Abstract
Objective: Idiopathic pulmonary fibrosis (IPF) is defined as a specific form of chronic, progressive fibrosing interstitial pneumonia with unknown cause. We analyzed the changed rate of pulmonary function and arterial blood gas in IPF patients, and evaluated their influence of changed rate to IPF prognosis. Methods: 81 patients with IPF were recruited successfully, they were followed-up at 6 and 12 months. Dyspnea score and respiratory assessment parameters including FVC, FEV1, TLC, SaO2, PA-aO2, and DLCO were evaluated at their 6 and 12 months follow-up. The changed value and changed rate of above parameters were calculated, and their treatment effects were divided into 3 subgroup: improved, stable and deteriorated group. Statistical analysis was performed between groups for survival and hazards regression analysis. Results: 55 of 81 patients were follow-up at 12 months. Dyspnea score and its changed rate, the changed value of FEV1%, FVC%, TLC%, DLCO%, and PaO2, SaO2, PA-aO2 were prognosis effect factors in IPF patients in 6 and 12 months group. The survival analysis of dyspnea scores, FVC%, TLC%, DLCO%, PaO2, SaO2 and PA-aO2 at K-M were all statistical significant (P < 0.05) in improved, stable and deteriorated group. Conclusion: FVC% changed rate, dyspnea score changed rate and PaO2 changed rate were IPF patient prognosis associated factors in 6 months group; and FVC% changed rate, DLCO% changed rate and TLC% changed rate were prognosis associated factors for IPF patient in 12 months group.
Keywords: Idiopathic pulmonary fibrosis, prognosis, survival analysis, respiratory assessment parameters
Introduction
Through reviewing the literature of idiopathic pulmonary fibrosis (IPF) before May 2010, the American thoracic society and European respiratory society, the Japanese respiratory society and Latin American thoracic society (ATS/ERS/JRS/ALAT) have redefined IPF based on evidence-based medicine [1]: IPF is defined as a specific form of chronic, progressive fibrosing interstitial pneumonia with unknown cause, which occurs primarily in older adults, limited to the lungs, and associated with the histopathologic and/or radiologic pattern of usual interstitial pneumonia (UIP). Based on the evidence published to date, there is no proven pharmacological therapy for IPF, and its prognosis is poor with a median survival of 2.8 years once diagnosed [2-4].
However, the survival time among individual are different, and it is very difficult to predict prognosis of IPF in each patients. There are many prognosic researches about clinic and lung function parameters, but these conclusions are controversial [5-10]. There were some reports indicated that changed value of clinic and lung function could affect the prognosis of IPF [11-13]. Collard HR, et al reported that evaluation of 6 and 12 months of physiological parameters are the factors that affect the prognosis of patients with IPF, but this conclusion is still controversial [14], because changing value of ten percent could be considered as that lung function parameter decreased from 80% to 70% or decreased from 60% to 50%, however, the changed rate was different. Thus this conclusion should be re-evaluated.
We hypothesized that after IPF patients followed up for 6 and 12 months, the parameters of changed rate is more prognostic than changed value form base-line value. To test this theory, we recruited patients according to 2011 ATS/ERS/JRS/ALAT diagnostic criteria, and strictly designed, retrospectively analyzed the changed rates of clinical, pulmonary function and arterial blood gas in IPF patients, and evaluated their influence of changed rates to IPF prognosis in 6 and 12 months.
Patients and methods
Patients
This retrospective study was approved by the hospital Ethics Committee in December 2003, all subjects signed written informed consent form. All works were undertaken following the provisions of the Declaration of Helsinki.
Patients with IPF were recruited at our university hospital from January 2004 until July 2007. In these patients, we further selected according to 2011 ATS/ERS/JRS/ALAT criteria. According to the criteria of follow-up time should not less than 4 months, a total of 81 patients were selected successfully. 52 of them have been followed up at least 9 months.
In this study, patients who was followed-up between 4-8 months after diagnosis were divided into 6-month group and patients who were followed-up between 9-15 months after diagnosis were divided into 12-month group.
Evaluated clinical parameters
Dyspnea score was evaluated in these patients and the degrees of dyspnea were scored from 0 to 20, higher score indicates more severe dyspnea. Pulmonary function was evaluated using the Vmax™ Encore PFT System (CA, USA). Arterial blood gas detection was performed as described before [15].
Other measured parameters were FVC, FEV1, TLC, SaO2, PA-aO2, and DLCO. The DLCO values were corrected for hemoglobin but not for alveolar gas volume. These values were expressed as percentages of the predicted values. Partial pressure of oxygen in artery (PaO2) was obtained when patients were seated at least 30 min and measured by blood gas analyzer (AVL 990, AVL AG, Switzerland).
In this study, changed value and changed rate were calculated as following: Changed value = Basic value - follow-up value; Changed rate = Changed value/basic value.
Sub group analysis for changed rate
According to ATS/ERS treatment criteria [16], changed rates were divided as 3 group: The improved group, stable group and deteriorated group. The standard values were considered as following: The changed rate of dyspnea score was 2 points; changed rate of TLC% and FVC% were 10%; changed rate of DLCO% was 15%; changed rate of SaO2% was 4% and changed rate of PA-aO2 was 4 mmHg.
