Abstract
Chronic myeloid leukemia (CML) is a clonal disease from hematopoietic stem cells. Surviving leukemia stem cells (LSCs) and progenitor cells are a potential source for CML relapse and progression. Recent data reported that IL-1 receptor accessory protein (IL1RAP) gene was differentially expressed in CML versus normal stem and progenitor cells. However, whether the level of IL1RAP is associated with clinical phases of CML, and correlations between IL1RAP expression and detections of diagnosis is still unclear. Here we demonstrated that IL1RAP was up-regulated in CD34+ and CD34+CD38- cells which highly enriched with stem cells. Furthermore, IL1RAP expression in CD34+CD38- cells was tightly consistent with the generation of BCR-ABL fusion gene and Philadelphia chromosome. Importantly, we found that the level of IL1RAP increased with disease progression from chronic phase (CP) into accelerated phase (AP) and blast phase (BP), which was investigated not only in new diagnosed CML patients but also in patients treated with tyrosine kinase inhibitors (TKI) and hydroxyurea. Negative correlation was detected between IL1RAP expression and neutrophil (NE), whereas no relation was found in white blood cell (WBC), lymphocyte (LY), red blood cell (RBC), platelet (PLT), age or gender of CML patients. In conclusion, we identified IL1RAP as a surface marker of LSCs may be a potential indicator for CML clinical phases.
Keywords: Chronic myeloid leukemia, leukemia stem cells, IL-1 receptor accessory protein
Introduction
Chronic myeloid leukemia (CML) is a myeloproliferative disease associated with the t (9; 22) chromosomal translocation resulting in the generation of the BCR-ABL fusion gene that expresses the constitutively activated tyrosine kinase BCR-ABL. This enzyme leads to the malignant transformation of primitive hematopoietic cells and to the consequent disease. The central role of BCR-ABL in the pathogenesis of CML culminated in the discovery of tyrosine kinase inhibitors (TKI), such as imatinib, against leukemogenic BCR-ABL kinase [1]. TKI has resulted in unprecedented responses and survival rates in patients with CML, which is currently the frontline therapy for CML [2]. Unfortunately, the initial enthusiasm generated by its high response rate has been dampened by the development of BCR-ABL-dependent resistance and BCR-ABL-independent resistance [3,4]. Additionally, TKI does not kill quiescent leukemia stem cells (LSCs), which thus persist in a majority of patients [5-8]. Moreover, the original leukemic clone in CML patients in complete molecular remission after imatinib is detected [9]. These lead to the persistence of minimal residual disease (MRD) despite continued TKI therapy, which is likely to be the culprits in disease relapse. Thus, to achieve a cure of CML, a desirable strategy is to efficiently target the LSCs.
From the viewpoint of LSC, many researchers devoted to the exploration of LSC markers. IL-1 receptor accessory protein (IL1RAP) was first identified as a subunit of the IL-1 type 1 receptor complex in mouse cells that contributes to the affinity of the receptor for IL-1 [10,11], and it has recently been shown to be required for IL-1 signal transduction [12-15]. Previous experimental evidence suggested that the human gene encoding the IL1RAP is on chromosome 3q28 [16]. Recently, IL1RAP protein was found to be overexpressed on the surface of LSCs of AML patients. Furthermore, high IL1RAP expression was independently associated with poor overall survival in AML patients [17]. Other reported that IL1RAP was induced as a consequence of retroviral P210 BCR/ABL expression and was also present on a population of CD34+CD38- cells from CML patients [18]. All these proof reveal that the expression of IL1RAP may participate in the pathogenesis of myelogenous disease. However, the association of IL1RAP expression with the progression and phases of CML have not yet been sufficiently elucidated. Furthermore, whether the level of IL1RAP is discrepant for different clinical therapies, which has remained obscure.
Here we collected samples from 67 CML patients at diagnosis, summarized and analyzed their characteristics. By analyzing IL1RAP expression in CD34+ cells, CD34+CD38+ cells, CD34+CD38- cells from normal and CML patients, we identified that IL1RAP may act as a cell surface biomarker for CML stem cells. Moreover, we demonstrated the high consistency of IL1RAP expression on LSC surface with generation of BCR-ABL fusion gene and Philadelphia (Ph) chromosome which were considered as molecular marker for CML. In addition, we found that the expression of IL1RAP in CD34+CD38- cells in Chronic phase (CP) of CML patients was the lowest and levels of IL1RAP were up-regulated when disease progressed to Accelerated phase (AP) and Blast phase (BP), which suggested that the detection of IL1RAP expression in CD34+CD38- cells may be helpful to monitor condition changes in CML patients and evaluate therapeutic effects in clinical practice.
Materials and methods
Patients and controls
A total of 67 CML patients (48 males and 19 females; median age 40 years, range 8-88 years) were enrolled by diagnostic criteria for CML. Regarding the disease phase according to the clinical diagnosis, 52 cases belonged to CP of CML, 8 cases belonged to AP and 7 cases belonged to BP. The control group composed of 15 healthy individuals including 4 males and 11 females with the median age of 56 (9-76) years. Informed consent was obtained from each participant. Ethical approval for the study was obtained from the Medical Ethical Committee of the Affiliated Hospital of Xuzhou Medical College. For CML patient detailed information, see Table S1.
Samples
Peripheral blood (PB) samples of 65 CML patients were collected. Bone marrow (BM) samples from 22 CML patients were obtained by routine bone marrow aspirations after signed informed consent. Some CML samples used for our analysis were collected at the time of diagnosis, and others were during treatment. Mononuclear cells were separated using lymphocyte separation medium by density gradient centrifugation from PB and BM samples respectively. Fresh isolated cells were used for Flow cytometry, RT-PCR, Florescence in situ hybridization and chromosome karyotype analysis, while the remaining cells were store at -80°C for further study.
Reagents and monoclonal antibodies
Lymphocyte separation medium was purchased from SCR (Shanghai, China). Monoclonal antibodies including APC conjugated anti-human CD34 (Cat: 343510) and FITC conjugated anti-human CD38 (Cat: 303504) were purchased from Biolegend (USA). PE conjugated anti-hIL-1RAcP monoclonal antibody was purchased from R&D Company (USA).
