Abstract
Objective: To assess clinical characteristics and treatment outcomes of patients with vascular injuries. Materials and methods: We retrospectively reviewed the medical records of 378 consecutive patients with vascular injuries treated at our hospital from January 2000 to December 2012. Basic characteristics (such as gender; age; cause, site, and type of injury; and concomitant injuries) were recorded, and efficacy was compared between treatments for same type/site injuries. Results: Vascular injuries occurred most frequently in patients aged 19-50 years, secondary to trauma, and in extremities (73%, 63%, and 84% of cases, respectively), particularly lower ones. Amputation was more common in popliteal artery injury (52.6% of cases); overall, inappropriate diagnosis or treatment or poor vascular anastomosis led to amputation in 17 cases. Extremity vascular patency, while comparable at 12 months, was significantly lower at 24 months after artificial blood-vessel implantation than autogenous vein grafting. Treatment of femoral artery pseudoaneurysm secondary to drug abuse yielded similar amputation but significantly lower limb ischemia rates after bypass graft surgery than arterial ligation. Conclusion: Initial and temporal outcome differentiation reported here for treatments for peripheral vascular injuries according to type and site underscores the importance of further defining treatment choice consequences, particularly long term ones because most affected patients are aged 19-50 years old.
Keywords: Vascular injury, epidemiology, interventional therapy, open surgery
Introduction
Vascular injury (especially in a major artery) is a common cause of disability and mortality, accounting in one report [1] for 20%-26% of trauma induced death. Repair and reconstruction of vascular injuries are determined according to type and site of injury, concomitant injury, presence of collateral circulation in the extremities, and available techniques and materials. Major or middle arterial injury is usually treated with covered stents or artificial blood vessels, while for middle to small blood vessel injury, autologous vein transplantation is recommended. To further understand the characteristics of vascular injuries and the impact of interventions used, we have retrospectively reviewed patients with vascular injuries treated at a Chinese center.
Material and methods
We retrospectively reviewed data on 378 consecutive patients with vascular injuries who were treated in our hospital from January 2000 to December 2012. Clinical information (such as gender, age, cause, site and type of injury and concomitant injuries) were collected and described with percentages. Therapeutic efficacy of treatment strategies for injuries of the same type and at the same site was compared using chi square test. A value of P < 0.05 was considered statistically significant.
Results
Patient and vascular injury characteristics
A total of 378 patients (309 men and 69 women; age range: 11-78 years) were recruited into the present study (Table 1). Cause, type and site of vascular injuries, therapeutic strategies and prognosis are shown in Table 2. Vascular injury was secondary to trauma in 238 patients, drug abuse in 88, and iatrogenic in 52 (accidental injury to the vessels in 12 and secondary to interventional therapy in 40). In terms of injury site, 9, 51, 88 and 230 patients had neck, trunk, and upper and lower extremity vascular injury, respectively. Injury type was classified as contusion with arterial thrombosis in 82 patients; complete vascular rupture in 96; partial vascular rupture in 200 (including 89 with drug abuse induced infectious pseudoaneurysm, and 46 who developed deep vein thrombosis); traumatic pseudoaneurysm in 90; and traumatic arteriovenous fistula in 22. Con- comitant injuries included subclavian vascular injury with hemopneumothorax in 3 patients; clavicular fracture in 7; intra-abdominal vascular injury with concomitant injury to the liver, spleen and intestine in 8; pelvic fracture in 7; and extremity vascular injuries with concomitant tissue injury, fracture and joint dislocation in 67. Therapeutic strategies were as follows: (1) covered stent: 3 patients with thoracic aortic rupture, 2 with abdominal aortic pseudoaneurysm (Figure 1), 13 with subclavian artery aneurysm (Figure 2), 4 with axillary artery injury, 3 with iliac artery pseudoaneurysm, and 9 with femoral artery pseudoaneurysm; (2) coil embolization: 3 patients with superior mesenteric artery pseudoaneurysm (Figure 3), 4 with internal iliac artery aneurysm and 3 with deep femoral artery aneurysm; (3) arterial ligation: 80 patients (ulnar/radial arterial injury in 24 and infectious femoral artery pseudoaneurysm in 56); (4) amputation: 23 patients (upper extremity in 3 and lower extremity in 20); and (5) vascular anastomosis: 229 (repair of arterial sidewall puncture in 80, end-end anastomosis in 66, autogenous vein grafting in 46 (Figures 4, 5) and artificial blood vessel implantation in 37).