When the dyspnea score and PA-aO2 were below standard value, TLC%, FVC%, DLCO% and SaO2% were over standard value, patient was divided into improved group; when the dyspnea score and PA-aO2 were over standard value, TLC%, FVC%, DLCO% and SaO2% were below standard value, patient was divided into deteriorated group; while when the above data between improved group and deteriorated group, patient was divided into stable group.
Statistical analysis
Statistical analysis was performed with the SPSS package software (SPSS13.0, Chicago, IL, USA). Data were expressed as mean ± standard deviation, or median and range. Continuous data were compared using paired Student t test. Survival was compared using the log rank test and displayed using Kaplan-Meier curves. Changed value and rate of groups compared with the base-line value were inserted in a multiple regression model as independent variables and stepwise multivariate analysis was performed, P < 0.05 was considered as statistically significant.
Results
Demographic data of recruited IPF patient
81 patients were included in 6 months group, their mean age at diagnosis was 60 years with 55 male and 26 female. We listed their clinical and physiological parameters in Table 1. A smoking history was found in 55.6% (45/81) of them. 55 patients were included in 12 months group, their mean age at diagnosis was 60 years with 35 male and 15 female, a smoking history was found in 42% (21/50) of them. Results demonstrated that dyspnea symptom and lung function parameter were worse in IPF patient at their follow-up. Compared with baseline value, the dyspnea score, TLC%, PaO2 and SaO2 were changed with statistical significance in 6 months group (P < 0.05). The FVC%, PaCO2 and PA-aO2 were changed significantly in 12 months group when compared with 6 months group (P < 0.05) (Table 2).
Table 1.
Comparison of 6 months followed up value with baseline value in 81 IPF patients
| Parameter | Cases (N) | Baseline value | 6 months value | T value | P value |
|---|---|---|---|---|---|
| Dyspnea scores | 80 | 5.7 ± 2.6 | 8.1 ± 5.6 | 4.61 | 0.00001** |
| FEV1, % | 81 | 67.2 ± 17.2 | 66.9 ± 19.3 | 0.2 | 0.841 |
| FVC, % | 81 | 67.1 ± 17.9 | 67.9 ± 22.9 | 0.53 | 0.531 |
| TLC, % | 81 | 58.3 ± 14.9 | 58.4 ± 18.9 | 5.03 | 0.00003** |
| RV, % | 81 | 56.5 ± 21.4 | 55.4 ± 21.6 | 0.48 | 0.634 |
| DLCO, % | 81 | 49.3 ± 14.9 | 46.6 ± 18.6 | 1.76 | 0.083 |
| PaO2, mmHg | 78 | 74.2 ± 13.9 | 71.9 ± 14.4 | 2.9 | 0.005 |
| PaCO2, mmHg | 78 | 35.5 ± 5.1 | 36.1 ± 3.7 | 1.81 | 0.078 |
| SaO2, % | 78 | 94 ± 3.3 | 92.9 ± 4.1 | 2.61 | 0.011* |
| PA-aO2, mmHg | 78 | 37 ± 14.2 | 37.4 ± 19.1 | 0.48 | 0.631 |
P < 0.05;
P < 0.001.
Table 2.
Comparison of 6 months followed up value with 12 months followed up value in IPF patients
| Parameter | Cases (N) | 6 months value | 12 months value | T values | P value |
|---|---|---|---|---|---|
| Dyspnea scores | 50 | 5 ± 4.8 | 5.2 ± 3.7 | 0.66 | 0.510 |
| FEV1, % | 50 | 76.4 ± 15.1 | 74.4± 13 | 1.84 | 0.072 |
| FVC, % | 48 | 80.1 ± 15.5 | 75 ± 17.3 | 4.51 | 0.0004** |
| TLC, % | 45 | 66.3 ± 16.6 | 65.6 ± 19.7 | 0.31 | 0.759 |
| RV, % | 52 | 52.7 ± 18.2 | 54.5 ± 15.9 | 1.63 | 0.110 |
| DLCO, % | 52 | 54.3 ± 17.6 | 54 ± 16.1 | 0.28 | 0.783 |
| PaO2, mmHg | 52 | 76.8 ± 13.5 | 76 ± 17 | 0.76 | 0.453 |
| PaCO2, mmHg | 52 | 37.2 ± 2.9 | 37.9 ± 2.8 | 2.99 | 0.04* |
| SaO2, % | 52 | 94.4 ± 3.8 | 94.1 ± 4.4 | 0.85 | 0.399 |
| PA-aO2, mmHg | 52 | 29.9 ± 17.6 | 30.1 ± 19 | 2.58 | 0.013* |
P < 0.05;
P < 0.001.
Survival time of 81 IPF patients
The follow-up time of patients 6 months group was ranged from 8 to 84.5 months with mean time of 33 months, their median survival time was 34.6 months (25-75% CI: 7.08 to 51.67 months). Follow-up time of 12 months group ranged from 13 to 84.5 months with mean time of 46 months, their median survival time was 51.13 months (25-75% CI: 21.67 to 55.6 months).
Single factor Cox proportional hazards regression analysis
We listed the hazards regression analysis results of changed value and changed rate of dyspnea score, pulmonary function and arterial blood gas value for prognosis of IPF patient (showed Tables 3 and 4). Because age, gender and smoking affect changing of dyspnea score, pulmonary function and arterial blood gas value, these 3 parameters need to be modify for prognostic evaluation in 6 and 12 months groups.
Table 3.
