Abstract
Background: Robot-assisted partial nephrectomy (RAPN) is being performed more frequently for the minimally invasive management of localized renal tumors. However, it’s unclear whether RAPN is more efficacious than the standard laparoscopic partial nephrectomy (LPN). The objective of this meta-analysis is to compare RAPN and LPN in terms of perioperative and oncologic outcomes for the treatment of localized renal tumors. Methods: A systematic search of electronic databases including MEDLINE, EMBASE and OVID was conducted. Comparative studies comparing RAPN and LPN for the treatment of localized renal tumors were regarded eligible. The mean difference (MD), odds ratio (OR) and their corresponding 95% confidence intervals (CI) were calculated for each outcome. The methodologic quality of the included studies was evaluated using the strict criteria of the Newcastle-Ottawa scale. Results: 14 comparative studies (n = 1539 participants) were included in the present meta-analysis. Operative time was similar for RAPN and LPN (MD = 6.33, 95% CI [-23.93, 36.59]), however, warm ischemia time favored RAPN (MD = -3.29, 95% CI [-6.47, -0.10]). There was no significant difference in estimated blood loss (EBL) (MD = -42.24, 95% CI [-87.10, 2.61]) and length of stay (LOS) (MD = -0.29, 95% CI [-0.89, 0.32]). The incidence of intraoperative complications was similar for RAPN and LPN (OR = 0.68, 95% CI [0.29, 1.58]), as well as incidence of postoperative minor complications (OR = 1.10, 95% CI [0.80, 1.51]) and postoperative major complications distributions by Clavien classification (OR = 0.99, 95% CI [0.61, 1.61]). In addition, no significant difference was found in terms of positive surgical margin rate (OR = 1.12, 95% CI [0.56, 2.25]). Conclusions: RAPN had similar operative time, LOS, EBL, and perioperative complications compared with LPN, as well as positive margin rates. RAPN appears to offer the advantage of decreased WIT compared with LPN. Studies with long-term follow up are needed to compare RAPN and LPN in terms of long-term complications and oncologic outcomes.
Keywords: Laparoscopic partial nephrectomy, meta-analysis, nephron-sparing surgery, robot-assisted partial nephrectomy
Introduction
The laparoscopic approach to nephron sparing surgery (NSS) was introduced to decrease the morbidity associated with traditional open surgery [1-3]. With the advances of laparoscopic surgery, LPN has become a technically feasible procedure. However, technical difficulty including intra-corporeal suturing and dissection is the largest obstacle to the widespread use of LPN [4]. Robotic minimally invasive surgery has several advantages, including three-dimensional imaging, tremor filtration, and augmented dexterity, allowing more precision in a smaller operative field and easier resection and repair [5-10]. In the last decade, some observational studies comparing RAPN and LPN for treating localized renal tumors were published. However, their results were inconsistent. As a result, it’s unclear whether RAPN is more efficacious than LPN. The objective of this meta-analysis is to compare RAPN and LPN in terms of perioperative and oncologic outcomes for the treatment of localized renal tumors.
Materials and methods
Data sources and searches
The meta-analysis was undertaken in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [11]. A literature search was carried out using MEDLINE, EMBASE and OVID to identify all articles published between January 1966 and January 2014 which compared RAPN and LPN in terms of perioperative outcomes and complications for the treatment of localized renal tumors. There were no restriction of origin and languages. We utilized the search terms “robotic”, “laparoscopic”, “partial nephrectomy”, “nephron-sparing surgery”, and “renal”, and we used both free text and MeSH searches for keywords. The reference list of each comparative study and previous reviews were manually examined to find additional relevant studies.
Study selection
Two reviewers independently selected eligible trials. Disagreement between the two reviewers was settled by discussing with the third reviewer. Inclusion criteria: comparative studies comparing RAPN and LPN for the treatment of localized renal tumors; studies with greater than 20 patients; patient age older than 16 years. Exclusion criteria: RAPN and LPN for the treatment of bilateral synchronous renal tumors; letters; single case reports; reviews; and studies containing previously published data.
Data extraction and quality assessment
The following data was collected by two reviewers independently using a purpose-designed form: name of first author, publishing time, journal, study design, number of patients, baseline data, warm ischemia time (WIT), operative time, length of stay (LOS), estimated blood loss (EBL), incidence of intraoperative complications, incidence of postoperative minor complications (Clavien 1 and 2), incidence of postoperative major complications (Clavien ≥ 3). We used Newcastle-Ottawa scale to assess the methodologic quality of each study. Two reviewers who were blinded regarding the source institution, the journal, and the authors for each included publication independently assess the methodologic quality. Disagreement between the two reviewers was settled by discussing with the third reviewer.