Flow cytometric analysis
Isolated mononuclear cells from PB and BM were stained with anti-CD34, CD38 and IL1RAcP antibodies and incubated for 15 min at room temperature in the dark. After incubation, the cells were washed, centrifuged and were finally re-suspended in 300 μL PBS. Cells were acquired on Flow cytometer (Calibur, BD, USA) and data were analyzed with the program Cellquest (BD, USA). Isotype controls were included in each staining.
RT-PCR
Bone marrow mononuclear cells from CML patients were isolated. The cells were lysed and mRNA was extracted by TRIzol (Invitrogen). RT-PCR for BCR-ABL fusion gene detection was performed with a standard procedure. The expression level of GAPDH was first evaluated as an internal control. The primer sequences were as follows: GAPDH, 5’-CTG GGC TAC ACT GAG CAC C-3’and 5’-AAG TGG TCG TTG AGG GCA ATG-3’; BCR, 5’-GAA GTG TTT CAG AAG CTT CTC C-3’; ABL, 5’-GTT TGG GCT TCA CAC CAT TCC-3’.
Florescence in situ hybridization (FISH)
Bone marrow (3-5 mL) samples from CML patients were processed using hypotonic solution to acquire nuclei. The nuclei were fixed in Carnoy solution. The following dual-color probe was used to detect BCR-ABL fusion gene: GLP BCR/ABL (DF) (Beijing GP Medical Technologies). FISH was performed according to the manufacturer’s instructions. Cells were counterstained with 4’, 6-diamidino-2-phenylindole (DAPI) and examined at room temperature on a microscope (BX 51 OLYMPUS, JAPAN). The images were acquired and analyzed using Cyto Vision 3.93 (APPLIED IMAGING).
Chromosome karyotype analysis
After bone marrow cells from CML patients were cultured in RPMI 1640 media (GIBCO BRL) for a period of time, dividing cells were arrested in metaphase by addition of colchicines (Sigma). The cells were next treated with a hypotonic solution to obtain nuclei, which were then treated with a chemical fixative, dropped on a glass slide, and stained with Giemsa dye (Applichem, Germany). R-banding was used in our lab. Once chromosomes were stained, we began to analyze the number and structure of chromosomes under the microscope (OLYMPUS, JAPAN).
Statistical analysis
SPSS16.0 software was used for statistical analysis. Data were expressed as mean values ± standard deviation (SD). Comparisons were carried out using Student’s t test if data conform to normality and homogeneity of variance, otherwise, using non-parametric two-tailed t-test for two-group comparisons. Multiple comparisons were performed by one-way ANOVA tests. The correlation between two parameters was expressed as Pearson or Spearman coefficient. X2 test was used for analyzing the consistency between expression of IL1RAP and BCR-ABL fusion gene, IL1RAP and Ph chromosome respectively. The P value < 0.05 was considered statistically significant.
Results
IL1RAP is overexpressed on stem and progenitor cells of CML patients
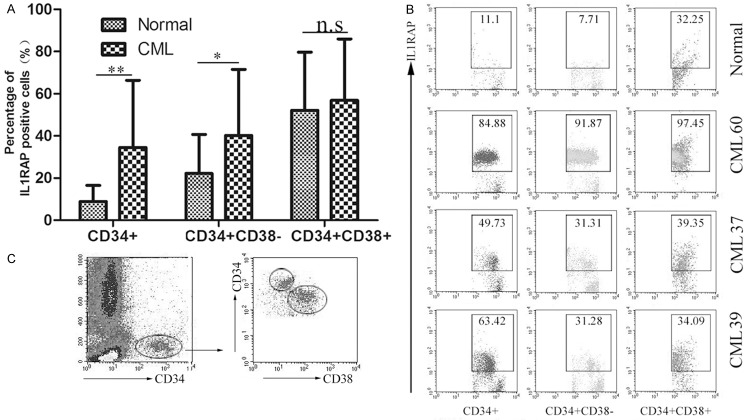
To analyze IL1RAP expression in normal and CML patients, mononuclear cells from peripheral blood were isolated and stained with anti-CD34, anti-CD38 and anti-IL1RAP Abs. Three cell subpopulations (CD34+, CD34+CD38+, CD34+CD38- subsets) were detected and analyzed by Flow cytometry. 62.84% of the total CD34+ cells were CD34+CD38- subpopulation. Results in Figure 1A showed that varying levels of IL1RAP expression was observed on the three subpopulations in CML patients and normal. For the total CD34+ cells, more increased IL1RAP expression were detected in CML group than normal group (P < 0.01). Similar phenomenon was observed for CD34+CD38- subpopulation and statistical analyzed difference was significant (P < 0.05). However, within the CD34+CD38+ subpopulation, although an increased tendency of IL1RAP expression was shown in CML patients compared with normal, this discrepancy was no significantly different. As shown in Figure 1B, representative samples showed that higher percentage of IL1RAP+ cells was detected in CML patients than that in healthy volunteers. Altogether, these results indicated that the expression of IL1RAP was up-regulated in CD34+ cell of CML patients, which was mainly reflected on CD34+CD38- subset enriched with LSCs. Our data is consistent with the previous study that IL1RAP may be a useful cell surface marker for LSC.
Figure 1.
IL1RAP was overexpressed on stem and progenitor cells of CML patients. Peripheral blood mononuclear cells of CML patients (n = 65) and healthy volunteers (n = 15) were isolated and stained for Flow cytometry analysis. A. Statistical analysis of IL1RAP expression in CD34+, CD34+CD38+, CD34+CD38- subsets from normal and CML patients respectively. *P < 0.05, **P < 0.01, n.s represents that there is no significant difference between groups. The bars represent the mean ± SD. B. FACS dot plots showed IL1RAP expression within three subsets from representative CML patients and normal blood samples. The data in each square presents the percentage of IL1RAP+ cells. C. An example of the gating strategy used for selecting CD34+CD38- and CD34+CD38+ cell population in normal and CML patients.