Single factor Cox proportional hazards regression analysis of parameter changed value in 6 and 12 months groups
| Parameter | Group (month) | -2likeihood | HR | 95% CI | Wald value | P value |
|---|---|---|---|---|---|---|
| Dyspnea scores | 6 | 47.5 | 1.012 | 1.008-1.016 | 41.656 | 0.00004** |
| 12 | 12.8 | 1.011 | 1.004-1.019 | 8.52 | 0.004* | |
| FEV1% | 6 | 27.4 | 0.937 | 0.911-0.963 | 21.71 | 0.00003** |
| 12 | 10 | 0.024 | 0.001-0.933 | 3.993 | 0.046* | |
| FVC% | 6 | 56 | 0.918 | 0.895-0.941 | 45.286 | 0.00002** |
| 12 | 30 | 0.894 | 0.852-0.934 | 20.713 | 0.00005** | |
| TLC% | 6 | 12 | 0.974 | 0.953-0.993 | 5.81 | 0.016* |
| 12 | 13 | 0.959 | 0.933-0.986 | 8.76 | 0.003 | |
| RV% | 6 | 9 | 0.993 | 0.985-1.001 | 3.028 | 0.082 |
| 12 | 6 | 1.007 | 0.993-1.022 | 0.922 | 0.337 | |
| DLCO% | 6 | 29 | 0.952 | 0.931-0.970 | 23.35 | 0.00001** |
| 12 | 26 | 0.932 | 0.906-0.959 | 23.44 | 0.00001** | |
| PaO2 (mmHg) | 6 | 21 | 0.928 | 0.895-0.962 | 16.25 | 0.00005** |
| 12 | 23 | 0.919 | 0.888-0.950 | 24.07 | 0.00009** | |
| PaCO2 (mmHg) | 6 | 5 | 0.997 | 0.97-1.025 | 1.806 | 0.098 |
| 12 | 3 | 1.015 | 0.962-1.139 | 0.775 | 0.644 | |
| SaO2% | 6 | 22 | 0.848 | 0.786-0.915 | 18.115 | 0.00002** |
| 12 | 17 | 0.803 | 0.716-0.901 | 14.033 | 0.0002** | |
| PA-aO2 (mmHg) | 6 | 62 | 1.177 | 1.12-1.237 | 41.566 | 0.00001** |
| 12 | 20 | 1.177 | 1.088-1.274 | 16.474 | 0.00005** |
P < 0.05, compared with base line value;
P < 0.001, compared with base line value.
Table 4.
Single factor Cox proportional hazards regression analysis of parameter changed rate in 6 and 12 months group
| Parameter | Group (month) | -2likeihood | HR | 95% CI | Wald value | P value |
|---|---|---|---|---|---|---|
| Dyspnea scores | 6 | 72.7 | 1.441 | 1.301-1.596 | 49.161 | 0.00002** |
| 12 | 25.3 | 1.62 | 1.30-2.03 | 18.48 | 0.00002** | |
| FEV1, % | 6 | 30.6 | 0.952 | 0.933-0.971 | 23.133 | 0.00002** |
| 12 | 13 | 0.923 | 0.872-0.977 | 7.523 | 0.006* | |
| FVC, % | 6 | 76.6 | 0.859 | 0.824-0.895 | 51.55 | 0.00007** |
| 12 | 40 | 0.874 | 0.829-0.913 | 32.65 | 0.00001** | |
| TLC, % | 6 | 13 | 0.978 | 0.963-0.994 | 7.665 | 0.006* |
| 12 | 15 | 0.968 | 0.948-0.998 | 9.415 | 0.002* | |
| RV, % | 6 | 7 | 0.956 | 0.945-1.012 | 2.028 | 0.182 |
| 12 | 7 | 1.017 | 0.983-1.122 | 1.922 | 0.937 | |
| DLCO, % | 6 | 38 | 0.962 | 0.949-0.976 | 29.506 | 0.00006** |
| 12 | 35 | 0.942 | 0.921-0.962 | 29.22 | 0.00007** | |
| PaO2, mmHg | 6 | 30 | 0.926 | 0.898-0.956 | 23.214 | 0.00001** |
| 12 | 27 | 0.928 | 0.9-0.956 | 24.084 | 0.00009** | |
| PaCO2, mmHg | 6 | 6 | 0.987 | 0.96-1.015 | 0.806 | 0.369 |
| 12 | 4 | 1.021 | 0.942-1.064 | 0.175 | 0.584 | |
| SaO2, % | 6 | 24 | 0.846 | 0.785-0.911 | 19.524 | 0.0001** |
| 12 | 19 | 0.806 | 0.721-0.9 | 14.507 | 0.0001** | |
| PA-aO2, mmHg | 6 | 68 | 1.054 | 1.038-1.07 | 46.944 | 0.00007** |
| 12 | 23 | 1.055 | 1.03-1.08 | 18.629 | 0.00009** |
P < 0.05, compared with base line value;
P < 0.001, compared with base line value.
We noticed that dyspnea score in 6 months group was prognostic factors for IPF patients (X 2 = 41.656, P = 0.00004). And after adjusted base-line value, it was still a prognostic factor (X 2 = 49.161, P = 0.00002), in addition, -2Likeihood value increased from 47.5 to 72.7, which means its prognostic significance increased. On the other hand, dyspnea score also was prognostic risk factors for IPF patient in 12 months groups (X 2 = 8.53, P = 0.004), its changed rate was also prognostic factors for IPF patient (X 2 = 18.48, P = 0.00002), and its -2Likeihood value increased 12.5.