Data synthesis and analysis
All the data analysis was carried out using professional meta-analysis software Review Manager (V5.1.0). Outcomes are baseline parameters including age, sex, body mass index (BMI), American Society of Anesthesi-ologist (ASA), tumor size, laterality; WIT; operative time; LOS; EBL; incidence of intraoperative complications, incidence of postoperative minor complications (Clavien 1 and 2); incidence of postoperative major complications (Clavien ≥ 3); positive surgical margin rate. The mean difference (MD), pooled odds ratio (OR) and their 95% confidence intervals (CI) were calculated for each outcome. I2 test was used to analyze heterogeneity of trials. Heterogeneity was interpreted as absent (I2: 0%-25%), low (I2: 25.1%-50%), moderate (I2: 50.1%-75%), or high (I2: 75.1%-100%) [12]. In the absence of a statistically significant heterogeneity (I2: 0%-25%), Mantel-Haenszel fixed model was used; otherwise, Mantel-Haenszel random model was performed. Sensitivity analysis were carried out by study design and tumor characteristics.
Results
Characteristics of studies included in the meta-analysis
Figure 1 shows the flow diagram for study inclusion. A total of 838 citations were identified during the initial search. On the basis of the title and abstract, we identified 16 papers. After reading the full manuscripts, two studies were excluded for reasons described in Figure 1. At last, 14 studies [13-26] published between 2006 and 2012 were included in the meta-analysis, involving 1539 participants, 704 participants in the robotic group and 835 participants in the laparoscopic group. All of the included studies were observational studies. Baseline data and NOS scores are shown in Table 1.
Figure 1.
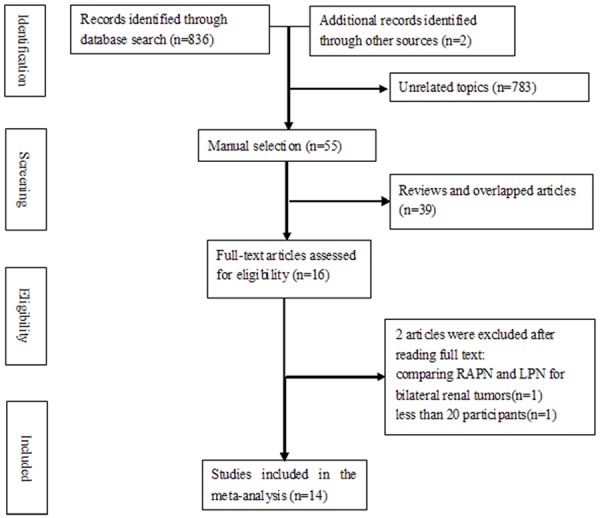
Flow diagram of screened, excluded, and analyzed publications.
Table 1.
Characteristics of participants in included studies. Data are presented as mean (SD)/(range)
| Author and year | Group | No of participants | Age, yr | No. male (%) | BMI, kg/m2 | ASA | Tumor size, cm | No. Left (%) | No. Right (%) | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|
| Ellison JS 2012 | RAPN | 108 | 59.4 (12.1) | 66 (61) | 30.9 (6.5) | NR | 2.9 (1.6) | 56 (52) | 52 (48) | 9 |
| LPN | 108 | 55.9 (10.6) | 62 (57) | 29.3 (6.1) | NR | 2.7 (1.4) | 51 (47) | 57 (53) | ||
| Long JA 2012 | RAPN | 199 | 58.5 (11.5) | 119 (59.8) | 30.7 (7.2) | NR | 3.8 (1.8) | 110 (55.3) | 89 (44.7) | 7 |
| LPN | 182 | 59.5 (13.0) | 112 (61.5) | 29.2 (5.3) | NR | 4.0 (1.7) | 96 (52.7) | 86 (47.3) | ||
| Williams SB 2011 | RAPN | 27 | 55.7 (11.2) | 17 (63) | 27.2 (3.74) | 2.2 (0.42) | 2.47 (1.18) | 14 (52) | 13 (48) | 9 |
| LPN | 59 | 54.6 (11.7) | 41 (70) | 28.9 (3.92) | 2.16 (0.37) | 3.08 (2.17) | 31 (53) | 28 (47) | ||
| Pierorazio PM 2011 | RAPN | 48 | 62 (27-77) | 27 (56.3) | 28.2 (17.8-40.5) | NR | 2.0 (0.9-6.0) | 26 (54.2) | 22 (45.8) | 7 |
| LPN | 102 | 56 (25-81) | 63 (61.8) | 30.3 (18.7-46.5) | NR | 2.2 (0.5-7.7) | 47 (46.1) | 55 (53.9) | ||
