Abstract
Aims
The Sprague–Dawley (SD) rat, an out-bred, all-purpose strain, has served well for lower urinary tract research. However, to test new cellular therapies for conditions such as stress urinary incontinence, an in-bred rat strain with immune tolerance, such as the Lewis rat, may be more useful. The objective of this study was to reveal any differences in lower urinary tract continence mechanisms between the Lewis and SD rat.
Methods
The contribution of (1) the striated and smooth muscle to the mechanical and functional properties of the urethra in vitro, and (2) the striated sphincter to leak point pressure (LPP) and reflex continence mechanisms in vivo were assessed in normal (control) Lewis and SD rats and in a model of stress urinary incontinence produced by bilateral pudendal nerve transection.
Results
Control, Lewis rats had significantly lower LPP, significantly less fast-twitch skeletal muscle and relied less on the striated sphincter for continence than control, SD rats, as indicated by the failure of neuromuscular blockade with alpha-bungarotoxin to reduce LPP. Nerve transection significantly decreased LPP in the SD rat, but not in the Lewis rat. Although the Lewis urethra contained more smooth muscle than the SD rat, it was less active in vitro as indicated by a low urethral baseline pressure and lack of response to phenylephrine.
Conclusions
We have observed distinct differences in functional and mechanical properties of the SD and Lewis urethra and have shown that the Lewis rat may not be suitable as a chronic model of SUI via nerve transection.
Keywords: Lewis, Sprague–Dawley, stress urinary incontinence, urethra
INTRODUCTION
Over the past decade various treatments for stress urinary incontinence, have emerged including injection of bulking agents,1 placement of synthetic slings,2 electrical stimulation and the injection of stem cells.3–6 As technology advances and more cell-based therapies are developed, it is imperative that these treatments are tested in appropriate animal models.
To date, the majority of lower urinary tract studies have been performed in the Sprague–Dawley (SD) rat.4,7–10 This is an out-bred, all-purpose strain used in many areas of research.11 However, as cellular treatments progress, a new model may be more appropriate for their initial validation and assessment. An in-bred animal would allow for fine tuning of the therapy in a more controlled in vivo study, without increased variability from genetic variation or immune responses by the test animal. The Lewis rat, an in-bred strain, is generally used in immunological and stem cell research studies, and could serve as a better model for investigating possible stem cell therapies to be used in the lower urinary tract.11 To this end, the lower urinary tract properties and response to models of urethral dysfunction would need to be verified in the Lewis rat.
Previous studies of stress urinary continence in rats produced by vaginal distension (VD) suggested that the Lewis11 and SD rats12 exhibit similar lower urinary tract dysfunctions. However the study in Lewis rats, which focused on an acute model of SUI, did not directly compare the two rat strains but only compared results in Lewis rats to those previously reported in SD rats. On the other hand, there have been several reports of differences in behavior, endocrine parameters, organ weight, and responses to pharmacological agents between SD and Lewis rats,13–15 emphasizing the need to validate the Lewis rat for urethral studies.
The objective of this study was to determine whether differences exist in the functional and mechanical properties of the urethra in SD and Lewis rats using established testing methods.
MATERIALS AND METHODS
Animals
Adult, female, SD, and Lewis rats (200–250 g) (Hilltop Lab Animals, Inc., Scottdale, PA) were used in this study. Animals were housed at the University of Pittsburgh under the supervision of the Department of Laboratory Animal Resources. The policies and procedures for the animal studies are in accordance with those detailed in the Guide for the Care and Use of Laboratory Animals, published by the US Department of Health and Human Services. Procedural protocols were approved by the University of Pittsburgh’s Institutional Animal Care and Use Committee.
Overview of Experiments
Five types of experiments were performed. Experiment 1 evaluated, in SD and Lewis rats, the effects of SUI induced by bilateral pudendal nerve transection on the leak point pressure (LPP), a common in vivo measure of urethral function.5,16 Experiment 2 compared the contribution of the striated sphincter to the continence mechanism in SD and Lewis rats using a specific inhibitor of skeletal muscle activity. Experiment 3 assessed common functional indices of the urethra during sneezing in continent SD and Lewis rats. Experiment 4 utilized our ex vivo urethral testing system8,10,17 to assess the mechanical properties of SD and Lewis urethras. Experiment 5 evaluated the ex vivo functional response of both SD and Lewis urethras to smooth and striated muscle agonists. Each experiment, as well as methodology common to them, are detailed below.
Surgical Procedures
The injured group for each strain in Experiment 1 was created by anesthetizing the animal with 4% isoflurane, and then transecting the pudendal nerves bilaterally using aseptic, surgical techniques near the internal iliac vessels, just distal to the bifurcation of the pelvic nerve from the L6-S1 nerve trunk.18 Since this common model for SUI19,20 utilizes complete transection of the nerve, it provides an immediate effect. However, animals were assessed one week following nerve transection.
For all in vivo experiments, rats were anesthetized by isoflurane inhalation, and the bladder was exposed via a mid-line incision. The ureters were cut bilaterally, urine was drained from the proximal ends to the abdominal cavity, and their distal ends were ligated to prevent the flow of urine into the bladder, which could alter intravesical pressure readings. The colon was cleared of feces through a small incision. Following all surgical procedures, isoflurane anesthetic was discontinued and replaced with urethane anesthetic (1.2 g/kg, subcutaneous, Sigma, St. Louis, MO). Additional doses of anesthetic were administered intravenously, as required, during experimentation.