IL1RAP is associated with generation of BCR-ABL fusion gene and Ph chromosome
CML is characterized by Ph chromosome formation, which is presented with the generation of BCR-ABL fusion gene at the molecular level. To date, it remains unclear whether the expression of IL1RAP as a cell surface marker for LSC of CML is consistent with Ph chromosome and BCR-ABL fusion gene as molecular markers of CML or not. Therefore, we analyzed the expression of IL1RAP in CD34+CD38- cells and BCR-ABL gene and Ph chromosome from blood and bone marrow samples of the same CML patients were detected simultaneously. Our data showed that 96.9% and 100% of CML cases was IL1RAP positive in CD34+CD38- cells in peripheral blood and bone marrow samples of BCR-ABL positive CML patients respectively (PB: n = 38, BM: n = 13, Figure 2A). Conversely, 86.1% and 84.6% of CML patients wi- th IL1RAP+CD34+CD38- cells in blood and bone marrow was BCR-ABL positive (Figure 2A). Then, we analyzed the correlation between IL1RAP expression in CD34+CD38- cells and Ph chromosome in CML patients. As shown in Figure 2B, 91.7% of cases in the Ph+ group was IL1RAP+ and 81.5% of cases in the IL1RAP+ group carried the Ph chromosome (n = 24). Furthermore, as for bone marrow samples, observations revealed that all of Ph+ CML cases were IL1RAP+, while 92.9% of CML patients with IL1RAP+CD34+CD38- cells presented Ph+ chromosome (n = 14). Collectively, the above data demonstrated the high consistency of IL1RAP as LSC surface marker with BCR-ABL expression and Ph chromosome as intracellular marker for CML. This implied that expression of IL1RAP in CD34+CD38- cells may be a potential and reliable indicator for the generation of BCR-ABL fusion gene and Ph chromosome in CML patients.
Figure 2.
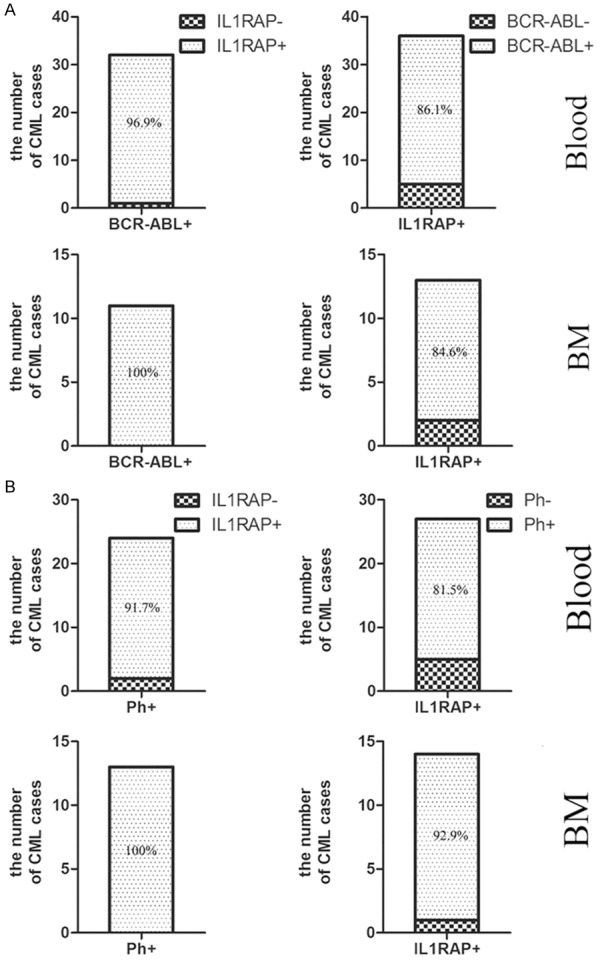
IL1RAP expression was strongly associated with BCR-ABL gene and Ph chromosome formation. IL1RAP+CD34+CD38- cell and IL1RAP-CD34+CD38- cell from blood and bone marrow were detected by Flow cytometry, while BCR-ABL fusion gene and Ph chromosome were detected by RT-PCR, FISH and chromosome karyotype analysis respectively. Stacked bars displayed the consistency between IL1RAP expression and BCR-ABL gene, IL1RAP expression and Ph chromosome respectively in blood and bone marrow samples from CML patients.
Level of IL1RAP were up-regulated with the development of clinical phase of CML patients
The disease progression of CML occurs in three stages including CP, AP and BP, which are driven by the oncogenic activity of the BCR-ABL fusion kinase. Previous and our studies have suggested that IL1RAP is a hallmark of CML CD34+ cells and IL1RAP is strongly correlated with the formation of BCR-ABL fusion gene [18]. Accordingly, we analyzed whether there was correlation between IL1RAP and clinical phases of CML. Mononuclear cells form peripheral blood were isolated and analyzed for CD34+, CD34+CD38- and CD34+CD38+ subpopulation by Flow cytometry respectively. Results in Figure 3A showed that the IL1RAP expression in total CD34+ subset from CP patients was obviously lower than that in AP and BP patients. However, there was no significant difference in CD34+ cells between AP and BP stages. Further study gated in CD34+CD38- and CD34+CD38+ cells showed that the diverse of IL1RAP expression presented only in CD34+CD38- subset considered as enriched LSC, but not CD34+CD38+ subset. Similar phenomenon was observed that no significant difference between AP and BP was detected both in CD34+CD38- and CD34+CD38+ subsets from AP and BP patients. Representative cases for CML patients (n = 3) were shown in Figure 3B, in which higher IL1RAP expression in CD34+ and CD34+CD38- cells from AP and BP patients were detected. Summarizing the results, we concluded that the expression of IL1RAP in CP stage was lowest and IL1RAP was up-regulated when disease progressed to AP and BP stages, which suggested that the level of IL1RAP in CD34+CD38- cells could be an indicator for disease progression.