| Seo IY 2011 | RAPN | 13 | 54.2 (12.4) | 10 (76.9) | 23.8 (2.3) | NR | 2.7 (1.2) | 4 (30.8) | 9 (69.2) | 5 |
| LPN | 14 | 53.9 (11.6) | 8 (57.1) | 24.6 (2.7) | NR | 2.0 (1.2) | 10 (71.4) | 4 (28.6) | ||
| Cho CL 2011 | RAPN | 10 | 63 (36-78) | 8 (80) | NR | 2.0 | 2.7 (1.7-5.0) | 7 (70) | 3 (30) | 5 |
| LPN | 10 | 56 (31-79) | 5 (50) | NR | 1.8 | 2.8 (1.2-5.0) | 5 (50) | 5 (50) | ||
| Haber GP 2010 | RAPN | 75 | 62.6 | 44 (58.7) | 30.1 | 2.4 | 2.8 | 39 (52) | 36 (48) | 9 |
| LPN | 75 | 60.0 | 40 (53.3) | 29.7 | 2.4 | 2.5 | 32 (42.7) | 43 (57.3) | ||
| DeLong JM 2010 | RAPN | 13 | 59.7 | 8 (61.5) | 28.9 | 2.3 | 2.6 | 6 (46.2) | 7 (53.8) | 5 |
| LPN | 15 | 53.6 | 8 (53.3) | 26.6 | 2.3 | 2.8 | 7 (46.7) | 8 (52.3) | ||
| Wang AJ 2009 | RAPN | 40 | 61.0 | NR | 29.7 | 2.3 | 2.5 | 23 (57.5) | 17 (42.5) | 9 |
| LPN | 62 | 58.0 | NR | 29.2 | 2.4 | 2.4 | 33 (53.2) | 29 (46.8) | ||
| Benway BM 2009 | RAPN | 129 | 59.2 | NR | 29.8 | NR | 2.9 | NR | NR | 9 |
| LPN | 118 | 59.2 | NR | 28.5 | NR | 2.6 | NR | NR | ||
| Kural AR 2009 | RAPN | 11 | 50.8 (13.2) | 8 (72.7) | 26.7 (3.8) | 1.54 (0.52) | 3.2 (2.0-4.1) | 8 (73) | 3 (27) | 7 |
| LPN | 20 | 58.9 (15.4) | 14 (70.0) | 27.8 (2.9) | 1.64 (0.63) | 3.2 (1.5-7.0) | 12 (60) | 8 (40) | ||
| Jeong W 2009 | RAPN | 31 | 53.4 | 15 (48.4) | 24.1 | NR | 3.4 | NR | NR | 7 |
| LPN | 26 | 58.7 | 13 (50) | 24.8 | NR | 2.4 | NR | NR | ||
| Aron M 2008 | RAPN | 12 | 64.0 (13.8) | 8 (66.7) | 29 (6.4) | 2 (1-3) | 2.4 (0.7) | 5 (41.7) | 7 (58.3) | 9 |
| LPN | 12 | 61.0 (13.8) | 8 (66.7) | 30 (6.4) | 2 (1-3) | 2.9 (0.7) | 5 (41.7) | 7 (58.3) | ||
| Caruso RP 2006 | RAPN | 10 | 58.0 | NR | 28.1 | NR | 2.0 | NR | NR | 7 |
| LPN | 10 | 61.0 | NR | 28.5 | NR | 2.2 | NR | NR |
ASA = American Society of Anesthesiologist; BMI = body mass index; LPN = laparoscopic partial nephrectomy; NOS = Newcastle-Ottawa scale; NR = not reported; RAPN = robot-assisted partial nephrectomy.
Results of the meta-analysis
Baseline parameters
There was no significant difference between the two groups for any of baseline parameters except for age (MD = 1.88, 95% CI [1.74, 2.02]) (shown in Table 2).
Table 2.
Meta-analysis of baseline parameters
| Outcomes | Odds Ratio [95% CI] or Mean Difference [95% CI] | Heterogeneity |
|---|---|---|
| Age | 1.88 [1.74, 2.02] | I2 = 99% |
| Sex | 1.04 [0.82, 1.32] | I2 = 0% |
| BMI | 0.10 [-0.75, 0.95] | I2 = 96% |
| ASA | -0.01 [-0.10, 0.08] | I2 = 0% |
| Tumor size | 0.06 [-0.14, 0.25] | I2 = 85% |
| Laterality | 1.16 [0.93, 1.46] | I2 = 0% |
ASA = American Society of Anesthesiologist; BMI = body mass index; CI = confidence intervals.
Operative time
There were nine studies investigating operative time. This result was influenced by heterogeneity (I2 = 97%), and so the meta-analysis was performed using a random model. Meta analysis results (Figure 2) showed that there was no significant difference in operative time (MD = 6.33, 95% CI [-23.93, 36.59]).
Figure 2.
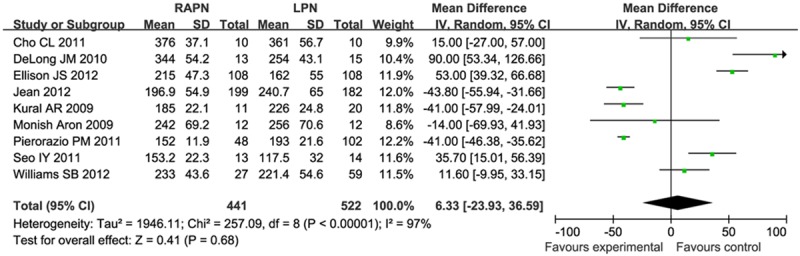
Forest plot of meta-analysis: Operative time. CI: confidence interval.