For pulmonary function parameters in IPF patients of 6 months group, the changed value of FEV1%, FVC%, TLC% and DLCO% were prognostic factors when compared with base-line value. After we adjusted base-line value, these 4 parameters still have are significant prognos tic factors (P value: 0.00002, 0.00007, 0.006 and 0.00006). And their prognosis strength were all increased, FVC% was the most remarkable among all (-2Likeihood value change: 20.6). The FEV1%, FVC%, TLC% and DLCO% were prognostic factors among lung function parameters changed rate of 12 months group when compared with base-line value. After adjusted base-line value, the 4 parameters still have were significant (P value: 0.006, 0.00001, 0.002, and 0.00007). And their prognosis strength were all increased, FVC% was the most remarkable among all (-2Likeihood value change: 10).
The PaO2, SaO2 and PA-aO2 were IPF patient prognostic factor in arterial blood gas parameters at 6 months group when compared with base-line value, and the after adjustment, the 3 parameters still were significant (P value: 0.00001, 0.0001 and 0.0000) when compared with base-line value. These 3 parameters were also prognostic factor for 12 months group after adjustment from base-line value with statistical significance (P value: 0.00009, 0.0001 and 0.00009).
Kaplan-Meier survival rate comparison result
Based on the changed rate of each parameter, IPF patients in 6 and 12 months groups were sub-divided into 3 sub-groups: improved group, stable group and deteriorated group. The survival analysis results of 3 sub-groups by Kaplan-Meier were demonstrated in Figures 1, 2, 3, 4, 5, 6 and 7. These parameter were all statistical significant (P < 0.05) except for TLC% in 6 month groups, which including dyspnea scores (Figure 1), FVC% (Figure 2), TLC% (Figure 3), DLCO% (Figure 4), PaO2 (Figure 5), SaO2 (Figure 6) and PA-aO2 (Figure 7).
Figure 1.
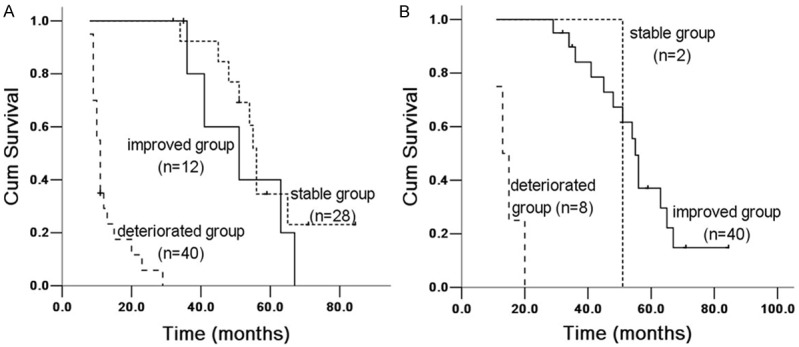
Kaplan Meier survival analysis of IPF patients with dyspnea score between 6 and 12 months groups. A. 6 months group: improved and stable group (X 2 = 1.546, P = 0.214), improved and deteriorated group (X 2 = 34.661, P = 0.00004); stable and deteriorated group (X 2 = 67.098, P = 0.00003). B. 12 months group: improved and stable group (X 2 = 1.047, P = 0.306), improved and deteriorated group (X 2 = 5.088, P = 0.024); stable and deteriorated group (X 2 = 68.341, P = 0.00001).
Figure 2.
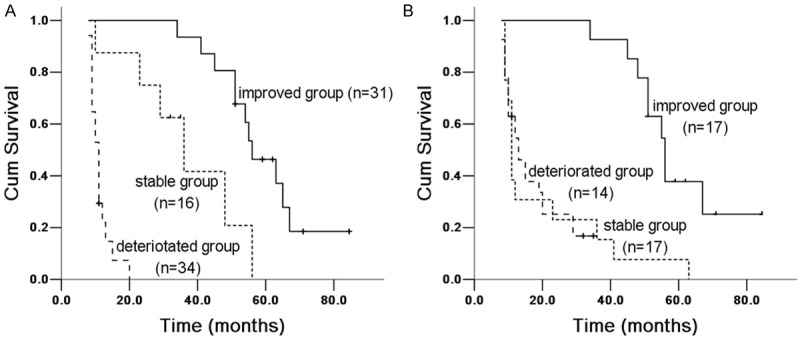
Kaplan Meier survival analysis of IPF patients with FVC% between 6 and 12 months groups. A. 6 months group: improved and stable group (X 2 = 16.197, P = 0.00006), improved and deteriorated group (X 2 = 67.727, P = 0.00002); stable and deteriorated group (X 2 = 30.623, P = 0.00003). B. 12 months group: improved and stable group (X 2 = 35.314, P = 0.00003), improved and deteriorated group (X 2 = 37.987, P = 0.0000); stable and deteriorated group (X 2 = 0.0004, P = 0.983).
Figure 3.