Table 1.
Cause of vascular injury by age group
| Age (yr) | Trauma (%) | Drug abuse (%) | Iatrogenic injury (%) | Total (%) |
|---|---|---|---|---|
| < 18 | 15 (3.97) | 3 (0.79) | 2 (0.53) | 20 (5.29) |
| 19-30 | 60 (15.87) | 30 (7.94) | 2 (0.53) | 92 (24.34) |
| 31-40 | 67 (17.72) | 43 (11.38) | 4 (1.06) | 114 (30.16) |
| 41-50 | 52 (13.76) | 7 (1.85) | 11 (2.91) | 70 (18.52) |
| 51-60 | 28 (7.41) | 5 (1.32) | 15 (3.97) | 48 (12.7) |
| > 61 | 16 (4.23) | 0 (0) | 18 (4.76) | 34 (8.99) |
Footnote: data are expressed as n (%).
Table 2.
Causes, types, treatments and outcomes of vascular injury in the 378 patients studied
| Site | Causes | Types | Therapies | Prognosis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Trauma | Iatrogenic injury | Drug abuse | Contusion | Partial rupture | Complete traverse | Open surgery | Interventional therapy | Death | Amputation | Success | |
| Neck | |||||||||||
| External carotid artery | 3 | 1 | 2 | 2 | 4 | 4 | |||||
| Common carotid artery | 2 | 3 | 4 | 1 | 5 | 5 | |||||
| Trunk | |||||||||||
| Subclavian artery | 14 | 4 | 3 | 11 | 4 | 5 | 13 | 2 | 16 | ||
| Descending aorta | 3 | 3 | 3 | 3 | |||||||
| Abdominal aorta | 5 | 5 | 3 | 2 | 3 | 2 | |||||
| Portal vein | 3 | 3 | 3 | 3 | |||||||
| Vena cava | 3 | 4 | 7 | 7 | 4 | 3 | |||||
| Superior mesenteric artery | 3 | 3 | 3 | 3 | |||||||
| Iliac artery | 9 | 3 | 4 | 8 | 5 | 7 | 12 | ||||
| Upper extremity | |||||||||||
| Axillary artery | 15 | 8 | 7 | 11 | 4 | 15 | |||||
| Brachial artery | 47 | 2 | 12 | 19 | 18 | 49 | 3 | 46 | |||
| Ulnar/radial artery | 19 | 5 | 14 | 10 | 24 | 24 | |||||
| Lower extremity | |||||||||||
| Femoral artery | 77 | 27 | 88 | 44 | 103 | 45 | 180 | 12 | 2 | 12 | 178 |
| Popliteal artery | 38 | 11 | 11 | 16 | 36 | 2 | 20 | 18 | |||
Figure 1.

A. Abdominal aortic pseudoaneurysm; B. Implantation of covered stent to isolate the pseudoaneurysm.
Figure 2.

A. Subclavian artery rupture; B Implantation of covered stent.
Figure 3.

A. Superior mesenteric artery pseudoaneurysm; B. Endovascular coil embolization and covered stent implantation.
Figure 4.

A. Restenosis of right femoral artery after artificial vascular grafting; B. Autologous saphenous vein grafting.
Figure 5.

A. Traumatic popliteal aneurysm; B. Autologous vein grafting after removal of popliteal aneurysm.
Age, causes and sites of vascular injury
Patients aged 19-50 years accounted for the highest proportion (73.02%) of vascular injuries. Iatrogenic injury predominantly occurred in patients older than 40 years (Figure 6). Vascular injury was trauma related in 238 patients (62.96%), drug abuse related in 88 (23.28%), and iatrogenic in 52 (13.76%). The proportion of trauma and iatrogenic injury increased over time while that related to intravenous drug use remained unchanged (Figure 7). Vascular injuries of extremities accounted for 84.13% (318/378) of cases. In addition, 230 patients (60.85%) and 88(23.28%) had vascular injury of lower and upper extremity, respectively.