Figure 3.
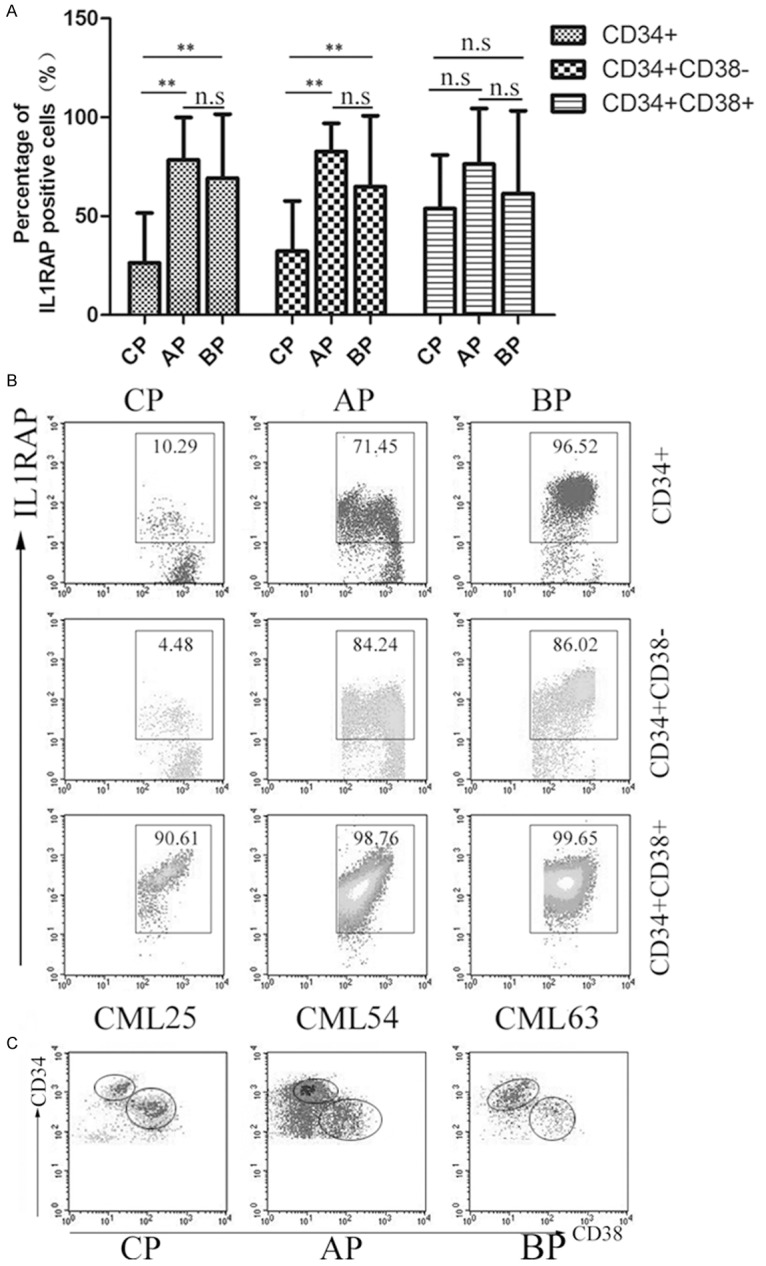
IL1RAP expression in different subpopulations from CP, AP and BP phases. Peripheral blood mononuclear cells were isolated and stained for Flow cytometry analysis. A. The percentage of IL1RAP+ cells at different phases in CD34+, CD34+CD38-, CD34+CD38+ subsets respectively. Bars represent mean ± SD. **P < 0.01, n.s P > 0.05. B. Representative dot plots from CP, AP and BP were present and IL1RAP expression was detected by Flow cytometry. C. Gating strategy for CD34+CD38+ and CD34+CD38- cells in each disease subtype.
Percentage of IL1RAP+ cells in CD34+CD38- cells from patients following HSCT, TKI and Hydroxyurea
Several treatments options in CML are available including interferon, chemotherapy, TKI, Hydroxyure, and allo-HSCT [19,20]. Our above studies have demonstrated that level of IL1RAP were up-regulated with the development of clinical phase of CML patients. Next, we would like to explore whether IL1RAP expression was still correlated with clinical stages after treatment. Here we analyzed the expression of IL1RAP in CD34+CD38- cells from CML patients following TKI (CP: n = 13; AP: n = 6; BP: n = 5), Hydroxyure (CP: n = 30) and allo-HSCT (n = 5) respectively, which were used the most common in clinical therapy. According to statistical analysis showed in Figure 4A, lower levels of IL1RAP in CD34+ CD38- cells from patients following HSCT were observed than that from patients in CP following Hydroxyure (P < 0.05). In contrast, although IL1RAP expression in CD34+ CD38- cells from patients following HSCT was lower compared with patients in CP following TKI, no statistically significant difference was detected (P > 0.05) (Figure 4B). Furthermore, we compared the expression of IL1RAP in CD34+CD38- cells from patients after TKI in different stages. Results in Figure 4B showed that IL1RAP expression in CD34+CD38- cells from patients after TKI in CP was obviously lower than that in AP (P < 0.01) and BP (P < 0.05). However, there was no statistical difference between AP and BP in CD34+ CD38- cells from patients after TKI. In combination with our above results, these data further proved that levels of IL1RAP were up-regulated with the development of clinical phases of CML patients.
Figure 4.