For Experiments 1 and 2, a catheter (PE-90) was inserted into the bladder dome for recording of intravesical pressure and then the spinal cord was transected at the T9-T10 level after laminectomy. A sterile sponge (Gelfoam, Upjohn, Kalamazoo, MI) was placed between the cut ends of the spinal cord and the overlying muscle and skin were sutured closed. Spinal transection eliminates the voiding reflex mediated by spinobulbospinal pathways, but still allows spinal urethral contractile reflexes via sympathetic and somatic nerves to remain active during bladder filling. In addition, for Experiment 2, catheters were placed in the carotid artery (PE-50) to measure blood pressure, in the jugular vein (PE-10) for administration of intravenous drugs, and in the trachea (PE-160) for artificial respiration. A small balloon-catheter was inserted into the abdominal cavity via the rectum and connected to a pressure transducer to record abdominal pressures (Pabd) during Experiment 3.
Experiment 1: Effects of Pudendal Nerve Injury on Sprague–Dawley and Lewis Rat Leak Point Pressure (LPP)
Control (untouched) and injured rats from each strain were compared. One week following injury, the LPP was assessed in each animal using the vertical tilt table method (V-LPP).21 Briefly, rats were mounted onto the tilt table, and the bladder catheter was attached to a pressure reservoir and a pressure transducer (BLPR, World Precision Instruments, Sarasota, FL) via a 3-way stopcock. Intravesical pressure was increased in 2 cm H2O increments until leakage was visible, while data were recorded with Chart™ software (AD Instruments, Castle Hill, NSW, Australia) on a computer with a PowerLab® analog-to-digital converter. The measure was repeated a minimum of two times. The average of the measurements was taken as the V-LPP for that animal.
Experiment 2: Contribution of the Striated Sphincter to the Continence Mechanism
Control SD and Lewis rats were prepared and tested for LPP as described above, but in the supine position (Su-LPP) as vertical positioning caused a significant decrease in blood pressure following intravenous injections of alpha-bungarotoxin, a neuromuscular blocking agent. Following initial Su-LPP testing, 333 μg/kg alpha-bungarotoxin dissolved in sterile saline was injected in divided doses at 10 min intervals, and the Su-LPP test was repeated. Animal respiration was maintained by a ventilator (3.5 ml/stroke, 80 strokes/min) following injection of alpha-bungarotoxin.22
Experiment 3: Assessment of Urethral Response During Sneeze
Control SD and Lewis rats were subjected to sneeze testing as previously reported.9 Briefly, bladders were emptied and a catheter with a side-mounted microtransducer, (SPR-524, Millar Instruments, Houston, TX) was inserted into the urethra via the external orifice. The sensor was positioned at the middle urethra on the inner urethral surface, based on previous studies.16 The sneeze reflex was induced by inserting a cut whisker into the animal’s nostril.9 Prior to and following initiation of the sneeze, urethral baseline pressure (UBP) and the change in abdominal pressure (ΔPabd) were measured in cm H2O.9 During sneeze, amplitude of the urethral pressure response above baseline (A-URS) and ΔPabd were measured in cm H2O.9 The average of at least 10 measurements of each metric was taken as the value for individual rats.9,16
Experiment 4: Ex Vivo Biomechanical Analysis
Following LPP measurements, the urethra was excised and tested as previously described by our group.8,10,17 Briefly, PE-50 tubing (Becton-Dickson, Franklin Lakes, NJ), was inserted through the urethra into the dome of the bladder. The bladder dome, as well as the external urethral meatus, was ligated around the catheter with 4-0 silk suture (Syneture, Mansfield, MA). The ureters were ligated and served as anatomical landmarks for the maintenance of in vivo length during mechanical testing. The urethra was then separated from the surrounding tissues and placed in cold, oxygenated media 199 (Sigma–Aldrich, St. Louis, MO), containing antibiotic/antimycotic and L-glutamine (Invitrogen, Carlsbad, CA) to prevent hypoxia of the tissue. Tissue was transported to our testing facility on ice.
The urethra was mounted onto stainless steel cannulae within a bathing chamber filled with media 199 and kept at physiologic conditions via a circulation loop and gaseous mixture of 95% O2 and 5% CO2. A hydrostatic reservoir was used to increase intraluminal pressure from 0 to 20 mm Hg in 2 mm Hg increments. Outer diameter and pressure were continuously measured and recorded over the duration of the experiments via a laser micrometer and pressure transducer, respectively. Due to the heterogeneity of the urethra, measurements were taken at proximal, middle, and distal segments along its length.10,17 Samples were assessed in both baseline (without pharmacological agents) and passive (in the presence of 3 mM EDTA) conditions.
Experiment 5: Ex Vivo Functional Analysis
Urethras isolated from control animals from each strain were mounted in our ex vivo testing system as described above, and subjected sequentially to: 0 and 8 mm Hg intralumenal pressures, Nω-nitro-L-arginine (NOSi; 100 μM; to remove endogenous nitric oxide synthase activity), phenylephrine (PE; 40 μM; to assess smooth muscle contraction), acetylcholine (ACh; 5 mM; to assess striated and smooth muscle contraction) and EDTA (3 mM; to inhibit muscular response) as previously described.8 Each stimulus was followed by a 30 min equilibration period, and the outer diameter of the middle portion of the urethra was continuously recorded at 1 Hz.