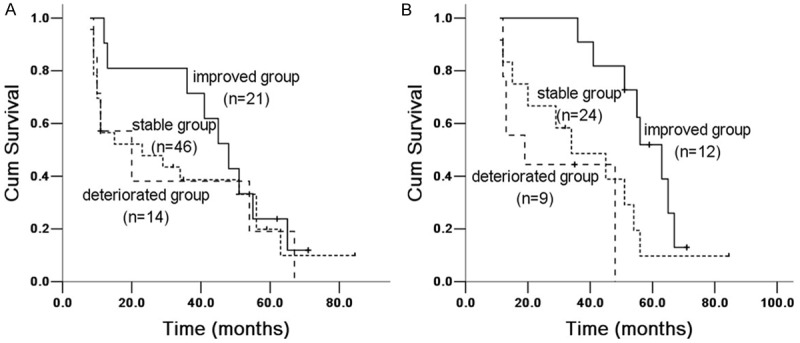
Kaplan Meier survival analysis of IPF patients with TLC% between 6 and 12 months groups. A. 6 months group: improved and stable group (X 2 = 1.5764, P = 0.209), improved and deteriorated group (X 2 = 1.774, P = 0.183); stability and deteriorating group (X 2 = 0.055, P = 0.814). B. 12 months group: improved and stable group (X 2 = 8.271, P = 0.004), improved and deteriorated group (X 2 = 19.677, P = 0.00009); stable and deteriorated group (X 2 = 1.355, P = 0.244).
Figure 4.
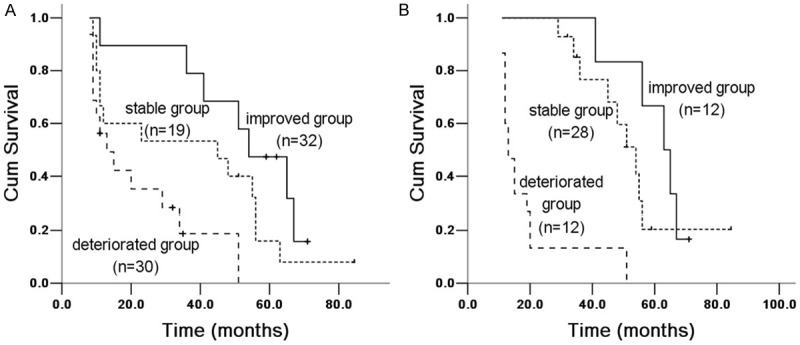
Kaplan Meier survival analysis of IPF patients with DLCO% between 6 and 12 months groups. A. 6 months group: improved and stable group (X 2 = 21.455, P = 0.00004), improved and deteriorated group (X 2 = 7.632, P = 0.006); stable and deteriorated group (X 2 = 4.016, P = 0.045). B. 12 months group: improved and stable group (X 2 = 3.025, P = 0.082), improved and deteriorated group (X 2 = 23.551, P = 0.00001); stable and deteriorated group (X 2 = 13.781, P = 0.00002).
Figure 5.
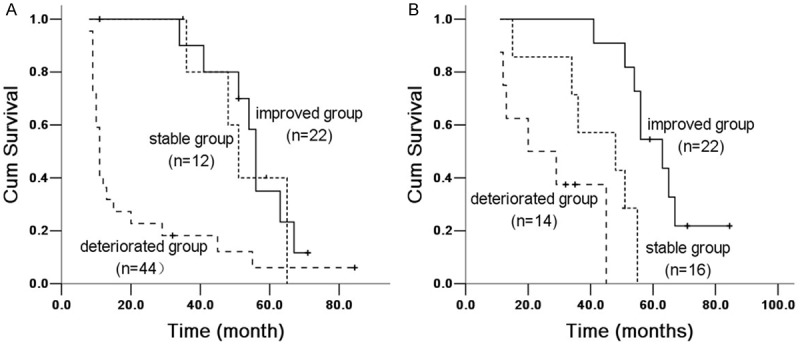
Kaplan Meier survival analysis of IPF patients with PaO2 between 6 and 12 months groups. A. 6 months group: improved and stable group (X 2 = 21.594, P = 0.00003), improved and deteriorated group (X 2 = 11.958, P = 0.001); stable and deteriorated group (X 2 = 0.45, P = 0.502). B. 12 months group: improved and stable group (X 2 = 12.344, P = 0.0004), improved and deteriorated group (X 2 = 29.527, P = 0.00006); stable and deteriorated group (X 2 = 10.29, P = 0.001).
Figure 6.
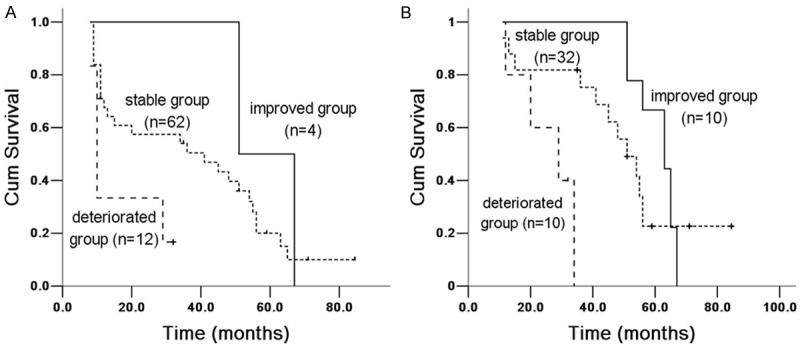
Kaplan Meier survival analysis of IPF patients with SaO2 between 6 and 12 months groups. A. 6 months group: improved and stable group (X 2 = 6.743, P = 0.009), improved and deteriorated group (X 2 = 9.968, P = 0.002); stable and deteriorated group (X 2 = 1.08, P = 0.299). B. 12 months group: improved and stable group (X 2 = 0.732, P = 0.392), improved and deteriorated group (X 2 = 5.523, P = 0.00008); stable and deteriorated group (X 2 = 12.431, P = 0.0004).