Warm ischemia time
There were nine studies investigating warm ischemia time. This result was influenced by heterogeneity (I2 = 82%), and so the meta-analysis was performed using a random model. Meta analysis results (Figure 3) showed that warm ischemia time were significantly shorter for RPN than for LPN (MD = -3.29, 95% CI [-6.47, -0.10]).
Figure 3.
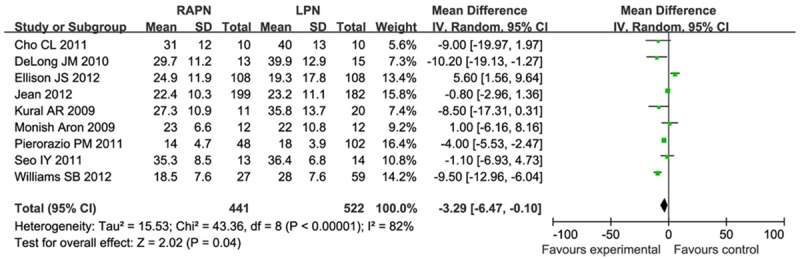
Forest plot of meta-analysis: Warm ischemia time. CI: confidence interval.
Estimated blood loss
There were nine studies investigating estimated blood loss. This result was influenced by heterogeneity (I2 = 41%), and so the meta-analysis was performed using a random model. Meta analysis results (Figure 4) showed that there was no significant difference in estimated blood loss (MD = -42.24, 95% CI [-87.10, 2.61]).
Figure 4.
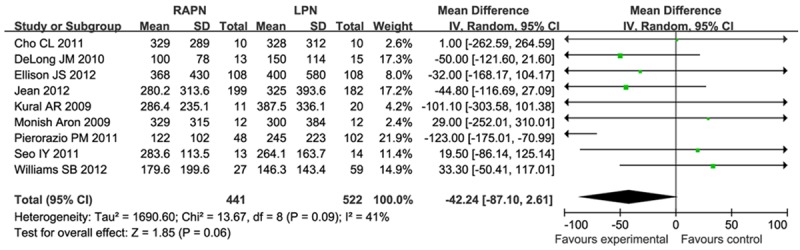
Forest plot of meta-analysis: Estimated blood loss. CI: confidence interval.
Length of stay
There were eight studies investigating length of stay. This result was influenced by heterogeneity (I2 = 86%), and so the meta-analysis was performed using a random model. Meta analysis results (Figure 5) showed that there was no significant difference in length of stay (MD = -0.29, 95% CI [-0.89, 0.32]).
Figure 5.
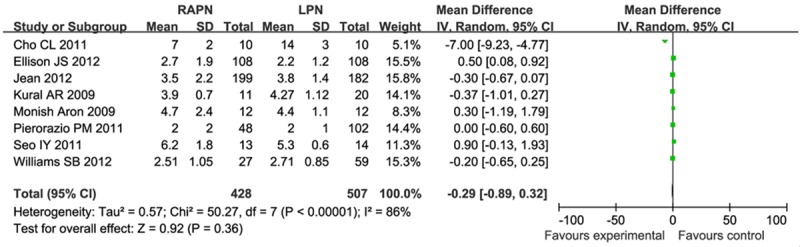
Forest plot of meta-analysis: Length of stay. CI: confidence interval.
Incidence of complications
There were six studies investigating incidence of intraoperative complications. A fixed-effect model was used due to the fact that there was no heterogeneity (I2 = 0%). Meta analysis results showed that there was no significant difference in incidence of intraoperative complications (OR = 0.68, 95% CI [0.29, 1.58]).
There were five studies investigating incidence of postoperative minor complications. A fixed-effect model was used due to the fact that there was no heterogeneity (I2 = 0%). Meta analysis results showed that there was no significant difference in incidence of postoperative minor complications (OR = 1.10, 95% CI [0.80, 1.51]).
There were five studies investigating incidence of postoperative major complications. A fixed-effect model was used due to the fact that there was no heterogeneity (I2 = 0%). Meta analysis results showed that there was no significant difference in incidence of postoperative major complications (OR = 0.99, 95% CI [0.61, 1.61]) (showed in Figure 6).
Figure 6.