Histology
Following each experiment, urethras were placed into 4% paraformaldehyde (Fisher, Pittsburgh, PA) followed by 30% sucrose. Urethras were divided into proximal, middle, and distal portions, each portion was cut in half, and each half processed into 10 μm paraffin or frozen sections.
Paraffin sections were dewaxed, rehydrated with an ethanol series, and stained with Lillie’s-modified Masson’s trichrome, hematoxylin and eosin (H&E), and picrosirius red (PSR). Frozen sections were stained for alpha-smooth muscle actin (SMA) and fast-twitch striated muscle myosin (SKEL; Sigma–Aldrich). Sections were permeabilized with 0.1% (v/v) Triton-X-100 in phosphate buffered saline (PBS) for 15 min and then blocked in PBS containing 0.02% (w/v) bovine serum albumin (Fraction V; Sigma–Aldrich) and 0.015% (w/v) glycine (Sigma–Aldrich) for 45 min. Sections were incubated with a primary antibody against either SMA (clone 1A4; Sigma–Aldrich; 1:500 in blocking solution) or SKEL (clone NOQ7.5.4D; Sigma–Aldrich; 1:250 in blocking solution) for 1 hr in a humidification chamber at room temperature. Unbound primary antibody was removed from sections by multiple washes with PBS. Next, sections were incubated with an Alexa 488-conjugated secondary antibody (Invitrogen; 1:500 in blocking solution) for 1 hr at room temperature in a humidification chamber. All sections were counterstained with DAPI for nuclear visualization and mounted with gelvatol. Sections were imaged with a color, digital camera (QImaging, Surrey, BC, Canada) on an E800 Nikon Eclipse microscope (Nikon, Melville, NY) using NIS Elements BR 3.0 software (Nikon).
Total cross-sectional area was determined for each section using the NIS-Elements BR 3.0 software (Nikon) area tool. The luminal and external circumference were manually outlined, and the difference between those areas was taken as the total cross sectional area. The total area stained positive for either SMA or SKEL was calculated using the NIS-Elements BR 3.0 software (Nikon) thresholding tool, which calculates the total area of fluorescence within a set intensity range. The intensity range was manually adjusted for each section such that only the visible fluorescence was incorporated into the measurement. The sum of the area associated with each fluorescent element was taken as the total positive area. The average percent of total area positively stained for each antibody is reported.
Geometric Estimation and Mechanical Characteristics
Inner diameters of tested urethras were estimated for proper calculation of various mechanical parameters.10 Paraffin sections stained with Lillie’s-modified Masson’s trichrome were imaged as described above and used for thickness assessment as previously described.10
Compliance, a measure of the distensibility of a tube, was calculated for each tested segment in Experiment #4 as previously described.10
Statistics
Data are represented as the average ± standard error of the mean. Statistical comparisons were performed using a Student’s t-test. For the alpha-bungarotoxin studies, a paired Student’s t-test was used to detect the difference before and after treatment within each strain. Significance was detected at P values less than 0.05.
RESULTS
In Vivo Leak Point Pressure in Control and Injured Animals
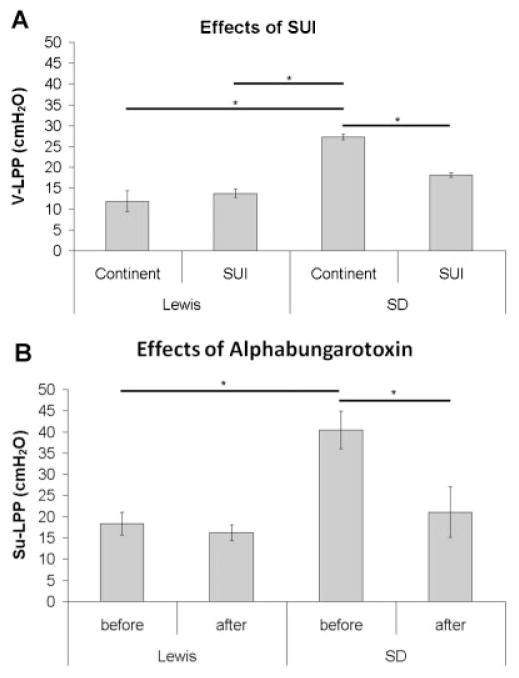
Injured SD animals had a significantly lower V-LPP than SD (n = 3) controls, but this difference was not detected in the Lewis rat (n = 3) (Fig. 1A). The control and injured Lewis rat also had a significantly lower V-LPP than the control SD rat (Fig. 1A). In the supine position, which was necessary to test the effect of alpha-bungarotoxin, the control SD rat (n = 4) maintained a significantly higher LPP than that of the Lewis rat (n = 7; Fig. 1 B). The injection of alpha-bungarotoxin (333 μg/kg, i.v.) significantly decreased Su-LPP in the SD rat, but did not alter Su-LPP in the Lewis rat (Fig. 1B).
Fig. 1.
A: Vertical leak point pressure (V-LPP) for both Lewis (n = 3) and Sprague–Dawley (n = 3) rats in continent and SUI states. B: Supine leak point pressure (Su-LPP) for both Lewis (n = 7) and Sprague–Dawley (n = 4) rats before and after injection with alpha-bungarotoxin. Data shown as average ± SEM, *P < 0.05.