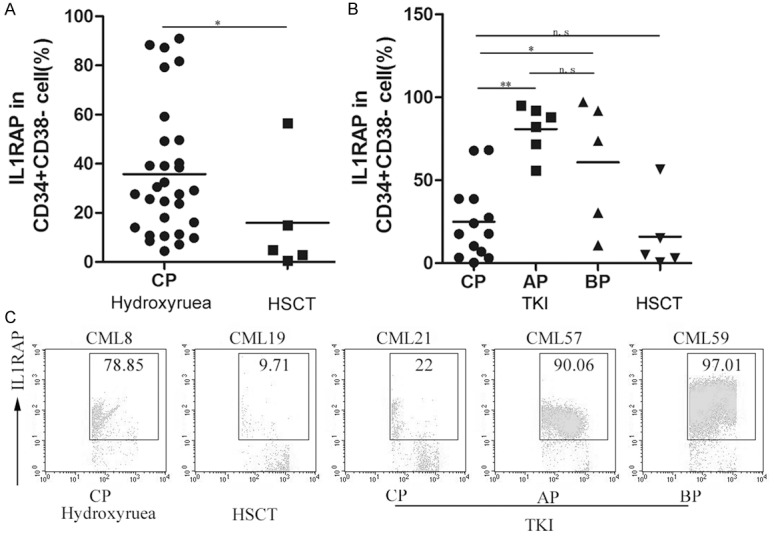
The levels of IL1RAP were up-regulated with the development of clinical phases of CML patients after treatment. A, B. The percentage of IL1RAP+CD34+CD38- cells from peripheral blood of CML patients in CP, AP and BP following HSCT, TKI and Hydroxyurea was analyzed respectively. Horizontal bars represent mean value; *P < 0.05; **P < 0.01; n.s means P > 0.05. Each symbol represents one data point from one patient. C. Representative dot plots showed IL1RAP expression within CD34+CD38- cells from peripheral blood of CML patients after treatment in different phases. The data in each square presents the percentage of IL1RAP+ cells.
IL1RAP expression is negatively correlated with NE
As we all know, hematopoietic stem cells (HSCs) are the origin of all kinds of blood cells in myeloid and lymphoid lineages. Previous and our above studies have demonstrated that IL1RAP may be a cell surface marker for stem cells of CML [18]. However, the relationship between the expression of IL1RAP in HSCs and all differentiated blood cells arising from HSCs was not clear so far. Thus, we analyzed the association between IL1RAP expression in CD34+CD38- cells and number of WBC, NE, LY, RBC, PLT. As shown in Figure 5, IL1RAP expression in CD34+CD38- cells was negatively correlated with NE in Blood (r = -0.533, P = 0.000) and in BM (r = -0.682, P = 0.004) from CML patients respectively, and the statistical analyzed difference was significant. In addition, for blood samples, IL1RAP was also negatively correlated with LY (r = -0.386, P = 0.007) and positively correlated with WBC (r = 0.341, P = 0.006), which was not observed for bone marrow samples. As for RBC and PLT, we did not find any relationship with IL1RAP regardless of blood or bone marrow.
Figure 5.
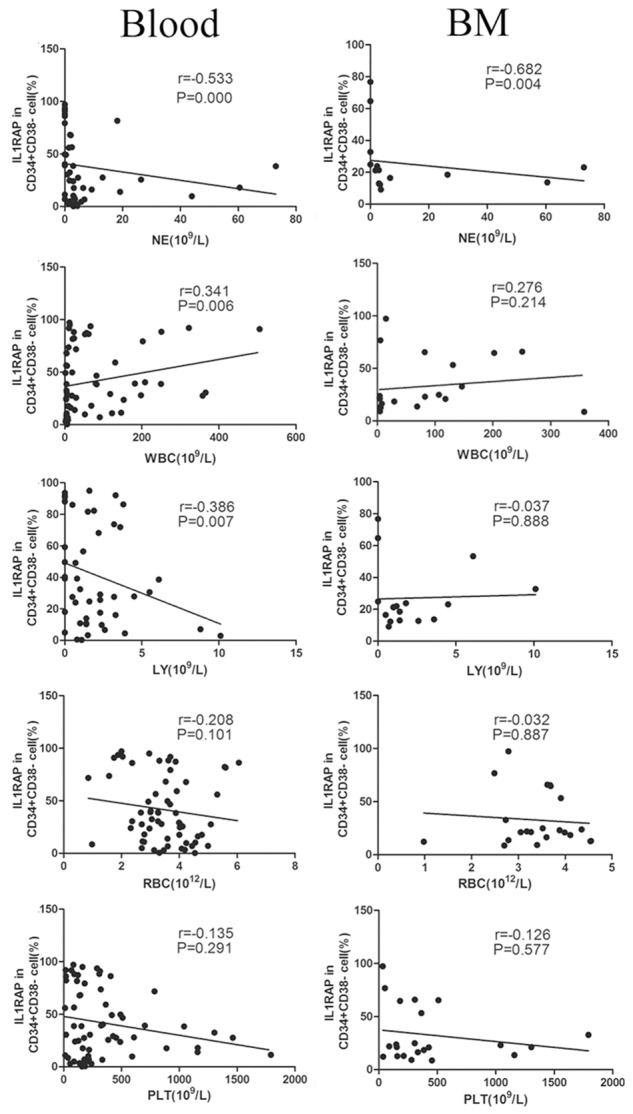
The correlation between expression of IL1RAP in CD34+CD38- cells and cells in blood and bone marrow. Expression of IL1RAP was gated on CD34+CD38- cells. Graphs in left and right columns showed samples from peripheral blood and bone marrow respectively. Each symbol represents one data point from one patient. The lines show trend line.
No statistical correlation between the level of IL1RAP and age or gender
Statistical data reported that the median age at diagnosis of CML is 55 years, with a third of all patients with CML older than 60 years [21]. Furthermore, it is reported that CML occurs more frequently in men than in women, with a male:female ratio of 1.3:1, and the incidence increases with age [22,23]. CML is a myeloproliferative disorder resulting from clonal expansion of transformed hematopoietic progenitor cells. However, the correlation of IL1RAP as a surface marker of LSC and patients’ age or gender is still not clear. Thus, next we analyzed the correlation of age and IL1RAP expression in CD34+CD38- cells from peripheral blood and bone marrow of patients respectively. Results in Figure 6A showed that no correlation between the percentage of IL1RAP positive CD34+CD38- cells in blood and age was observed (n = 65, r = 0.052, P = 0.682). The same phenomenon was obtained for bone marrow cells (n = 22, r = -0.111, P = 0.622). Furthermore, we compared the IL1RAP levels in CD34+CD38- cells in blood and bone marrow samples from male and female patients respectively. As shown in Figure 6B, although a increased tendency of the mean percentage of IL1RAP+CD34+CD38- cells was observed in male patients, no statistical significant difference was detected between male and female in blood group and BM group (P > 0.05). Our results indicated that the expression of IL1RAP in CD34+CD38- subsets was not correlated with patients’ age or gender. In other words, although CML is common in old people and occurs more frequently in men than in women, after the onset of CML, there is no correlation between IL1RAP level in LSC of patients and age or gender.