Figure 7.
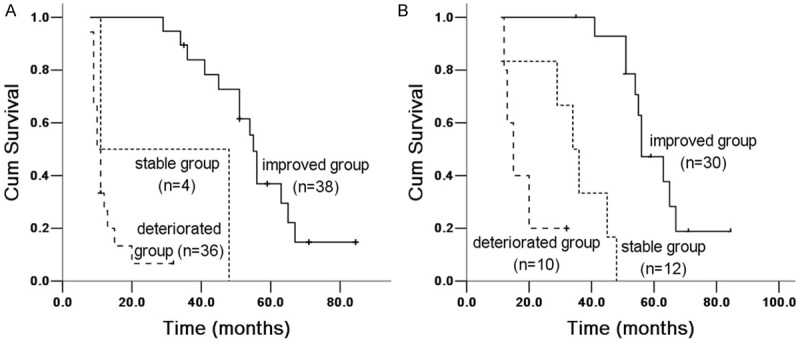
Kaplan Meier survival analysis of IPF patients with PA-aO2 between 6 and 12 months groups. A. 6 months group: improved and stable group (X 2 = 68.618, P = 0.00007), improved and deteriorated group (X 2 = 3.435, P = 0.064); stable and deteriorated group (X 2 = 11.52, P = 0.001). B. 12 months group: improved and stable group (X 2 = 46.678, P = 0.00008), improved and deteriorated group (X 2 = 36.539, P = 0.00001); stable and deteriorated group (X 2 = 5.079, P = 0.024).
Multivariable Cox proportional hazards regression analysis
We analyzed multifactor prognostic parameters which were significant in single factor Cox proportional hazards. Result showed that FVC% changed rate (HR = 0.899, Wald value = 17.104, P = 0.00004), dyspnea scores change rated (HR = 1.131, Wald value = 4.919, P = 0.027), PaO2 changed rate (HR = 0.957, Wald value = 4.489, P = 0.034) were prognostic associated factors in 6 months group; and FVC% changed rate (HR = 0.838, Wald value = 17.336, P = 0.00003), DLCO% changed rate (HR = 0.932, Wald value = 15.709, P = 0.00007) and TLC% changed rate (HR = 0.962, Wald value = 3.895, P = 0.048) were prognosis associated factors for IPF patient in 12 months group.
Discussion
Researches about prognosis factors for IPF patients were generally given priority to series of baseline variables, which including: age, gender, smoking history, dyspnea score, FEV1, FVC, TLC, TGV, RV, DLCO, PaO2, PA-aO2, X-ray chest radiograph lesion degree, sports physiology, BALF and lung tissue pathology [3,5-10,17-20]. However, their conclusions were inconsistent. Research performed by King et al mentioned baseline variables for a comprehensive scoring system, which was composed by clinical, medical imageology and physiology components, and they indicated that this comprehensive scoring system is prognosis factor for sensitive IPF patients [7], however, the imaging analysis and lung function test in this scoring system were hard to be performed, thus the clinical application of this system was restrictive.
We hypothesized that after IPF patients followed up for 6 and 12 months, the changed rate and changed value of parameters were more prognostic than base-line value of parameters, we selected patients according to the diagnosis of IPF, 2011ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis to testify this hypothesis. Our study found that clinic and lung function parameters were deteriorated in IPF patients after followed up for 6 and 12 months; their survival rate at dyspnea score, pulmonary function parameters (except the RV) and arterial blood gas (except PaCO2) between groups were statistically significant; The changed rate of dyspnea score, and PaO2 in 6 months group and FVC%, DLCO, TLC% in 12 months group were prognostic factors for IPF patient; these changed rates were more valuable than changed value of them and have stronger prognostic significance than changed value, among these factors; the changed rate of FVC% had better prognostic significance.
In Multivariable Cox proportional hazards regression analysis, we found that changed value of FVC% in 6 and 12 months were the strongest prognostic factors for IPF patients. FVC% test has the character of simple and excellent repetition, this test is usually used as an index for evaluating patients with lung disease [21], and also is provided for the prognostic information for patients with IPF. Our study demonstrated that FVC% changed rate also could predict the prognosis of IPF after adjustment of variable; this indicates that the disease progression and initial lesion of patient were independent, they all were factors affected the prognosis of patients with IPF.
There were many studies focused on whether FVC% changed values affect IPF patient prognosis [17,22]. Compelling studies reported that declined FVC% value may lead to increased mortality when compared with value before follow-up [3,17,18,23-25]. Hanson D, et al [10] discovered that the prognosis is poor when FVC% dropped more than 10% in IPF patient after followed-up for 1 year. Some research took FVC% changed values of 10-15% as group standard [10,23,26-28]. We found that survival analysis of FVC% in improved group (drop 10%), stable group (between decreased 10% and increased 10%) and deteriorated group (decreased more than 10%) among 3 sub-groups have obvious difference (P < 0.05), the mortality rate increased in deteriorated group.