Urethral Pressure During Sneeze
In the supine position the Lewis rats (n = 8) had a significantly lower urethral baseline pressure than the SD rats (n = 10) prior to and during sneeze (Fig. 2). However, there was no difference between the two strains of rats in the change in either urethral pressure response or abdominal pressure response during sneeze.
Fig. 2.
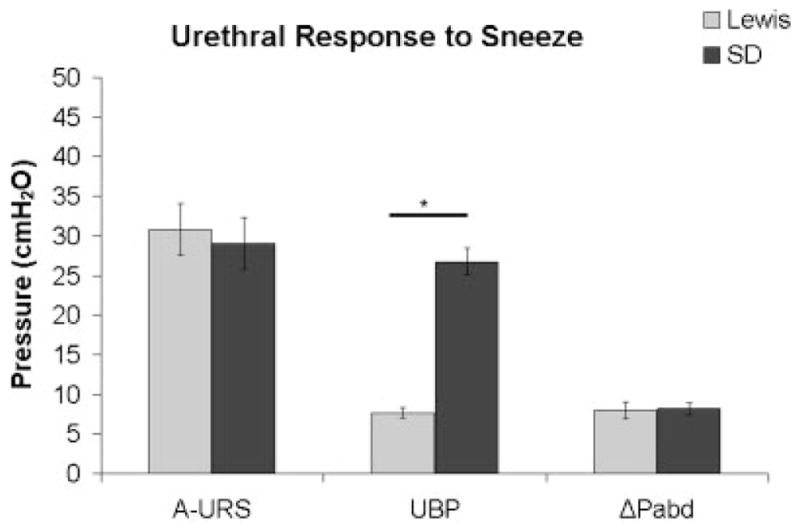
Change in urethral pressure response (increase from baseline; A-URS) during sneeze, urethral baseline pressure (UBP) and change in abdominal pressure (ΔPabd) for both Lewis (n = 8) and Sprague–Dawley (n = 10) rats. Data shown as average ± SEM, *P < 0.05.
Ex Vivo Mechanical Characteristics
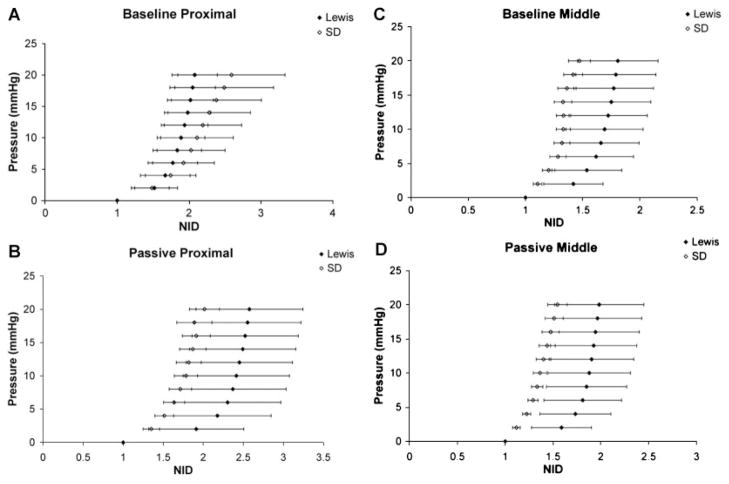
Pressure–diameter (P–D) curves obtained from ex vivo mechanical testing of the urethras in their baseline conditions are shown in Figure 3, where the normalized inner diameter (NID) is the pressurized inner diameter divided by the inner diameter at 0 mm Hg. In the proximal urethra the P–D curve for SD rats (n = 6) was shifted to the right of the curve for Lewis rats (n = 4); representing a slightly larger compliance with increasing pressure in SD rats (Fig. 3A). The Lewis middle urethra showed a larger distension at low pressures, indicating an increased compliance compared to the SD mid-urethra (Fig. 3C). There were no differences between the strains in the distal portion in the baseline state (data not shown).
Fig. 3.
A,C: Baseline and (B,D) passive pressure–diameter curves from the proximal and middle portions of continent Lewis (n = 4) and Sprague–Dawley (n = 6) rats. Data shown as average ± SEM, no significant differences were noted.
In the passive state, the P–D curves for both the proximal and middle portions of the Lewis urethra (n = 4) were shifted to the right of the curves for SD rats (n = 6; Fig. 3B,D). Calculated compliance values were greater in the Lewis urethra (n = 4) at low pressures (0–6 mm Hg) in the middle portion in the baseline state as well as in the proximal and middle portions in the passive state (data not shown). The passive, distal, SD urethra (n = 6) was more compliant than the corresponding Lewis urethra, although not significantly (data not shown).
Ex Vivo Functional Characteristics of the Middle Urethra of Control Animals
In SD urethras (n = 6) exposed to steady 8 mm Hg intralumenal pressure, a NOSi, did not elicit a consistent change in outer diameter; whereas PE and ACh both produced a contraction. Subsequent addition of EDTA completely reversed the contractile response (Fig. 4). Lewis urethras (n = 5), which exhibited a large increase in outer diameter in response to an 8 mm Hg pressure, were not as responsive to the drugs tested as the SD urethras (Fig. 4). Additionally, the Lewis urethras behaved in an unpredictable manner with the diameter tending to drift over the duration of the experiment (data not shown). Contractile responses to PE and ACh agonists in SD and Lewis urethras were compared using two methods for quantification: (1) the difference between the outer diameter at 8 mm Hg prior to any drug treatment and the outer diameter after an agonist and (2) the difference between the outer diameter immediately before and after administration of the agonists. SD urethras (n = 6) had a significantly greater contractile response than Lewis urethras (n = 5) to both PE and ACh using method 1 (data not shown) and to PE using method 2 (Fig. 4).