Figure 6.

Causes of vascular injury in 378 patients by age group.
Figure 7.
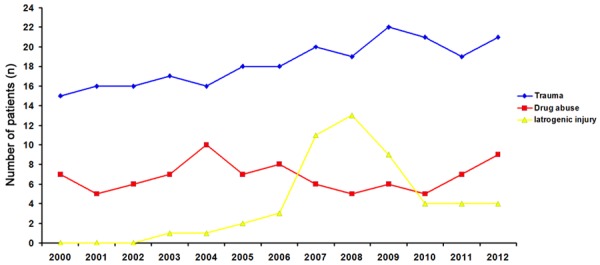
Number of patients with vascular injury in each year.
Death and amputation
In this study, 14 patients died, 12 from chest or abdominal vascular injury (10 hemorrhagic shock and 2 ARDS or MODS within 48 h after surgery) and 2 from femoral artery injury (infectious pseudoaneurysm rupture secondary to intravenous drug use with simultaneous infection, hemorrhagic shock and poor systemic condition). Difference in mortality rate between chest or abdominal and extremity vascular injury was statistically significant (P < 0.001).
Thirty-five patients underwent amputation: 3 in upper and 32 in lower extremities, with proportion of patients being significantly higher in the latter group and in cases of popliteal artery injury (20/38, 52.63%). Overall, 17 patients underwent amputation secondary to misdiagnosis, inappropriate treatment or poor vascular anastomosis.
Follow-up after interventional therapy or open surgery
Vascular patency during 2-24-month follow-up was assessed via telephone or hospital visit. Of all studied patients, 297 patients underwent follow-up (90.27%), and 32 patients were lost to follow-up after favorable recovery from ligation of middle to small arteries or direct vascular repair (9.73%).
For patients receiving interventional therapy, there were 2 cases of stent thrombosis of the femoral artery within 1 month post procedure, with blood flow reestablished after thrombolytic therapy. One patient underwent a second procedure because of stent displacement and aneurysm rupture. Stent exposure was found in 1 patient; the stent was removed followed by femoral artery ligation. Intra-stent stenosis or recurrence of vascular injury was not observed in the remaining patients.
Restenosis occurred in 16 patients who underwent vascular grafting of the femoral artery: 5 received autogenous vein grafting and 11 artificial vascular grafting (2 had concomitant infection). Patency rate 12 months post procedure was comparable between patients treated with autogenous vein grafting and artificial vascular grafting (81.08% vs. 89.13%, P > 0.05). However, 24 months post procedure, a marked difference was noted in the patency rate between these patients (70.27% vs. 89.13%, P < 0.05). The patency rate of patients receiving artificial vascular grafting was significantly lower than that of patients undergoing autogenous vein grafting (Table 3).
Table 3.
Patency rate of 83 patients treated for vascular injury of lower extremity with vascular grafting
| Patency rate | Autogenous vein grafting | Artificial vascular grafting | P |
|---|---|---|---|
| 12 months | 41 (89.13) | 30 (81.08) | > 0.05 |
| 24 months | 41 (89.13) | 26 (70.27) | < 0.05 |
Footnote: data are expressed as n (%).
Of 88 patients with femoral artery pseudoaneurysm secondary to intravenous drug use, artificial vascular bypass grafting and covered stent implantation were performed in 32 patients; artificial vascular infection was noted in 9 patients within 1 week after surgery, and stent exposure in 1 patient (Figure 8). In the latter 10 patients, stent was removed and femoral artery ligated; of these, 5 patients underwent amputation, 4 developed intermittent claudication. Smooth blood flow and no symptoms of lower extremity ischemia were observed in the remaining patients. In addition, 56 patients received ligation of external iliac artery or femoral artery and removal of aneurysm, 2 patients died of hemorrhagic shock and infection, 7 patients received high-level amputation due to ischemic gangrene and 47 received arterial ligation (21 patients developed intermittent claudication and low skin temperature although there were no symptoms of extremity ischemia). Despite comparable amputation rate (P > 0.05), incidence of extremity ischemia in patients treated with arterial ligation was markedly higher in patients with arterial ligation than in those treated with vascular bypass grafting (P < 0.01) (Table 4).
