, Hickey-Dwyer M. Intravitreal bevacizumab in the treatment of neovascular age-related macular degeneration, 6- and 9-month results. Eye (Lond) 2008;22(1):82–86. doi: 10.1038/sj.eye.6702936. [DOI] [PubMed] [Google Scholar]
- 172.Kook D, Wolf A, Neubauer AS, et al. [Retinal pigment epithelial tears after intravitreal injection of bevacizumab for AMD. Frequency and progress] Ophthalmologe. 2008;105(2):158–164. doi: 10.1007/s00347-007-1561-6. [DOI] [PubMed] [Google Scholar]
- 173.Ronan SM, Yoganathan P, Chien FY, Corcóstegui IA, et al. Retinal pigment epithelium tears after intravitreal injection of bevacizumab (Avastin) for neovascular age-related macular degeneration. Retina. 2007;27(5):535–540. doi: 10.1097/IAE.0b013e3180cc2645. [DOI] [PubMed] [Google Scholar]
- 174.Chan CK, Meyer CH, Gross JG, Abraham P, et al. Retinal pigment epithelial tears after intravitreal bevacizumab injection for neovascular age-related macular degeneration. Retina. 2007;27(5):541–551. doi: 10.1097/IAE.0b013e3180cc2612. [DOI] [PubMed] [Google Scholar]
- 175.Chang LK, Sarraf D. Tears of the retinal pigment epithelium: an old problem in a new era. Retina. 2007;27(5):523–534. doi: 10.1097/IAE.0b013e3180a032db. [DOI] [PubMed] [Google Scholar]
- 176.Leitritz M, Gelisken F, Inhoffen W, Voelker M, Ziemssen F. Can the risk of retinal pigment epithelium tears after bevacizumab treatment be predicted? An optical coherence tomography study. Eye. 2008;22(12):1504–1507. doi: 10.1038/eye.2008.145. [DOI] [PubMed] [Google Scholar]
- 177.Chiang A, Chang LK, Yu F, Sarraf D. Predictors of anti-VEGF-associated retinal pigment epithelial tear using FA and OCT analysis. Retina. 2008;28(9):1265–1269. doi: 10.1097/IAE.0b013e31817d5d03. [DOI] [PubMed] [Google Scholar]
- 178.Lesniak SP, Fine HF, Prenner JL, Roth DB. Long-term follow-up of spontaneous retinal pigment epithelium tears in age-related macular degeneration treated with anti-VEGF therapy. Eur J Ophthalmol. 2011;21(1):73–76. doi: 10.5301/ejo.2010.2285. [DOI] [PubMed] [Google Scholar]
- 179.Gutfleisch M, Heimes B, Schumacher M, et al. Long-term visual outcome of pigment epithelial tears in association with anti-VEGF therapy of pigment epithelial detachment in AMD. Eye. 2011;25(9):1181–1186. doi: 10.1038/eye.2011.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Coco RM, Sanabria MR, Hernandez AG, Fernandez Munoz M. Retinal pigment epithelium tears in age-related macular degeneration treated with antiangiogenic drugs: a controlled study with long follow-up. Ophthalmologica. 2012;228(2):78–83. doi: 10.1159/000338730. [DOI] [PubMed] [Google Scholar]
- 181.Saito M, Kano M, Itagaki K, Oguchi Y, Sekiryu T. Retinal pigment epithelium tear after intravitreal aflibercept injection. Clin Ophthalmol. 2013;7:1287–1289. doi: 10.2147/OPTH.S47735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Bertelmann T, Sekundo W, Wenner Y. [Tear in the retinal pigment epithelium by intravitreal injection of aflibercept] Ophthalmologe. 2014;111:775–777. doi: 10.1007/s00347-013-2954-3. [DOI] [PubMed] [Google Scholar]
- 183.Cunningham ET, Jr, Feiner L, Chung C, Tuomi L, Ehrlich JS. Incidence of retinal pigment epithelial tears after intravitreal ranibizumab injection for neovascular age-related macular degeneration. Ophthalmology. 2011;118(12):2477–2452. doi: 10.1016/j.ophtha.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 184.Saint-Geniez M, Kurihara T, Sekiyama E, Maldonado AE, D’Amore PA. An essential role of RPE-derived soluble VEGF in the maintenance of choriocapillaris. Proc Natl Acad Sci U S A. 2009;106(44):18751–18756. doi: 10.1073/pnas.0905010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Rosenfeld PJ, Shapiro H, Tuomi L, et al. MARINA and ANCHOR Study Groups Characteristics of patients losing vision after 2 years of monthly dosing in the phase III ranibizumab clinical trials. Ophthalmology. 2011;118(3):523–530. doi: 10.1016/j.ophtha.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 186.Olsen TW. Treatment of exudative age-related macular degeneration: many factors to consider. Am J Ophthalmol. 2007;144(2):281–283. doi: 10.1016/j.ajo.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Curtis LH, Hammill BG, Schulman KA, Cousin SW. Risks of mortality, myocardial infarction, bleeding, and stroke associated with therapies for age-related macular degeneration. Arch Ophthalmol. 2010;128(10):1273–1279. doi: 10.1001/archophthalmol.2010.223. [DOI] [PubMed] [Google Scholar]
- 188.Van Der Reis MI, La Heij EC, De Jong-Hesse Y, Ringens PJ, Hendrikse F, Schouten JSAG. A systemic review of the adverse events of intravitreal anti-vascular endothelial growth factor injections. Retina. 2011;31(8):1449–1469. doi: 10.1097/IAE.0b013e3182278ab4. [DOI] [PubMed] [Google Scholar]
- 189.Tolentino M. Systemic and ocular safety of intravitreal anti-VEGF therapies for ocular neovascular disease. Surv Ophthalmol. 2011;56(2):95–113. doi: 10.1016/j.survophthal.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 190.Semeraro F, Morescalchi F, Parmeggiani F, Arcidiacono B, Costagliola C. Systemic adverse drug reactions secondary to anti-VEGF intravitreal injection in patients with neovascular age-related macular degeneration. Curr Vasc Pharmacol. 2011;9(5):629–646. doi: 10.2174/157016111796642670. [DOI] [PubMed] [Google Scholar]
- 191.Costagliola C, Agnifili L, Arcidiacono B, et al. Systemic thromboembolic adverse events in patients treated with intravitreal anti-VEGF drugs for neovascular age-related macular degeneration. Expert Opin Biol Ther. 2012;12(10):1299–1313. doi: 10.1517/14712598.2012.707176. [DOI] [PubMed] [Google Scholar]