Fig. 4.
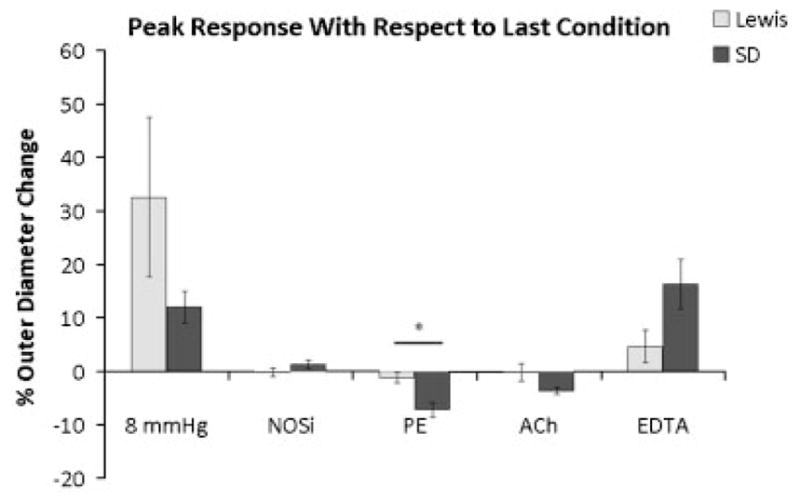
Responses of the middle urethra of continent Lewis (n = 5) and Sprague-Dawley (n = 6) rats to various agonists and antagonists (100 μM Nω-nitro-L-arginine, 40 μM phenylephrine, 5 mM acetylcholine and 3 mM EDTA). Responses are represented as percentage change in outer diameter from the outer diameter just prior to a change in pressure or administration of each drug. Data were digitized at rate of 1 Hz with 30 minute equilibrations at each condition. Values reported as average ± SEM, *P < 0.05.
Histology
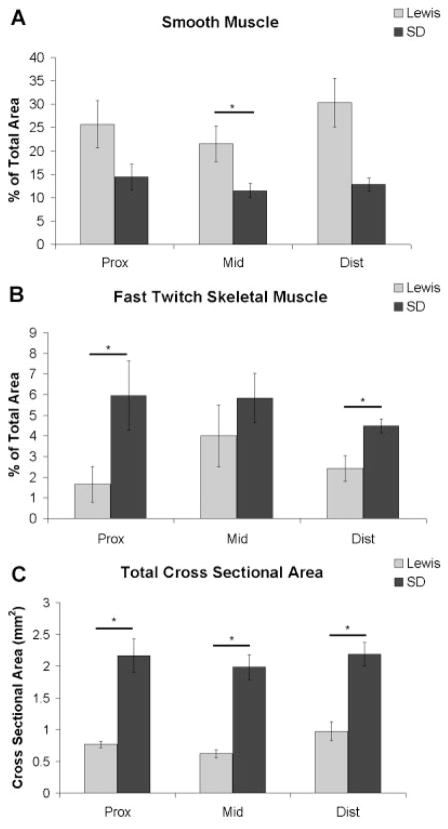
Semi-quantification revealed that all portions of the Lewis urethra (n = 7) have a higher percentage of smooth muscle than the SD urethra (n = 4–7), with the middle portion being significantly greater (Fig. 5A), while all portions of the SD urethra contain a higher percentage of fast-twitch striated muscle than the Lewis urethra, with proximal and distal portions being significantly different (Fig. 5B). It was also observed that the SD rats had a significantly larger total cross-sectional area in all three portions of the urethra, than the Lewis rat (Fig. 5C).
Fig. 5.
Percent of the total cross-sectional area staining positive for (A) smooth muscle actin. B: Fast twitch skeletal muscle, or (C) total cross-sectional area of both the Lewis (n = 7) and Sprague–Dawley (n = 4–7) urethra. Data shown as average ± SEM, *P < 0.05.
DISCUSSION
The in vivo and ex vivo experiments reported here revealed that the normal properties of the urethra and the response of the urethra to bilateral pudendal nerve transection are markedly different in the Lewis and SD rats. Lewis rats exhibited a significantly lower LPP and intraurethral baseline pressure in vivo (Fig. 2) and higher compliance (Fig. 3) at the middle urethra in vitro compared to the measurements in SD rats. In addition Lewis rat LPP was unaffected by pudendal nerve transection, whereas LPP was significantly reduced by nerve transection in SD rats (Fig. 1). Injection of alpha-bungarotoxin also had no effect on LPP in Lewis rats, while significantly decreasing the LPP in SD rats (Fig. 1), indicating a greater contribution of striated muscle to the maintenance of continence in the SD rat, compared to the Lewis rat. The SD urethra had a significantly greater contractile response than the Lewis urethra in response to both PE and ACh (Fig. 4). The SD rat urethra had a larger cross sectional area in comparison to the Lewis rat urethra at all three segmental levels and had a muscular layer that exhibited a significantly greater proportion of striated versus smooth muscle (see Fig. 5). These differences in the cross-sectional area of the urethra between strains (Fig. 5) could contribute to the differences seen in LPP (Fig. 1) since LPP values were not normalized to organ weight. However, animal age and weight were consistent for both rat strains. These functional, morphological and mechanical differences suggest that the Lewis rat has a lower level of intrinsic and neurally mediated contractile activity of the urethral muscle; and therefore may not be a useful model for SUI induced by neural injury.