Figure 8.

Covered stent exposed at 3 months after stent implantation for femoral artery pseudoaneurysm secondary to intravenous drug use.
Table 4.
Amputation rate and incidence of extremity ischemia in 86 patients with postprocedural infectious femoral artery pseudoaneurysm
| Vascular bypass grafting | Arterial ligation | P | |
|---|---|---|---|
| Amputation rate | 5 (15.62) | 7 (12.5) | > 0.05 |
| Incidence of extremity ischemia | 4 (14.81) | 21 (44.68) | < 0.01 |
Footnote: data are expressed as n (%).
Discussion
In the present study, trauma was the major cause of vascular injury, more commonly in males than females and most likely secondary to increased chance of trauma exposure driven by profession and/or lifestyle (driving and drinking). Patients aged 19-50 had the highest proportion of vascular injuries, also most likely secondary to exposure to high-risk activities. The latter finding is consistent with that of Perkins et al [2] who reported that patients aged 22-43 years were more likely to suffer trauma-induced vascular injury. In this study, intravenous drug use was another cause of vascular injury in predominantly males aged 19-40 years.
Johnson et al [3-7] reported a 4-13% incidence of iatrogenic vascular injury. In this study, iatrogenic vascular injury mainly occurred in patients older than 40 years, which might be attributed to older age; clinicians being unfamiliar with anatomic structure; accidental carotid artery dissection during radical surgery for parotid tumor; extent of radical surgery for advanced malignancy [8-10] which may invade or encapsulate important blood vessels; and common use of clinical techniques (such as diagnostic puncture and interventional therapy) likely to increase vascular injury incidence. Iatrogenic vascular injury incidence increased from 2007 to 2009, consistent with the learning curve for interventional therapy.
The higher mortality rate seen in this study among patients with chest or abdominal injury (23.53%) as compared to those with vascular injury of extremities is consistent with the reported range of 23-60% in other studies [11,12], and might be explained by chest or abdominal injury usually being: 1. associated with damage to major blood vessels, possibly resulting in death soon after injury; 2. hard to identify; and 3. associated with delayed hemostasis [13] and major surgery with greater likelihood of complications.
Previous studies [14,15] have also reported a predominance of extremity, lower more common than upper, vascular injury. This study found preponderance of amputation among lower as compared to upper extremity vascular injury, particularly popliteal artery injury. Anatomically, the popliteal artery is a terminal artery with poor collateral circulation and small diameter which render vascular anastomosis challenging. As also documented in our study, many factors may lead to amputation. Extremity vascular injury with concomitant bone fracture, nerve injury and massive tissue injury makes revascularization impossible and forces amputation with its high degree of disability [16,17]. Incorrect emergent hemostasis and poor vascular anastomosis may also lead to amputation. Incorrect rubber tourniquet use may result in prolonged ischemia in turn leading to extremity edema and possibly tissue necrosis. In our study, although vascular anastomosis had been performed in other hospitals and a second surgery was successfully performed when there was no apparent blood flow to the distal end, prolonged ischemia would ultimately result in limb necrosis, making amputation mandatory. The latter evolution would suggest the need for a second surgical exploration when distal arterial pulse is not palpable or tissue edema deteriorates after revascularization.
Delayed diagnosis is another important cause of amputation. Investigators [18-20] have proposed that delayed diagnosis accounts for 85% of amputations. In our study, delayed diagnosis of vascular injury was noted in 3 patients with knee dislocation and popliteal artery injury. When these patients were transferred into our hospital, limb necrosis was present. In addition, for patients with hemorrhagic shock, clinicians should pay attention to vital signs and the signs of focal ischemia of the extremities. Timely surgical exploration is particularly required in patients with comminuted fracture, open fractures, segmental diaphyseal fractures, or dislocations due to blunt trauma or crush injury [21]. However, the order of fracture fixation and vascular repair remains controversial [22,23]. We favor Hossny et al’s [24] (transient shunt for vascular injury with concomitant bone fracture reduces ischemia time of extremity) proposal to promptly perform surgical exploration to control bleeding followed by repair of blood vessels and finally fracture fixation.