Figure 6.
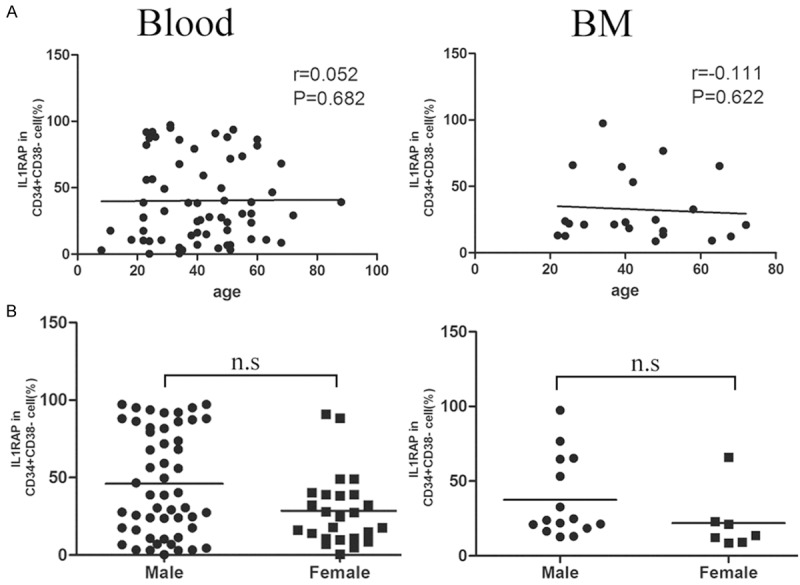
IL1RAP expression in CD34+CD38- subsets was not correlated with age or gender. A. Correlation between the percentage of IL1RAP+CD34+CD38- cells and age in patients’ Blood and BM. Each symbol represents one data point from one patient. The lines show trend line. B. The correlation of IL1RAP expression in CD34+CD38- cells from peripheral blood and bone marrow with gender was analyzed respectively. Horizontal bars represent mean value; n.s means P > 0.05.
Discussion
Chronic myeloid leukemia accounts for 15-20% of newly diagnosed cases of leukemia in adults. The majority of CML cases are characterized by cells carrying the Ph chromosome, which contains the oncogenic BCR-ABL fusion protein. Treatment with imatinib mesylate and other TKI revolutionized the therapy of CML by inhibiting the BCR-ABL tyrosine kinase [24-26]. However, CML patients require chronic daily treatment with TKIs, and interruption or discontinuation of therapy almost invariably results in disease relapse. Moreover, it also alone does not cure this disease because of the persistence of MRD which is thought to reside in LSC despite continued TKI therapy. Because TKIs does not kill quiescent LSCs, which thus persist in a majority of patients and are likely to be the culprits in disease relapse [4,27,28]. Therefore, some other means are essential and ultimately the goal for the development of new CML treatments will be to target TKI-resistant cells, which are the LSCs of CML.
IL-1RAP is identified as a subunit of membrane-bound form of the IL-1 receptor [29,30]. It have been described as candidate hepatocellular carcinoma markers in several microarray-based studies [31]. Furthermore, studies have shown that IL1RAP overexpression is implicated in the molecular pathogenesis of different myeloid malignancies, including CML and subsets of AML and MDS [17,32]. In order to further validate the role of IL1RAP in CML, we compared IL1RAP expression on stem and progenitor cells from CML patients and normal. Our data show that IL1RAP is up-regulated in CD34+ and CD34+CD38- subsets of CML patients. This is consistent with previous studies that IL1RAP is overexpression on LSC surface [18]. Moreover, we analyzed the correlation between IL1RAP and BCR-ABL fusion gene, Ph as characteristic mark of CML. As demonstrated in this study, IL1RAP is closely associated with BCR-ABL and Ph generation. Although large prospective studies are needed in our ongoing research, in collaboration with previous findings we can draw a conclusion that IL1RAP as cell surface marker may be a potential indicator for reflecting the generation of BCR-ABL and Ph as intracellular marker of CML. Furthermore, targeting IL1RAP may open up a previously unexplored avenue for therapy of CML.
Notwithstanding the remarkable success in treating patients in CML-CP with TKI, to which some patients acquire resistance or intolerance, culminating in disease progression from CML-CP to the AP and BP. However, mechanisms responsible for the disease progression remain largely unknown and the current literature on the changing of patients’ clinical, laboratory and molecular data when they transform CP to AP or BP is little. Our above results have demonstrated that IL1RAP as a cell surface marker distinguish normal stem cell and LSC of CML. Thus, we next explored the correlation of IL1RAP with CML disease progression. As shown in our study, for the total CD34+ and CD34+CD38- cells, IL1RAP levels in patients with AP and BP are significantly higher than in patients with CP (P < 0.05). However, there is no significant difference between IL1RAP levels in patients with AP and BP. It suggests that higher levels of IL1RAP are associated with poor course of the disease or transformation into AP or BP. Thus we hope that IL1RAP may become an advantageous tool to help predict the severity of disease in the clinic. However, it is noteworthy that because approximately 90% of CML patients are in CP at diagnosis, we do not have sufficient AP and BP samples now. Therefore, the levels of IL1RAP elevated could indicate transformation into AP or BP need to be cleared by expanding our samples.