This study also found that mortality risk of IPF patients increased significantly when DLCO% decreased over 15%. Although measurement standards of DLCO% in European and American countries have been accepted [29], changed rate of DLCO% were fluctuated more than FVC%, thus changed rate of 15% was considered as clinically significant improvement criteria [10,26,27].
Literature reports indicated that DLCO% in improved group dropped over 20% compared with stable group [10], this has better prognosis after a year of treatment for IPF patients, if considering both the changing of FVC% and DLCO%, the prognosis would be better.
We also found that survival analysis of TLC, PaO2, dyspnea score, SaO2 and PA-aO2 between groups were meaningful. Changed rate of dyspnea scores, PaO2 in 6 months group and TLC% in 12 months group were prognostic factors for IPF patient. However, they were not yet confirmed by other studies, and further researches are needed.
Our article still exist the following deficiencies: 1. The selection bias, we required that all selected cases should survival more than 6 or 12 months and could be followed-up at 6 and 12 months, but many patients failed in visiting; 2. Treatment bias: we were familiar with these IPF patients in our center, and they received regular treatment which might be different in some clinical cases.
Conclusion
Our research demonstrated that clinical parameters and lung function parameters were easily tested and have good repeatability, they could be used as monitoring indicators for the prognosis of IPF patients, changed rate of parameters in 6 and 12 months have better prognostic significance than changed value, but these conclusion still need be verified in further study.
Disclosure of conflict of interest
None.
References
- 1.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE Jr, Kondoh Y, Myers J, Müller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schünemann HJ ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicholson AG, Colby TV, du Bois RM, Hansell DM, Wells AU. The prognostic significance of the histologic pattern of interstitial pneumonia in patients presenting with the clinical entity of cryptogenic fibrosing alveolitis. Am J Respir Crit Care Med. 2000;162:2213–2217. doi: 10.1164/ajrccm.162.6.2003049. [DOI] [PubMed] [Google Scholar]
- 3.Mapel DW, Hunt WC, Utton R, Baumgartner KB, Samet JM, Coultas DB. Idiopathic pulmonary fibrosis: survival in population based and hospital based cohorts. Thorax. 1998;53:469–476. doi: 10.1136/thx.53.6.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjoraker JA, Ryu JH, Edwin MK, Myers JL, Tazelaar HD, Schroeder DR, Offord KP. Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1998;157:199–203. doi: 10.1164/ajrccm.157.1.9704130. [DOI] [PubMed] [Google Scholar]
- 5.Hubbard R, Johnston I, Britton J. Survival in patients with cryptogenic fibrosing alveolitis: a population-based cohort study. Chest. 1998;113:396–400. doi: 10.1378/chest.113.2.396. [DOI] [PubMed] [Google Scholar]
- 6.Gay SE, Kazerooni EA, Toews GB, Lynch JP 3rd, Gross BH, Cascade PN, Spizarny DL, Flint A, Schork MA, Whyte RI, Popovich J, Hyzy R, Martinez FJ. Idiopathic pulmonary fibrosis: predicting response to therapy and survival. Am J Respir Crit Care Med. 1998;157:1063–1072. doi: 10.1164/ajrccm.157.4.9703022. [DOI] [PubMed] [Google Scholar]
- 7.King TE Jr, Tooze JA, Schwarz MI, Brown KR, Cherniack RM. Predicting survival in idiopathic pulmonary fibrosis: scoring system and survival model. Am J Respir Crit Care Med. 2001;164:1171–1181. doi: 10.1164/ajrccm.164.7.2003140. [DOI] [PubMed] [Google Scholar]
- 8.Mogulkoc N, Brutsche MH, Bishop PW, Greaves SM, Horrocks AW, Egan JJ Greater Manchester Pulmonary Fibrosis Consortium. Pulmonary function in idiopathic pulmonary fibrosis and referral for lung transplantation. Am J Respir Crit Care Med. 2001;164:103–108. doi: 10.1164/ajrccm.164.1.2007077. [DOI] [PubMed] [Google Scholar]
- 9.Mogulkoc N, Brutsche MH, Bishop PW, Murby B, Greaves MS, Horrocks AW, Wilson M, McCullough C, Prescott M, Egan JJ Greater Manchester Pulmonary Fibrosis Consortium. Pulmonary (99m) Tc-DTPA aerosol clearance and survival in usual interstitial pneumonia (UIP) Thorax. 2001;56:916–923. doi: 10.1136/thorax.56.12.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanson D, Winterbauer RH, Kirtland SH, Wu R. Changes in pulmonary function test results after 1 year of therapy as predictors of survival in patients with idiopathic pulmonary fibrosis. Chest. 1995;108:305–310. doi: 10.1378/chest.108.2.305. [DOI] [PubMed] [Google Scholar]
- 11.Leslie KO, Cool CD, Sporn TA, Curran-Everett D, Steele MP, Brown KK, Wahidi MM, Schwartz DA. Familial idiopathic interstitial pneumonia: histopathology and survival in 30 patients. Arch Pathol Lab Med. 2012;136:1366–1376. doi: 10.5858/arpa.2011-0627-OAI. [DOI] [PubMed] [Google Scholar]