Delayed treatment of post-operative complications might also lead to amputation. After revascularization, there might be ischemia/reperfusion injury, bone compartment syndrome and thrombosis, which may cause disordered blood flow and limb necrosis requiring amputation [25]. In the present study, amputation was performed in 6 patients who developed bone compartment syndrome after revascularization resulting in limb necrosis due to ischemia. We agree with the view proposed in a recent study that prophylactic bone compartment decompression is helpful to improve the outcome and shorten the hospital stay when the arterial injury is treated at > 8 h after injury [26].
Vascular injury is usually a component of severe or multiple injuries. Thus, it is necessary to perform step-by-step therapy according to the principles for damage control surgery (DCS). Bleeding should be promptly controlled and vascular repair done after resuscitation in the ICU.
Endovascular interventional therapy has been the treatment of choice among vascular surgeons for injury to the thoracoabdominal aorta, subclavian artery, superior mesenteric artery and iliac vessels. In particular, covered stent implantation and coil embolization maximize procedural success. Ferreira et al [27-29] reported that patients receiving interventional therapy had higher survival and lower complication rates. The present study also documented low complication rates for endovascular interventional therapy and open surgery. Interventional therapy, with its limited invasiveness, short procedural time, favorable safety and acceptable effectiveness, is preferred for patients with hard-to-expose injury to blood vessels.
Prior to endovascular interventional therapy, patients should be evaluated by clinical and imaging examination [30] to assess for presence of blood vessel rupture (pseudoaneurysm, arteriovenous fistula) or dissection and distal end outlet, and for patient tolerance to the procedure. During endovascular interventional therapy, a femoral artery approach is usually used with length of covered stent 2-3 cm longer than that of the vascular crevasse. Longer stents are more likely to be associated with stent thrombosis while shorter stents may cause internal leakage. Stent diameter should be slightly larger than that of the injured artery (about 10-20% larger). Obstruction of major branches should be avoided during stent implantation.
Clinicians should be aware if interventional therapy complications. In the present study, postprocedural complications included stent thrombosis (n = 2), stent exposure (n = 1) and stent displacement (n = 1). Thus, stent implantation for the treatment of vascular injury still has complications, especially in juvenile patients and those with injury to middle to small blood vessels or blood vessels spanning the joint. In addition, selection of endovascular interventional therapy should be carefully considered [31,32]. Endovascular stenting is unfeasible when there is longitudinal or transverse injury to the blood vessels, no anchor region around the injured site, or presence of important branches.
In the present experience, vascular repair was performed with direct suture, vein patch repair and end-end anastomosis. For patients with injured vessel > 2 cm, autologous saphenous vein grafting was done in 46 patients, and artificial vascular grafting in 37 patients, aiming to avoid anastomotic tension after vascular anastomosis. Although at 12 months after surgery, patency rate was comparable between patients treated with autologous vein grafting and artificial vascular grafting, a marked difference was noted at 24 months after surgery. In this study, a male patient aged 23 years underwent ipsilateral autogenous vein grafting for trauma-induced femoral artery injury which had occurred 10 years earlier. One year before, artificial vascular grafting was performed for venous aneurysm at another hospital. Vascular restenosis occurred at 1 year after surgery, and was treated with saphenous vein grafting. This patient was followed up for 2 years, and vascular patency was noted. This case is consistent with the conclusion that long-term patency rate after artificial vascular grafting was lower than that after autologous vein grafting [33]. Thus, autologous vein grafting is preferred for patients with vascular injury of extremities requiring vascular grafting [34,35].
There remains controversy on vascular grafting of femoral artery pseudoaneurysm secondary to intravenous drug use. When the pre-operative evaluation shows poor collateral circulation and an autogenous vein is unavailable, artificial vascular bypass grafting or covered stent implantation is preferred. After arterial ligation, artificial vascular bypass grafting and covered stent implantation were associated with comparable amputation rate, while incidence of extremity ischemia was lower after vascular bypass grafting. Thus, for patients with femoral artery pseudoaneurysm secondary to intravenous drug use, arterial ligation is feasible [36], however, incidence of post-operative extremity ischemia is high and patients are at high risk for amputation.