To our knowledge, only one other publication11 has described the properties of the urethra in the Lewis rat. In this study, LPP was measured in normal animals and in the vaginal distension model of SUI. It was reported that vaginal distension, a transient and quickly reversible model of SUI, did result in a significant decrease in LPP, but almost the same decrease in LPP occurred in the sham group, which received placement of the balloon catheter into the vagina but not distension. In addition the continent Lewis rats had a noticeably lower LPP compared to SD rats,11 which is consistent with our other results.
In our current study, we chose a chronic model of SUI: bilateral pudendal nerve transection.16,20 The pudendal nerve innervates the external urethral striated sphincter and plays a major role in maintaining continence.23 Following nerve transection, there is a significant decrease in LPP that persists for 6 weeks20 in contrast to the rapid recovery that occurs in the vaginal distension model.16,20,24 Lewis rats were unaffected by pudendal nerve transection and had significantly lower LPP values than those in SD rats (Fig. 1), which were similar to LPP values previously published in SD rats.4,16,25 The lack of a lower LPP following pudendal nerve transection in the Lewis rat indicates that the bladder to external urethral sphincter reflex is weaker or non-existent in the Lewis rat under the conditions of the experiments. However, these results vary from reports that SUI, due to vaginal distension, significantly lowers LPP in both strains.11,26 Thus it is possible that the vaginal distension model produces a more extensive injury to smooth muscle and blood vessels as well as nerves in the urethra and therefore produces a larger change in LPP.7,25,26 Urethrolysis, which is the separation of the urethra from the surrounding tissue, has shown promise as an alternative method to produce chronic SUI in SD rats for up to 24 weeks.27–29 While this method has not been successfully reported in the Lewis rat, it may yield more promising results as a model of SUI in the Lewis rat as it is based more on urethral mobility and the smooth muscle components of the urethra without a focus on the innervation and striated muscle of the urethra.
The unaltered LPP following alpha-bungarotoxin in the Lewis rats (Fig. 1) indicates that the striated sphincter does not contribute to urethral outlet resistance or the maintenance of continence under the conditions of our experiments. This contrasts with the prominent lowering of LPP by alpha-bungarotoxin in the SD rat in the same experimental model (see also a previous report).30 The more abundant striated muscle in the SD rat (Fig. 5) is consistent with the greater contribution of the striated sphincter to LPP in this strain. In addition it is probable that the reflex activation of the striated sphincter muscle is lower in Lewis rats than in SD rats because transection of the pudendal nerve, which innervates and activates the striated sphincter, did not affect LPP in the Lewis rat but significantly reduced it in the SD rat. This could be further explored by performing combined cystometry and external urethral sphincter electromyography to compare reflex response in continent animals from both strains.31
Although LPP and UBP were lower in the continent Lewis rat compared to the SD rat (Fig. 2) the mid-urethral pressure responses to sneeze, measured by microtransducer-tipped catheters, were not different in Lewis and SD rats. This indicates that the ability of both the pudendal nerve pathway and the external urethral sphincter muscle to control the urethra are equal in the two rat strains. As reported previously, sneeze-induced urethral closure mechanisms are mediated by somatic nerve-induced reflex contractions of external urethral sphincter and pelvic floor striated muscles, and occur without intravesical pressure increases.9,16 LPP is most likely regulated by the passive properties of the urethra and/or by bladder-to-urethral reflexes evoked by distension of the bladder. Thus the striated muscle-mediated urethral continence reflex induced by strong, phasic stimuli such as sneeze seems to be well maintained, especially at the mid-urethral level, in the Lewis rat.
Because smooth muscle represents a greater proportion of the muscle mass in the Lewis rat urethra, it might be expected that smooth muscle activity would make a greater contribution to the continence mechanism. However, the lower UBP indicates a decreased activity of the urethral smooth muscle in the Lewis rat compared to SD (Fig. 2), and may explain the increased compliance of the Lewis urethra compared to the SD urethra (Fig. 3). This decreased smooth muscle activity was confirmed in our Experiment #5, which showed a significantly greater contraction to PE and a greater contraction to the combined administration of PE and ACh in the SD urethra compared to the Lewis urethra (Fig. 4). The failure to detect a significant difference in the ACh-induced contraction (Fig. 4), which might consist of both striated and smooth muscle components, could be explained by the PE-induced smooth muscle contraction occluding the smooth muscle component of ACh contraction (Fig. 4). Nevertheless, we can conclude from these data that urethral smooth muscle contractility in response to PE is significantly lower in Lewis rats compared to SD rats.
There are many possible reasons for the differences found in urethral function between these animals. First, receptor expression in the smooth and/or striated muscle cells could be lower, thereby diminishing the response to contractile agents. Second the Lewis rats may have an increased sensitivity to the anesthetic used in the in vivo experiments which would reduce reflex activity of the sphincter. However, no obvious differences in the depth of anesthesia were observed between the two strains in our experiments. Furthermore, the same dose of anesthetic was used in experiments in a different laboratory when SUI induced by vaginal distension reduced LPP in the Lewis rat.11 Although this study systematically attempted to determine the cause of differences in urethral function between these strains, further studies are needed to address these issues. A better understanding of the neural control of the urethra could be obtained by quantifying the density and type of innervation of the urethra in the two strains by using immunohistochemistry to identify cholinergic and adrenergic nerves. In addition, assessing the expression of various transmitter receptors using molecular techniques and the effects of electrical stimulation of nerves could be useful. All of our work was performed in anesthetized animals. Assessing micturition frequency and volume in a metabolic cage32 or performing awake cystometry33 might eliminate any functional differences as a result of anesthesia although the LPP measurements are technically difficult to perform in awake conditions.