- 12.Ley B, Ryerson CJ, Vittinghoff E, Ryu JH, Tomassetti S, Lee JS, Poletti V, Buccioli M, Elicker BM, Jones KD, King TE Jr, Collard HR. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med. 2012;156:684–691. doi: 10.7326/0003-4819-156-10-201205150-00004. [DOI] [PubMed] [Google Scholar]
- 13.Soares Pires F, Caetano Mota P, Melo N, Costa D, Jesus JM, Cunha R, Guimaraes S, Souto-Moura C, Morais A. Idiopathic pulmonary fibrosis--clinical presentation, outcome and baseline prognostic factors in a Portuguese cohort. Rev Port Pneumol. 2013;19:19–27. doi: 10.1016/j.rppneu.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Collard HR, King TE Jr, Bartelson BB, Vourlekis JS, Schwarz MI, Brown KK. Changes in clinical and physiologic variables predict survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2003;168:538–542. doi: 10.1164/rccm.200211-1311OC. [DOI] [PubMed] [Google Scholar]
- 15.Sun WX, Jin D, Li Y, Wang RT. Increased arterial stiffness in stable and severe asthma. Respir Med. 2014;108:57–62. doi: 10.1016/j.rmed.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 16.American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS) Am J Respir Crit Care Med. 2000;161:646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz DA, Van Fossen DS, Davis CS, Helmers RA, Dayton CS, Burmeister LF, Hunninghake GW. Determinants of progression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1994;149:444–449. doi: 10.1164/ajrccm.149.2.8306043. [DOI] [PubMed] [Google Scholar]
- 18.Erbes R, Schaberg T, Loddenkemper R. Lung function tests in patients with idiopathic pulmonary fibrosis. Are they helpful for predicting outcome? Chest. 1997;111:51–57. doi: 10.1378/chest.111.1.51. [DOI] [PubMed] [Google Scholar]
- 19.King TE Jr, Schwarz MI, Brown K, Tooze JA, Colby TV, Waldron JA Jr, Flint A, Thurlbeck W, Cherniack RM. Idiopathic pulmonary fibrosis: relationship between histopathologic features and mortality. Am J Respir Crit Care Med. 2001;164:1025–1032. doi: 10.1164/ajrccm.164.6.2001056. [DOI] [PubMed] [Google Scholar]
- 20.McCormack FX, King TE Jr, Bucher BL, Nielsen L, Mason RJ. Surfactant protein A predicts survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1995;152:751–759. doi: 10.1164/ajrccm.152.2.7633738. [DOI] [PubMed] [Google Scholar]
- 21.Lung function testing: selection of reference values and interpretative strategies. American Thoracic Society. Am Rev Respir Dis. 1991;144:1202–1218. doi: 10.1164/ajrccm/144.5.1202. [DOI] [PubMed] [Google Scholar]
- 22.Tukiainen P, Taskinen E, Holsti P, Korhola O, Valle M. Prognosis of cryptogenic fibrosing alveolitis. Thorax. 1983;38:349–355. doi: 10.1136/thx.38.5.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rudd RM, Haslam PL, Turner-Warwick M. Cryptogenic fibrosing alveolitis. Relationships of pulmonary physiology and bronchoalveolar lavage to response to treatment and prognosis. Am Rev Respir Dis. 1981;124:1–8. doi: 10.1164/arrd.1981.124.1.1. [DOI] [PubMed] [Google Scholar]
- 24.Jezek V, Fucik J, Michaljanic A, Jezkova L. The prognostic significance of functional tests in cryptogenic fibrosing alveolitis. Bull Eur Physiopathol Respir. 1980;16:711–720. [PubMed] [Google Scholar]
- 25.Hubbard R, Venn A, Smith C, Cooper M, Johnston I, Britton J. Exposure to commonly prescribed drugs and the etiology of cryptogenic fibrosing alveolitis: a case-control study. Am J Respir Crit Care Med. 1998;157:743–747. doi: 10.1164/ajrccm.157.3.9701093. [DOI] [PubMed] [Google Scholar]
- 26.Douglas WW, Ryu JH, Swensen SJ, Offord KP, Schroeder DR, Caron GM, DeRemee RA. Colchicine versus prednisone in the treatment of idiopathic pulmonary fibrosis. A randomized prospective study. Members of the Lung Study Group. Am J Respir Crit Care Med. 1998;158:220–225. doi: 10.1164/ajrccm.158.1.9709089. [DOI] [PubMed] [Google Scholar]
- 27.Raghu G, Depaso WJ, Cain K, Hammar SP, Wetzel CE, Dreis DF, Hutchinson J, Pardee NE, Winterbauer RH. Azathioprine combined with prednisone in the treatment of idiopathic pulmonary fibrosis: a prospective double-blind, randomized, placebo-controlled clinical trial. Am Rev Respir Dis. 1991;144:291–296. doi: 10.1164/ajrccm/144.2.291. [DOI] [PubMed] [Google Scholar]
- 28.Raghu G, Johnson WC, Lockhart D, Mageto Y. Treatment of idiopathic pulmonary fibrosis with a new antifibrotic agent, pirfenidone: results of a prospective, open-label Phase II study. Am J Respir Crit Care Med. 1999;159:1061–1069. doi: 10.1164/ajrccm.159.4.9805017. [DOI] [PubMed] [Google Scholar]
- 29.American Thoracic Society. Single-breath carbon monoxide diffusing capacity (transfer factor). Recommendations for a standard technique-1995 update. Am J Respir Crit Care Med. 1995;152:2185–2198. doi: 10.1164/ajrccm.152.6.8520796. [DOI] [PubMed] [Google Scholar]