Taken together, major or middle vascular injury (such as thoracoabdominal aorta, subclavian artery and superior/inferior vena cava) is a major cause of death. Incidence of middle to small arterial injury of extremities is high, and amputation rate after surgery for lower extremity vascular injury (especially popliteal artery injury) is higher than that after surgery for upper extremity vascular injury. Vascular repair and reconstruction should be done according to the site and type of vascular injury, concomitant injuries, collateral circulation, available therapeutic techniques and materials. For major to middle vascular injury, covered stent implantation or artificial vascular grafting is preferred; for middle to small vascular injury, autogenous vein grafting is preferred. Professional vascular surgical techniques and experience are crucial to reduce mortality and amputation rate.
This study is limited by its retrospective nature and by only reflecting experience at one Chinese center. Further studies are warranted to confirm the hypotheses raised by the observations described.
Disclosure of conflict of interest
None.
References
- 1.Loh SA, Rockman CB, Chung C, Maldonado TS, Adelman MA, Cayne NS, Pachter HL, Mussa FF. Existing trauma and critical care scoring systems underestimate mortality among vascular trauma patients. J Vasc Surg. 2011;53:359–366. doi: 10.1016/j.jvs.2010.08.074. [DOI] [PubMed] [Google Scholar]
- 2.Perkins ZB, De’Ath HD, Aylwin C, Brohi K, Walsh M, Tai NR. Epidemiology and outcome of vascular trauma at a British Major Trauma Centre. Eur J Vasc Endovasc Surg. 2012;44:203–209. doi: 10.1016/j.ejvs.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Johnson CA. Endovascular management of peripheral vascular trauma. Semin Intervent Radiol. 2010;27:38–43. doi: 10.1055/s-0030-1247887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kouvelos GN, Papas NK, Arnaoutoglou EM, Papadopoulos GS, Matsagkas MI. Endovascular repair of profunda femoral artery false aneurysms using covered stents. Vascular. 2011;19:51–54. doi: 10.1258/vasc.2010.cr0224. [DOI] [PubMed] [Google Scholar]
- 5.Momiy J, Vasquez J. Iatrogenic vertebral artery pseudoaneurysm due to central venous catheterization. Proc (Bayl Univ Med Cent) 2011;24:96–100. doi: 10.1080/08998280.2011.11928692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee BK, Jeung KW, Min YI, Heo T, Ryu HH, Jeong IS. A case of iatrogenic ilio-iliac arteriovenous fistula after percutaneous cardiopulmonary support in a patient with a tortuous iliac artery. J Artif Organs. 2011;14:151–154. doi: 10.1007/s10047-010-0545-5. [DOI] [PubMed] [Google Scholar]
- 7.de Troia A, Tecchio T, Azzarone M, Biasi L, Piazza P, Franco Salcuni P. Endovascular treatment of an innominate artery iatrogenic pseudoaneurysm following subclavian vein catheterization. Vasc Endovascular Surg. 2011;45:78–82. doi: 10.1177/1538574410388308. [DOI] [PubMed] [Google Scholar]
- 8.Mandolfino T, Canciglia A, Taranto F, D’Alfonso M, Tonante A, Mamo M, Sturniolo G. Outcome of iatrogenic injuries to the abdominal and pelvic veins. Surg Today. 2008;38:1009–1012. doi: 10.1007/s00595-008-3793-8. [DOI] [PubMed] [Google Scholar]
- 9.Clarke B, McCluggage WG. Iatrogenic lesions and artefacts in gynaecological pathology. J Clin Pathol. 2009;62:104–112. doi: 10.1136/jcp.2008.061424. [DOI] [PubMed] [Google Scholar]