CONCLUSIONS
Studies in the out-bred, all-purpose SD rat have provided considerable insight into the function of the lower urinary tract. However, as stem-cell therapies continue to develop for urethral dysfunctions, an in-bred strain with less genetic variation may be more suitable to use for initial validation to avoid rejection issues. The work reported here has provided strong evidence that functional and mechanical characteristics of the urethra are markedly different in Lewis and SD rats. We have also shown that pudendal nerve transection may not be an appropriate model of chronic SUI in the Lewis rat. Taken together, these results indicate that the Lewis rat may not be a suitable model for testing cell-based therapies in a chronic model of SUI via pudendal nerve transection, however an alternative method such as urethrolysis should be further explored.
Acknowledgments
The authors wish to thank the members of the Vascular Bio-engineering Laboratory at the University of Pittsburgh. Funding for this work was provided by NIH R21EB6318-2, T32EB001026 and DK067226, and the University of Pittsburgh Provost’s Development Fund.
Footnotes
Conflict of interest: none.
References
- 1.Nygaard IE, Heit M. Stress urinary incontinence. Obstet Gynecol. 2004;104:607–20. doi: 10.1097/01.AOG.0000137874.84862.94. [DOI] [PubMed] [Google Scholar]
- 2.Tsui KP, Ng SC, Tee YT, et al. Complications of synthetic graft materials used in suburethral sling procedures. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:165–7. doi: 10.1007/s00192-004-1229-6. [DOI] [PubMed] [Google Scholar]
- 3.Aboushwareb T, Atala A. Stem cells in urology. Nat Clin Pract Urol. 2008;5:621–31. doi: 10.1038/ncpuro1228. [DOI] [PubMed] [Google Scholar]
- 4.Cannon TW, Lee JY, Somogyi G, et al. Improved sphincter contractility after allogenic muscle-derived progenitor cell injection into the denervated rat urethra. Urology. 2003;62:958–63. doi: 10.1016/s0090-4295(03)00679-4. [DOI] [PubMed] [Google Scholar]
- 5.Lee JY, Cannon TW, Pruchnic R, et al. The effects of periurethral muscle-derived stem cell injection on leak point pressure in a rat model of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2003;14:31–7. doi: 10.1007/s00192-002-1004-5. discussion 7. [DOI] [PubMed] [Google Scholar]
- 6.Strasser H, Berjukow S, Marksteiner R, et al. Stem cell therapy for urinary stress incontinence. Exp Gerontol. 2004;39:1259–65. doi: 10.1016/j.exger.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Damaser MS, Whitbeck C, Chichester P, et al. Effect of vaginal distension on blood flow and hypoxia of urogenital organs of the female rat. J Appl Physiol. 2005;98:1884–90. doi: 10.1152/japplphysiol.01071.2004. [DOI] [PubMed] [Google Scholar]
- 8.Jankowski RJ, Prantil RL, Chancellor MB, et al. Biomechanical characterization of the urethral musculature. Am J Physiol Renal Physiol. 2006;290:F1127–34. doi: 10.1152/ajprenal.00330.2005. [DOI] [PubMed] [Google Scholar]
- 9.Miyazato M, Kaiho Y, Kamo I, et al. Effect of duloxetine, a norepinephrine and serotonin reuptake inhibitor, on sneeze-induced urethral continence re-flex in rats. Am J Physiol Renal Physiol. 2008;295:F264–71. doi: 10.1152/ajprenal.90241.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prantil RL, Jankowski RJ, Kaiho Y, et al. Ex vivo biomechanical properties of the female urethra in a rat model of birth trauma. Am J Physiol Renal Physiol. 2007;292:F1229–37. doi: 10.1152/ajprenal.00292.2006. [DOI] [PubMed] [Google Scholar]
- 11.Woo LL, Hijaz A, Pan HQ, et al. Simulated childbirth injuries in an inbred rat strain. Neurourol Urodyn. 2009;28:356–61. doi: 10.1002/nau.20644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woo LL, Hijaz A, Kuang M, et al. Over expression of stem cell homing cytokines in urogenital organs following vaginal distention. J Urol. 2007;177:1568–72. doi: 10.1016/j.juro.2006.11.047. [DOI] [PubMed] [Google Scholar]
- 13.Hlavacova N, Bakos J, Jezova D. Differences in home cage behavior and endocrine parameters in rats of four strains. Endocr Regul. 2006;40:113–8. [PubMed] [Google Scholar]
- 14.Kaminsky O, Klenerova V, Stohr J, et al. Differences in the behaviour of Sprague–Dawley and Lewis rats during repeated passive avoidance procedure: Effect of amphetamine. Pharmacol Res. 2001;44:117–22. doi: 10.1006/phrs.2001.0848. [DOI] [PubMed] [Google Scholar]
- 15.Pill J, Volkl A, Hartig F, et al. Differences in the response of Sprague–Dawley and Lewis rats to bezafibrate: The hypolipidemic effect and the induction of peroxisomal enzymes. Arch Toxicol. 1992;66:327–33. doi: 10.1007/BF01973627. [DOI] [PubMed] [Google Scholar]