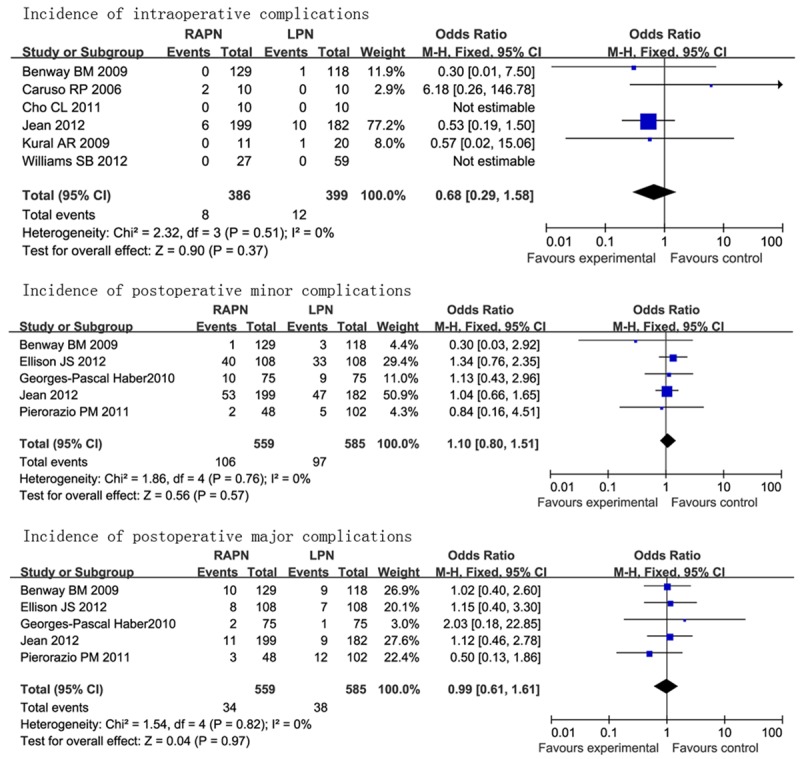
Forest plot of meta-analysis: incidence of complications. CI: confidence interval.
Positive surgical margin rate
There were 11 studies investigating positive surgical margin rate. A fixed-effect model was used due to the fact that there was no heterogeneity (I2 = 0). Meta analysis results (Figure 7) showed that there was no significant difference in positive surgical margin rate (OR = 1.12, 95% CI [0.56, 2.25]).
Figure 7.
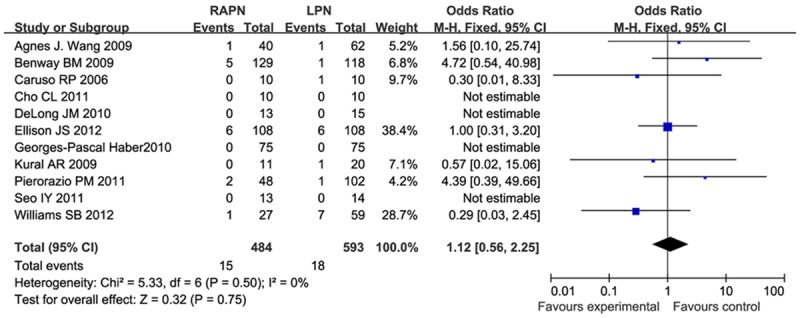
Forest plot of meta-analysis: Positive surgical margin rate. CI: confidence interval.
Discussion
Result explanation
The incidence of localized renal tumors increases steadily due in large part to the increasing use and sophistication of cross-sectional abdominal imaging with technological advancements [27]. Radical nephrectomy (RN) has traditionally been considered the standard of care for localized renal tumors [28]. However, it has demonstrated worse outcomes for overall mortality than PN for patients with small renal masses [29,30]. NSS has complemented RN as a standard of care for localized renal tumors now. It preserved healthy renal parenchyma as well as provided similar oncological outcomes compared to radical nephrectomy [30-33]. With the advances of laparoscopic surgery, LPN has become a safe and effective treatment modality [34-36]. However, technical difficulty including intra-corporeal suturing, dissection, and repairing the defect under the time constraints of warm ischemia is the largest obstacle to the widespread use of LPN [35]. Advantages of the robot are three-dimensional imaging with high definition quality, stable motion without tremor, seven degrees of freedom, which allow more precision in a smaller operative field and easier resection and repair [5,6]. Our meta-analysis results showed that there was no significant difference in most perioperative outcomes between RAPN and LPN, including operative time, estimated blood loss, length of stay, intraoperative complications, postoperative minor complications, and postoperative major complications. Further, no statistical significance was found regarding positive surgical margin rate. However, the warm ischemia time favored RAPN. We think that the shorter warm ischemia time is related to the improved visualization and ease of tumor excision/reconstruction allowed by the articulating robotic instruments, because the technique for LPN and RALPN was otherwise very similar and performed by the same surgical team. It has been demonstrated that there is a significant decrease in eGFR when the warm ischemia time was longer than 30 min [37]. Some authors recommended that the pedicle clamping necessary during partial nephrectomy should be limited to 20 min of warm ischemia [38,39]. Minimizing warm ischemia times and preserving renal parenchyma are favorable for avoiding chronic renal disease and the associated morbidity [40]. In the study of Ellison JS et al [24], the operations were performed by experienced laparoscopic surgeon who performed LPN and a heterogeneous group of robotic surgeons who performed RAPN early in their adoption of the robotic technique, which may influence the result. So we made a sensitivity analysis by excluding this study, however, all the results did not changed significantly, which revealed that RAPN was easier to learn. Meta-analysis of baseline parameters showed that the participants in the RAPN group is significantly older than participants in the LPN group, revealing that RAPN can safely accommodate a wider age range than LPN.