- 10.Anaya-Ayala JE, Charlton-Ouw KM, Kaiser CL, Peden EK. Successful emergency endovascular treatment for superior vena cava injury. Ann Vasc Surg. 2009;23:139–141. doi: 10.1016/j.avsg.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 11.Barmparas G, Inaba K, Talving P, David JS, Lam L, Plurad D, Green D, Demetriades D. Pediatric vs adult vascular trauma: a National Trauma Databank review. J Pediatr Surg. 2010;45:1404–1412. doi: 10.1016/j.jpedsurg.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 12.Steenburg SD, Ravenel JG, Ikonomidis JS, Schonholz C, Reeves S. Acute traumatic aortic injury: imaging evaluation and management. Radiology. 2008;248:748–762. doi: 10.1148/radiol.2483071416. [DOI] [PubMed] [Google Scholar]
- 13.Kelly JF, Ritenour AE, McLaughlin DF, Bagg KA, Apodaca AN, Mallak CT, Pearse L, Lawnick MM, Champion HR, Wade CE, Holcomb JB. Injury severity and causes of death from Operation Iraqi Freedom and Operation Enduring Freedom: 2003-2004 versus 2006. J Trauma. 2008;64:S21–26. doi: 10.1097/TA.0b013e318160b9fb. discussion S26-27. [DOI] [PubMed] [Google Scholar]
- 14.Fowler J, Macintyre N, Rehman S, Gaughan JP, Leslie S. The importance of surgical sequence in the treatment of lower extremity injuries with concomitant vascular injury: A meta-analysis. Injury. 2009;40:72–76. doi: 10.1016/j.injury.2008.08.043. [DOI] [PubMed] [Google Scholar]
- 15.Franz RW, Goodwin RB, Hartman JF, Wright ML. Management of upper extremity arterial injuries at an urban level I trauma center. Ann Vasc Surg. 2009;23:8–16. doi: 10.1016/j.avsg.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 16.Pape HC, Probst C, Lohse R, Zelle BA, Panzica M, Stalp M, Steel JL, Duhme HM, Pfeifer R, Krettek C, Sittaro NA. Predictors of late clinical outcome following orthopedic injuries after multiple trauma. J Trauma. 2010;69:1243–1251. doi: 10.1097/TA.0b013e3181ce1fa1. [DOI] [PubMed] [Google Scholar]
- 17.Feliciano DV, Moore FA, Moore EE, West MA, Davis JW, Cocanour CS, Kozar RA, McIntyre RC Jr. Evaluation and management of peripheral vascular injury. Part 1. Western Trauma Association/critical decisions in trauma. J Trauma. 2011;70:1551–1556. doi: 10.1097/TA.0b013e31821b5bdd. [DOI] [PubMed] [Google Scholar]
- 18.Green NE, Allen BL. Vascular injuries associated with dislocation of the knee. J Bone Joint Surg Am. 1977;59:236–239. [PubMed] [Google Scholar]
- 19.Patterson BM, Agel J, Swiontkowski MF, Mackenzie EJ, Bosse MJ. Knee dislocations with vascular injury: outcomes in the Lower Extremity Assessment Project (LEAP) Study. J Trauma. 2007;63:855–858. doi: 10.1097/TA.0b013e31806915a7. [DOI] [PubMed] [Google Scholar]
- 20.Stayner LR, Coen MJ. Historic perspectives of treatment algorithms in knee dislocation. Clin Sports Med. 2000;19:399–413. doi: 10.1016/s0278-5919(05)70214-3. [DOI] [PubMed] [Google Scholar]
- 21.McHenry TP, Holcomb JB, Aoki N, Lindsey RW. Fractures with major vascular injuries from gunshot wounds: implications of surgical sequence. J Trauma. 2002;53:717–721. doi: 10.1097/00005373-200210000-00016. [DOI] [PubMed] [Google Scholar]
- 22.Huynh TT, Pham M, Griffin LW, Villa MA, Przybyla JA, Torres RH, Keyhani K, Safi HJ, Moore FA. Management of distal femoral and popliteal arterial injuries: an update. Am J Surg. 2006;192:773–778. doi: 10.1016/j.amjsurg.2006.08.043. [DOI] [PubMed] [Google Scholar]