Our examination of clinical samples reveal that the percentage of IL1RAP+ cells in CD34+CD38-subset is markedly lower in HSCT group compared to in Hydroxyurea group. We all know that patients after HSCT are similar to normal and our above findings have indicated that IL1RAP levels on the total CD34+ cells and CD34+CD38- cells are significantly lower in normal versus CML patients. Moreover, IL1RAP expression in CD34+CD38- cells from patients after TKI treatment in CP is obviously lower than that in AP and BP. So these data confirm again that IL1RAP expression is tightly associated with CML clinical phases and might be an effect indicator for disease progression. Now we are prolonging our study and following up patients until they obtain complete remission to better explore the association of IL1RAP with therapy.
Besides, to better understand other factors of affecting IL1RAP, we performed multivariate analysis. The multivariate analysis studying age, sex, blood components of CML patients showed that only a negative significant correlation of IL1RAP with NE in blood and in bone marrow was detected simultaneously. In the classical CML, only the myeloid and megakaryotytic compartments show a neoplastic expansion, and the granulocytic progenitors have a moderate degree of impairment of their differentiation and maturation capacity, whereas the erythroid, monocytic and B- and T-lymphoid lineages do not reveal any functional damage [33]. This might at least in part account for our finds.
Acknowledgements
We would like to thank all patients for their collaboration in this study. We also thank the staff at the Xuzhou Medical College/Laboratory of Transplantation and Immunology for their support during the study. This work was supported by the National Natural Sciences Foundation of China (No. 81200376), Jiangsu Special Grant of Clinical Science (BL2012022 and BL2013010), Foundation of Jiangsu Province Six Talents Peak (2012-WSN-082) and Talents Foundation of Xuzhou Medical College (2012KJZ13).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Schenone S, Bruno O, Radi M, Botta M. New insights into small-molecule inhibitors of Bcr-Abl. Med Res Rev. 2011;31:1–41. doi: 10.1002/med.20175. [DOI] [PubMed] [Google Scholar]
- 2.Holtz MS, Slovak ML, Zhang F, Sawyers CL, Forman SJ, Bhatia R. Imatinib mesylate (STI571) inhibits growth of primitive malignant progenitors in chronic myelogenous leukemia through reversal of abnormally increased proliferation. Blood. 2002;99:3792–800. doi: 10.1182/blood.v99.10.3792. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia R, Holtz M, Niu N, Gray R, Snyder DS, Sawyers CL, Arber DA, Slovak ML, Forman SJ. Persistence of malignant hematopoietic progenitors in chronic myelogenous leukemia patients in complete cytogenetic remission following imatinib mesylate treatment. Blood. 2003;101:4701–7. doi: 10.1182/blood-2002-09-2780. [DOI] [PubMed] [Google Scholar]
- 4.Jiang X, Zhao Y, Smith C, Gasparetto M, Turhan A, Eaves A, Eaves C. Chronic myeloid leukemia stem cells possess multiple unique features of resistance to BCR-ABL targeted therapies. Leukemia. 2007;21:926–35. doi: 10.1038/sj.leu.2404609. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Peng C, Sullivan C, Li D, Li S. Critical molecular pathways in cancer stem cells of chronic myeloid leukemia. Leukemia. 2010;24:1545–54. doi: 10.1038/leu.2010.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Copland M, Hamilton A, Elrick LJ, Baird JW, Allan EK, Jordanides N, Barow M, Mountford JC, Holyoake TL. Dasatinib (BMS-354825) targets an earlier progenitor population than imatinib in primary CML but does not eliminate the quiescent fraction. Blood. 2006;107:4532–9. doi: 10.1182/blood-2005-07-2947. [DOI] [PubMed] [Google Scholar]
- 7.Graham SM, Jorgensen HG, Allan E, Pearson C, Alcorn MJ, Richmond L, Holyoake TL. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood. 2002;99:319–25. doi: 10.1182/blood.v99.1.319. [DOI] [PubMed] [Google Scholar]
- 8.Holyoake T, Jiang X, Eaves C, Eaves A. Isolation of a highly quiescent subpopulation of primitive leukemic cells in chronic myeloid leukemia. Blood. 1999;94:2056–64. [PubMed] [Google Scholar]
- 9.Sobrinho-Simoes M, Wilczek V, Score J, Cross NC, Apperley JF, Melo JV. In search of the original leukemic clone in chronic myeloid leukemia patients in complete molecular remission after stem cell transplantation or imatinib. Blood. 2010;116:1329–35. doi: 10.1182/blood-2009-11-255109. [DOI] [PubMed] [Google Scholar]
- 10.Greenfeder SA, Nunes P, Kwee L, Labow M, Chizzonite RA, Ju G. Molecular cloning and characterization of a second subunit of the interleukin 1 receptor complex. J Biol Chem. 1995;270:13757–65. doi: 10.1074/jbc.270.23.13757. [DOI] [PubMed] [Google Scholar]
- 11.Subramaniam S, Stansberg C, Cunningham C. The interleukin 1 receptor family. Dev Comp Immunol. 2004;28:415–28. doi: 10.1016/j.dci.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 12.Korherr C, Hofmeister R, Wesche H, Falk W. A critical role for interleukin-1 receptor accessory protein in interleukin-1 signaling. Eur J Immunol. 1997;27:262–7. doi: 10.1002/eji.1830270139. [DOI] [PubMed] [Google Scholar]
- 13.Jensen LE, Muzio M, Mantovani A, Whitehead AS. IL-1 signaling cascade in liver cells and the involvement of a soluble form of the IL-1 receptor accessory protein. J Immunol. 2000;164:5277–86. doi: 10.4049/jimmunol.164.10.5277. [DOI] [PubMed] [Google Scholar]