Limitations of the included studies and meta-analysis
There are several limitations to the included studies. Firstly, There are no randomized controlled trials (RCTs) comparing RAPN with LPN until now. The nonrandomized nature of the observational study leaves it open to selection bias, confounding bias known or unknown, and reporting bias, all of which have effects on any conclusions drawn from the meta-analysis compiled from these studies. The second limitation is that most of the current studies didn’t do sub-analysis of different tumor characteristics such as different TNM classifications and tumor anatomic complexity, so we can not compare long-term oncologic outcomes between RAPN and LPN for treating localized renal tumors of specific tumor characteristics. The third limitation is that the majority of the comparative studies included in this meta-analysis were from University hospitals from western Europe and north America. The participants included in the studies underwent RAPN or LPN by world leaders in their speciality. As a result, the findings may not be applicable to small centers and the rest of the world.
Implications for future research
As designing and carrying out RCTs may be difficult for surgical practice, future research efforts should focus on improvement in research methodology of observational studies. The recommendation for the reporting of observation studies can be used as a guideline for improvement in research methodology [41]. Researchers should compare RAPN and LPN for treating localized renal tumors of specific tumor characteristics such as different TNM classifications and tumor anatomic complexity. Additionally, studies with long-term follow up are needed to compare RAPN and LPN in terms of long-term complications and oncologic outcomes.
Conclusions
RAPN had similar operative time, LOS, EBL, and perioperative complications compared with LPN, as well as positive margin rates. RAPN appears to offer the advantage of decreased WIT compared with LPN. Studies with long-term follow up are needed to compare RAPN and LPN in terms of long-term complications and oncologic outcomes.
Disclosure of conflict of interest
None.
References
- 1.MacLennan S, Imamura M, Lapitan MC, Omar MI, Lam TB, Hilvano-Cabungcal AM, Royle P, Stewart F, MacLennan G, MacLennan SJ, Dahm P, Canfield SE, McClinton S, Griffiths TR, Ljungberg B, N’Dow J UCAN Systematic Review Reference Group; EAU Renal Cancer Guideline Panel. Systematic review of perioperative and quality-of-life outcomes following surgical management of localised renal cancer. Eur Urol. 2012;62:1097–1117. doi: 10.1016/j.eururo.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 2.MacLennan S, Imamura M, Lapitan MC, Omar MI, Lam TB, Hilvano-Cabungcal AM, Royle P, Stewart F, MacLennan G, MacLennan SJ, Canfield SE, McClinton S, Griffiths TR, Ljungberg B, N’Dow J UCAN Systematic Review Reference Group; EAU Renal Cancer Guideline Panel. Systematic review of oncological outcomes following surgical management of localised renal cancer. Eur Urol. 2012;61:972–993. doi: 10.1016/j.eururo.2012.02.039. [DOI] [PubMed] [Google Scholar]
- 3.Leslie S, Goh AC, Gill IS. Partial nephrectomy-contemporary indications, techniques and outcomes. Nat Rev Urol. 2013;10:275–83. doi: 10.1038/nrurol.2013.69. [DOI] [PubMed] [Google Scholar]
- 4.Woldrich JM, Palazzi K, Stroup SP, Sur RL, Parson JK, Chang D, Derweesh IH. Trends in the surgical management of localized renal masses: thermal ablation, partial and radical nephrectomy in the USA, 1998-2008. BJU Int. 2013;111:1261–8. doi: 10.1111/j.1464-410X.2012.11497.x. [DOI] [PubMed] [Google Scholar]
- 5.Guillonneau B, Jayet C, Tewari A, Vallancien G. Robot assisted laparoscopic nephrectomy. J Urol. 2001;166:200–201. [PubMed] [Google Scholar]
- 6.Gettman MT, Blute ML, Peschel R, Bartsch G. Current status of robotics in urologic laparoscopy. Eur Urol. 2003;43:106–112. doi: 10.1016/s0302-2838(02)00579-1. [DOI] [PubMed] [Google Scholar]