- 16.Kamo I, Torimoto K, Chancellor MB, et al. Urethral closure mechanisms under sneeze-induced stress condition in rats: A new animal model for evaluation of stress urinary incontinence. Am J Physiol Regul Integr Comp Physiol. 2003;285:R356–65. doi: 10.1152/ajpregu.00010.2003. [DOI] [PubMed] [Google Scholar]
- 17.Jankowski RJ, Prantil RL, Fraser MO, et al. Development of an experimental system for the study of urethral biomechanical function. Am J Physiol Renal Physiol. 2004;286:F225–32. doi: 10.1152/ajprenal.00126.2003. [DOI] [PubMed] [Google Scholar]
- 18.Manzo J, Vazquez MI, Cruz MR, et al. Fertility ratio in male rats: Effects after denervation of two pelvic floor muscles. Physiol Behav. 2000;68:611–8. doi: 10.1016/s0031-9384(99)00219-x. [DOI] [PubMed] [Google Scholar]
- 19.Hijaz A, Daneshgari F, Sievert KD, et al. Animal models of female stress urinary incontinence. J Urol. 2008;179:2103–10. doi: 10.1016/j.juro.2008.01.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng CW, Chen JJ, Chang HY, et al. External urethral sphincter activity in a rat model of pudendal nerve injury. Neurourol Urodyn. 2006;25:388–96. doi: 10.1002/nau.20229. [DOI] [PubMed] [Google Scholar]
- 21.Conway DA, Kamo I, Yoshimura N, et al. Comparison of leak point pressure methods in an animal model of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:359–63. doi: 10.1007/s00192-004-1263-4. [DOI] [PubMed] [Google Scholar]
- 22.Yoshiyama M, deGroat WC, Fraser MO. Influences of external urethral sphincter relaxation induced by alpha-bungarotoxin a neuromuscular junction blocking agent on voiding dysfunction in the rat with spinal cord injury. Urology. 2000;55:956–60. doi: 10.1016/s0090-4295(00)00474-x. [DOI] [PubMed] [Google Scholar]
- 23.Ahn H, Lin DL, Esparza N, et al. Short-term timecourse of bilateral pudendal nerve injury on leak-point pressure in female rats. J Rehabil Res Dev. 2005;42:109–14. [PubMed] [Google Scholar]
- 24.Bakircioglu ME, Sievert KD, Lau A, et al. The effect of pregnancy delivery on the function ultrastructure of the rat bladder urethra. BJU Int. 2000;85:350–61. doi: 10.1046/j.1464-410x.2000.00435.x. [DOI] [PubMed] [Google Scholar]
- 25.Damaser MS, Broxton-King C, Ferguson C, et al. Functional and neuroanatomical effects of vaginal distention and pudendal nerve crush in the female rat. J Urol. 2003;170:1027–31. doi: 10.1097/01.ju.0000079492.09716.43. [DOI] [PubMed] [Google Scholar]
- 26.Cannon TW, Wojcik EM, Ferguson CL, et al. Effects of vaginal distension on urethral anatomy function. BJU Int. 2002;90:403–7. doi: 10.1046/j.1464-410x.2002.02918.x. [DOI] [PubMed] [Google Scholar]
- 27.Kinebuchi Y, Aizawa N, Imamura T, et al. Autologous bone-marrow-derived mesenchymal stem cell transplantation into injured rat urethral sphincter. Int J Urol. 2010;17:359–68. doi: 10.1111/j.1442-2042.2010.02471.x. [DOI] [PubMed] [Google Scholar]
- 28.Phull H, Salkini M, Escobar C, et al. The role of angiotensin II in stress urinary incontinence: A rat model. Neurourol Urodyn. 2007;26:81–8. doi: 10.1002/nau.20339. discussion 9. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez LV, Chen S, Jack GS, et al. New objective measures to quantify stress urinary incontinence in a novel durable animal model of intrinsic sphincter deficiency. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1332–8. doi: 10.1152/ajpregu.00760.2004. [DOI] [PubMed] [Google Scholar]
- 30.Torimoto K, Hirao Y, Matsuyoshi H, et al. alpha1-Adrenergic mechanism in diabetic urethral dysfunction in rats. J Urol. 2005;173:1027–32. doi: 10.1097/01.ju.0000146268.45662.36. [DOI] [PubMed] [Google Scholar]
- 31.Chang HY, Cheng CL, Chen JJ, et al. Serotonergic drugs spinal cord transections indicate that different spinal circuits are involved in external urethral sphincter activity in rats. Am J Physiol Renal Physiol. 2007;292:F1044–53. doi: 10.1152/ajprenal.00175.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chuang YC, Fraser MO, Yu Y, et al. Analysis of the afferent limb of the vesicovascular reflex using neurotoxins, resiniferatoxin, capsaicin. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1302–10. doi: 10.1152/ajpregu.2001.281.4.R1302. [DOI] [PubMed] [Google Scholar]
- 33.Ogawa T, Sasatomi K, Hiragata S, et al. Therapeutic effects of endothelin-A receptor antagonist on bladder overactivity in rats with chronic spinal cord injury. Urology. 2008;71:341–5. doi: 10.1016/j.urology.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]