- 14.Smith DE, Hanna R, Della F, Moore H, Chen H, Farese AM, MacVittie TJ, Virca GD, Sims JE. The soluble form of IL-1 receptor accessory protein enhances the ability of soluble type II IL-1 receptor to inhibit IL-1 action. Immunity. 2003;18:87–96. doi: 10.1016/s1074-7613(02)00514-9. [DOI] [PubMed] [Google Scholar]
- 15.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–32. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dale M, Hammond DW, Cox A, Nicklin MJ. The human gene encoding the interleukin-1 receptor accessory protein (IL1RAP) maps to chromosome 3q28 by fluorescence in situ hybridization and radiation hybrid mapping. Genomics. 1998;47:325–6. doi: 10.1006/geno.1997.5113. [DOI] [PubMed] [Google Scholar]
- 17.Barreyro L, Will B, Bartholdy B, Zhou L, Todorova TI, Stanley RF, Ben-Neriah S, Montagna C, Parekh S, Pellagatti A, Boultwood J, Paietta E, Ketterling RP, Cripe L, Fernandez HF, Greenberg PL, Tallman MS, Steidl C, Mitsiades CS, Verma A, Steidl U. Overexpression of IL-1 receptor accessory protein in stem and progenitor cells and outcome correlation in AML and MDS. Blood. 2012;120:1290–8. doi: 10.1182/blood-2012-01-404699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaras M, Johnels P, Hansen N, Agerstam H, Tsapogas P, Rissler M, Lassen C, Olofsson T, Bjerrum OW, Richter J, Fioretos T. Isolation and killing of candidate chronic myeloid leukemia stem cells by antibody targeting of IL-1 receptor accessory protein. Proc Natl Acad Sci U S A. 2010;107:16280–5. doi: 10.1073/pnas.1004408107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cortes J, Kantarjian H. How I treat newly diagnosed chronic phase CML. Blood. 2012;120:1390–7. doi: 10.1182/blood-2012-03-378919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kantarjian HM, O’Brien S, Cortes JE, Shan J, Giles FJ, Rios MB, Faderl SH, Wierda WG, Ferrajoli A, Verstovsek S, Keating MJ, Freireich EJ, Talpaz M. Complete cytogenetic and molecular responses to interferon-alpha-based therapy for chronic myelogenous leukemia are associated with excellent long-term prognosis. Cancer. 2003;97:1033–41. doi: 10.1002/cncr.11223. [DOI] [PubMed] [Google Scholar]
- 21.Brave M, Goodman V, Kaminskas E, Farrell A, Timmer W, Pope S, Harapanhalli R, Saber H, Morse D, Bullock J, Men A, Noory C, Ramchandani R, Kenna L, Booth B, Gobburu J, Jiang X, Sridhara R, Justice R, Pazdur R. Sprycel for chronic myeloid leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia resistant to or intolerant of imatinib mesylate. Clin Cancer Res. 2008;14:352–9. doi: 10.1158/1078-0432.CCR-07-4175. [DOI] [PubMed] [Google Scholar]
- 22.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 23.Faderl S, Talpaz M, Estrov Z, Kantarjian HM. Chronic myelogenous leukemia: biology and therapy. Ann Intern Med. 1999;131:207–19. doi: 10.7326/0003-4819-131-3-199908030-00008. [DOI] [PubMed] [Google Scholar]
- 24.Cortes J, Hochhaus A, Hughes T, Kantarjian H. Front-line and salvage therapies with tyrosine kinase inhibitors and other treatments in chronic myeloid leukemia. J. Clin. Oncol. 2011;29:524–31. doi: 10.1200/JCO.2010.31.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Hare T, Zabriskie MS, Eiring AM, Deininger MW. Pushing the limits of targeted therapy in chronic myeloid leukaemia. Nat Rev Cancer. 2012;12:513–26. doi: 10.1038/nrc3317. [DOI] [PubMed] [Google Scholar]
- 26.Shami PJ, Deininger M. Evolving treatment strategies for patients newly diagnosed with chronic myeloid leukemia: the role of second-generation BCR-ABL inhibitors as first-line therapy. Leukemia. 2012;26:214–24. doi: 10.1038/leu.2011.217. [DOI] [PubMed] [Google Scholar]
- 27.Savona M, Talpaz M. Getting to the stem of chronic myeloid leukaemia. Nat Rev Cancer. 2008;8:341–50. doi: 10.1038/nrc2368. [DOI] [PubMed] [Google Scholar]
- 28.Quintas-Cardama A, Kantarjian H, Cortes J. Imatinib and beyond--exploring the full potential of targeted therapy for CML. Nat Rev Clin Oncol. 2009;6:535–43. doi: 10.1038/nrclinonc.2009.112. [DOI] [PubMed] [Google Scholar]
- 29.Wesche H, Korherr C, Kracht M, Falk W, Resch K, Martin MU. The interleukin-1 receptor accessory protein (IL-1RAcP) is essential for IL-1-induced activation of interleukin-1 receptor-associated kinase (IRAK) and stress-activated protein kinases (SAP kinases) J Biol Chem. 1997;272:7727–31. doi: 10.1074/jbc.272.12.7727. [DOI] [PubMed] [Google Scholar]
- 30.Volpe F, Clatworthy J, Kaptein A, Maschera B, Griffin AM, Ray K. The IL1 receptor accessory protein is responsible for the recruitment of the interleukin-1 receptor associated kinase to the IL1/IL1 receptor I complex. FEBS Lett. 1997;419:41–4. doi: 10.1016/s0014-5793(97)01426-9. [DOI] [PubMed] [Google Scholar]
- 31.Kim JW, Ye Q, Forgues M, Chen Y, Budhu A, Sime J, Hofseth LJ, Kaul R, Wang XW. Cancer-associated molecular signature in the tissue samples of patients with cirrhosis. Hepatology. 2004;39:518–27. doi: 10.1002/hep.20053. [DOI] [PubMed] [Google Scholar]
- 32.Pellicano F, Sinclair A, Holyoake TL. In search of CML stem cells’deadly weakness. Curr Hematol Malig Rep. 2011;6:82–7. doi: 10.1007/s11899-011-0085-y. [DOI] [PubMed] [Google Scholar]
- 33.Pane F, Intrieri M, Quintarelli C, Izzo B, Muccioli GC, Salvatore F. BCR/ABL genes and leukemic phenotype: from molecular mechanisms to clinical correlations. Oncogene. 2002;21:8652–67. doi: 10.1038/sj.onc.1206094. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.