- 7.Gettman MT, Blute ML, Chow GK, Neururer R, Bartsch G, Peschel R. Robotic-assisted laparoscopic partial nephrectomy: technique and initial clinical experience with DaVinci robotic system. Urology. 2004;64:914–918. doi: 10.1016/j.urology.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 8.Ho H, Schwentner C, Neururer R, Steiner H, Bartsch G, Peschel R. Robotic-assisted laparoscopic partial nephrectomy: surgical technique and clinical outcomes at 1 year. BJU Int. 2009;103:663–668. doi: 10.1111/j.1464-410X.2008.08060.x. [DOI] [PubMed] [Google Scholar]
- 9.Autorino R, Khalifeh A, Laydner H, Samarasekera D, Rizkala E, Eyraud R, Haber GP, Stein RJ, Kaouk JH. Repeat robot-assisted partial nephrectomy (RAPN): feasibility and early outcomes. BJU Int. 2013;111:767–772. doi: 10.1111/j.1464-410X.2013.11800.x. [DOI] [PubMed] [Google Scholar]
- 10.Babbar P, Hemal AK. Robot-assisted partial nephrectomy: current status, techniques, and future directions. Int Urol Nephrol. 2012;44:99–109. doi: 10.1007/s11255-011-9900-6. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aron M, Koenig P, Kaouk JH, Nguyen MM, Desai MM, Gill IS. Robotic and laparoscopic partial nephrectomy: a matched-pair comparison from a high-volume centre. BJU Int. 2008;102:86–92. doi: 10.1111/j.1464-410X.2008.07580.x. [DOI] [PubMed] [Google Scholar]
- 14.Benway BM, Bhayani SB, Rogers CG, Dulabon LM, Patel MN, Lipkin M, Wang AJ, Stifelman MD. Robot assisted partial nephrectomy versus laparoscopic partial nephrectomy for renal tumors: a multi-institutional analysis of perioperative outcomes. J Urol. 2009;182:866–872. doi: 10.1016/j.juro.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 15.Caruso RP, Phillips CK, Kau E, Taneja SS, Stifelman MD. Robot assisted laparoscopic partial nephrectomy: initial experience. J Urol. 2006;176:36–39. doi: 10.1016/S0022-5347(06)00499-X. [DOI] [PubMed] [Google Scholar]
- 16.DeLong JM, Shapiro O, Moinzadeh A. Comparison of laparoscopic versus robotic assisted partial nephrectomy: one surgeon’s initial experience. Can J Urol. 2010;17:5207–5212. [PubMed] [Google Scholar]
- 17.Jeong W, Park SY, Lorenzo EI, Oh CK, Han WK, Rha KH. Laparoscopic partial nephrectomy versus robot-assisted laparoscopic partial nephrectomy. J Endourol. 2009;23:1457–1460. doi: 10.1089/end.2009.0302. [DOI] [PubMed] [Google Scholar]
- 18.Kural AR, Atug F, Tufek I, Akpinar H. Robot-assisted partial nephrectomy versus laparoscopic partial nephrectomy: comparison of outcomes. J Endourol. 2009;23:1491–1497. doi: 10.1089/end.2009.0377. [DOI] [PubMed] [Google Scholar]
- 19.Wang AJ, Bhayani SB. Robotic partial nephrectomy versus laparoscopic partial nephrectomy for renal cell carcinoma: single-surgeon analysis of > 100 consecutive procedures. Urology. 2009;73:306–310. doi: 10.1016/j.urology.2008.09.049. [DOI] [PubMed] [Google Scholar]
- 20.Haber GP, White WM, Crouzet S, White MA, Forest S, Autorino R, Kaouk JH. Robotic versus laparoscopic partial nephrectomy: single-surgeon matched cohort study of 150 patients. Urology. 2010;76:754–758. doi: 10.1016/j.urology.2010.03.058. [DOI] [PubMed] [Google Scholar]
- 21.Cho CL, Ho KL, Chu SS, Tam PC. Robot-assisted versus standard laparoscopic partial nephrectomy: comparison of perioperative outcomes from a single institution. Hong Kong Med J. 2011;17:33–38. [PubMed] [Google Scholar]
- 22.Pierorazio PM, Patel HD, Feng T, Yohannan J, Hyams ES, Allaf ME. Robotic-assisted versus traditional laparoscopic partial nephrectomy: comparison of outcomes and evaluation of learning curve. Urology. 2011;78:813–819. doi: 10.1016/j.urology.2011.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seo IY, Choi H, Boldbaatr Y, Lee JW, Rim JS. Operative outcomes of robotic partial nephrectomy: a comparison with conventional laparoscopic partial nephrectomy. Korean J Urol. 2011;52:279–283. doi: 10.4111/kju.2011.52.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ellison JS, Montgomery JS, Wolf JS Jr, Hafez KS, Miller DC, Weizer AZ. A matched comparison of perioperative outcomes of a single laparoscopic surgeon versus a multisurgeon robot-assisted cohort for partial nephrectomy. J Urol. 2012;188:45–50. doi: 10.1016/j.juro.2012.02.2570. [DOI] [PubMed] [Google Scholar]