- 23.Hossny A. Blunt popliteal artery injury with complete lower limb ischemia: is routine use of temporary intraluminal arterial shunt justified? J Vasc Surg. 2004;40:61–66. doi: 10.1016/j.jvs.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Kim JY, Buck DW 2nd, Forte AJ, Subramanian VS, Birman MV, Schierle CF, Kloeters O, Mattox KL, Wall MJ, Epstein MJ. Risk factors for compartment syndrome in traumatic brachial artery injuries: an institutional experience in 139 patients. J Trauma. 2009;67:1339–1344. doi: 10.1097/TA.0b013e318197b999. [DOI] [PubMed] [Google Scholar]
- 25.Farber A, Tan TW, Hamburg NM, Kalish JA, Joglar F, Onigman T, Rybin D, Doros G, Eberhardt RT. Early fasciotomy in patients with extremity vascular injury is associated with decreased risk of adverse limb outcomes: a review of the National Trauma Data Bank. Injury. 2012;43:1486–1491. doi: 10.1016/j.injury.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feliciano DV. Evaluation and treatment of vascular injuries. In: Browner BD, Levine AM, Jupiter JB, Trafton P, Krettek C, editors. Skeletal Trauma: Basic Science, Management, and Reconstruction. Philadelphia: WB Saunders Company; 2008. [Google Scholar]
- 27.Puech-Leao P, Wolosker N, Zerati AE, Nascimento LD. Impact of endovascular technique in vascular surgery training at a large university hospital in Brazil. J Surg Educ. 2011;68:19–23. doi: 10.1016/j.jsurg.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Wolosker N, Mendes Cde A, Jacob CE, Wolosker AM, Puech-Leao P. Endovascular infrarenal aortic aneurysm repair combined with laparoscopic cholecystectomy. Clinics (Sao Paulo) 2010;65:743–744. doi: 10.1590/S1807-59322010000700015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferreira J, Canedo A, Brandao D, Maia M, Braga S, Chaparro M, Barreto P, Vaz G. Isolated iliac artery aneurysms: six-year experience. Interact Cardiovasc Thorac Surg. 2010;10:245–248. doi: 10.1510/icvts.2009.218305. [DOI] [PubMed] [Google Scholar]
- 30.Starnes BW, Arthurs ZM. Endovascular management of vascular trauma. Perspect Vasc Surg Endovasc Ther. 2006;18:114–129. doi: 10.1177/1531003506293418. [DOI] [PubMed] [Google Scholar]
- 31.Cina CS, Moore R, Maggisano R, Kucey D, Dueck A, Rapanos T. Endovascular repair of popliteal artery aneurysms with Anaconda limbs: technique and early results. Catheter Cardiovasc Interv. 2008;72:716–724. doi: 10.1002/ccd.21706. [DOI] [PubMed] [Google Scholar]
- 32.Tielliu IF, Zeebregts CJ, Vourliotakis G, Bekkema F, van den Dungen JJ, Prins TR, Verhoeven EL. Stent fractures in the Hemobahn/Viabahn stent graft after endovascular popliteal aneurysm repair. J Vasc Surg. 2010;51:1413–1418. doi: 10.1016/j.jvs.2009.12.071. [DOI] [PubMed] [Google Scholar]
- 33.Johnson WC, Lee KK. A comparative evaluation of polytetrafluoroethylene, umbilical vein, and saphenous vein bypass grafts for femoral-popliteal above-knee revascularization: a prospective randomized Department of Veterans Affairs cooperative study. J Vasc Surg. 2000;32:268–277. doi: 10.1067/mva.2000.106944. [DOI] [PubMed] [Google Scholar]
- 34.Doody O, Given MF, Lyon SM. Extremities--indications and techniques for treatment of extremity vascular injuries. Injury. 2008;39:1295–1303. doi: 10.1016/j.injury.2008.02.043. [DOI] [PubMed] [Google Scholar]
- 35.Hafez HM, Woolgar J, Robbs JV. Lower extremity arterial injury: results of 550 cases and review of risk factors associated with limb loss. J Vasc Surg. 2001;33:1212–1219. doi: 10.1067/mva.2001.113982. [DOI] [PubMed] [Google Scholar]
- 36.Salimi J, Shojaeefar A, Khashayar P. Management of infected femoral pseudoaneurysms in intravenous drug abusers: a review of 57 cases. Arch Med Res. 2008;39:120–124. doi: 10.1016/j.arcmed.2007.07.004. [DOI] [PubMed] [Google Scholar]