- 25.Long JA, Yakoubi R, Lee B, Guillotreau J, Autorino R, Laydner H, Eyraud R, Stein RJ, Kaouk JH, Haber GP. Robotic versus laparoscopic partial nephrectomy for complex tumors: comparison of perioperative outcomes. Eur Urol. 2012;61:1257–1262. doi: 10.1016/j.eururo.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Williams SB, Kacker R, Alemozaffar M, Francisco IS, Mechaber J, Wagner AA. Robotic partial nephrectomy versus laparoscopic partial nephrectomy: a single laparoscopic trained surgeon’s experience in the development of a robotic partial nephrectomy program. World J Urol. 2013;31:793–8. doi: 10.1007/s00345-011-0648-5. [DOI] [PubMed] [Google Scholar]
- 27.Hock LM, Lynch J, Balaji KC. Increasing incidence of all stages of kidney cancer in the last 2 decades in the United States: an analysis of surveillance, epidemiology and end results program data. J Urol. 2002;167:57–60. [PubMed] [Google Scholar]
- 28.Robson CJ, Churchill BM, Anderson W. The results of radical nephrectomy for renal cell carcinoma. J Urol. 1969;101:297–301. doi: 10.1016/s0022-5347(17)62331-0. [DOI] [PubMed] [Google Scholar]
- 29.Huang WC, Elkin EB, Levey AS, Jang TL, Russo P. Partial nephrectomy versus radical nephrectomy in patients with small renal tumors--is there a difference in mortality and cardiovascular outcomes? J Urol. 2009;181:55–61. doi: 10.1016/j.juro.2008.09.017. discussion 61-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson RH, Boorjian SA, Lohse CM, Leibovich BC, Kwon ED, Cheville JC, Blute ML. Radical nephrectomy for pT1a renal masses may be associated with decreased overall survival compared with partial nephrectomy. J Urol. 2008;179:468–471. doi: 10.1016/j.juro.2007.09.077. discussion 472-463. [DOI] [PubMed] [Google Scholar]
- 31.Lau WK, Blute ML, Weaver AL, Torres VE, Zincke H. Matched comparison of radical nephrectomy vs nephron-sparing surgery in patients with unilateral renal cell carcinoma and a normal contralateral kidney. Mayo Clin Proc. 2000;75:1236–1242. doi: 10.4065/75.12.1236. [DOI] [PubMed] [Google Scholar]
- 32.Pettus JA, Jang TL, Thompson RH, Yossepowitch O, Kagiwada M, Russo P. Effect of baseline glomerular filtration rate on survival in patients undergoing partial or radical nephrectomy for renal cortical tumors. Mayo Clin Proc. 2008;83:1101–1106. doi: 10.4065/83.10.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thrasher JB, Robertson JE, Paulson DF. Expanding indications for conservative renal surgery in renal cell carcinoma. Urology. 1994;43:160–168. doi: 10.1016/0090-4295(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 34.Lane BR, Gill IS. 5-Year outcomes of laparoscopic partial nephrectomy. J Urol. 2007;177:70–74. doi: 10.1016/j.juro.2006.08.093. discussion 74. [DOI] [PubMed] [Google Scholar]
- 35.Gill IS, Kavoussi LR, Lane BR, Blute ML, Babineau D, Colombo JR Jr, Frank I, Permpongkosol S, Weight CJ, Kaouk JH, Kattan MW, Novick AC. Comparison of 1,800 laparoscopic and open partial nephrectomies for single renal tumors. J Urol. 2007;178:41–46. doi: 10.1016/j.juro.2007.03.038. [DOI] [PubMed] [Google Scholar]
- 36.Allaf ME, Bhayani SB, Rogers C, Varkarakis I, Link RE, Inagaki T, Jarrett TW, Kavoussi LR. Laparoscopic partial nephrectomy: evaluation of long-term oncological outcome. J Urol. 2004;172:871–873. doi: 10.1097/01.ju.0000134292.36152.fa. [DOI] [PubMed] [Google Scholar]
- 37.Gill IS, Kamoi K, Aron M, Desai MM. 800 Laparoscopic partial nephrectomies: a single surgeon series. J Urol. 2010;183:34–41. doi: 10.1016/j.juro.2009.08.114. [DOI] [PubMed] [Google Scholar]
- 38.Thompson RH, Lane BR, Lohse CM, Leibovich BC, Fergany A, Frank I, Gill IS, Blute ML, Campbell SC. Every minute counts when the renal hilum is clamped during partial nephrectomy. Eur Urol. 2010;58:340–345. doi: 10.1016/j.eururo.2010.05.047. [DOI] [PubMed] [Google Scholar]
- 39.Becker F, Van Poppel H, Hakenberg OW, Stief C, Gill I, Guazzoni G, Montorsi F, Russo P, Stöckle M. Assessing the impact of ischaemia time during partial nephrectomy. Eur Urol. 2009;56:625–634. doi: 10.1016/j.eururo.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 40.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 41.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]


