Abstract
In this study, we report an active targeting liposomal formulation directed by a novel peptide (T7) that specifically binds to the transferrin receptor (TfR) overexpressed on ovarian carcinoma cells. The objectives of this study were to evaluate the in vitro and in vivo tumor drug targeting delivery of T7-anchored liposomes on A2780 cells. T7 conjugated to the distal end of DSPE-PEG2000-maleimide was incorporated into the liposomes via a post-insertion method, the liposome could keep stability in 50% FBS for more than 24 h. The uptake efficiency of T7-LP was 3.7 times higher than that of LP on A2780 cells. The anti-proliferative activity of T7-LP-PTX against A2780 cells was much stronger compared to that of LP-PTX and free PTX, respectively. The homing specificity and anticancer efficacy of T7-LP-PTX were also evaluated on the tumor spheroids, which revealed that T7-LP-PTX was more efficaciously internalized into tumor cells than LP. Compared to LP, T7-LP-PTX showed the highest accumulation capability into tumor spheroids, and the greatest tumor growth inhibitory effect in vitro. In the in vivo study, the T7-LP-PTX showed the best inhibition effect of the tumor growth for the A2780-bearing mice and tumor accumulation. In brief, the T7-LP may be an efficient targeting drug delivery system for ovarian carcinoma.
Keywords: Ovarian carcinoma, transferrin receptor, liposome, tumor targeting
Introduction
Ovarian carcinoma is the sixth most common cancer in women and the leading reason of death among all gynecologic malignancies in Western countries and in China [1,2]. In 2011, it was estimated that approximately 21,880 new cases of ovarian cancer were diagnosed in the United States [3]. In the past several years, although progress has been made in the treatment of ovarian cancer by improved debulking surgery and the introduction of platinum-taxane regimens, the long-term survival rate of patients with advanced ovarian cancer remains less than 29% [4]. PTX, as one of common anticancer drugs, has been found to possess activity of interest as a single agent [5-7]. Specifically, PTX not only promotes the assembly and stabilization of microtubules but also interferes with essential cellular functions including mitosis, cell transport, and cell motility [8-10]. Because of its poor solubility in water, PTX is currently formulated in a 1:1 mixture of Cremophor EL (apolyethoxylated castor oil) and ethanol to create Taxol. However, Cremophor EL has been reported that it would lead to various serious side effects, including acute hypersensitivity reactions, nephrotoxicity, neurotoxicity, and cardio toxicity [11].
In the last two decades, liposomal drug delivery systems hold extraordinary potential for delivery of therapeutics to tumor, various strategies have been used to improve their targeting specificity and cellular uptake. PEGylation has been extensively employed to enhance the accumulation of liposomes in tumor tissues through enhanced permeability and retention (EPR) effects, which was the passive form of targeting. In attempts to increase the specificity of interaction between liposomes and tumor cells, recent efforts in the liposome field have been focusing on the development of the active tumor targeted liposomes, which were modified with some specific ligands such as Tf [12], folic acid [13], peptides [14] or antibodies [15], and could selectively recognize and bind to the specific receptor over-expressed on tumor cells, resulting in increased targeting efficiency and less toxicity. Transferrin receptors are highly expressed on tumor cells. Peptide T7 (sequenced HAIYPRH) was screened by a phage display system on the cells expressing human transferrin receptor (TfR) [16]. The high affinity for TfR was comparable to that of transferrin (Tf), with Kd of w10 nM. Recently, the internalization of the complex formed after T7 binding with TfR was found to be facilitated by endogenous Tf [17]. Thus, for TfR highly-expressed tumors, T7 may be a potential ligand for targeting delivery of agents.
In this paper, T7 was modified by covalent linkage bond on the surface of liposome through bifunctional polyethyleneglycol (PEG), yielding T7-LP. The ovarian carcinoma targeting efficiency of T7-LP as drug delivery vectors was evaluated in vitro and in vivo. Furthermore, the anti-tumor effect of T7-LP-PTX was systematically evaluated in vitro and in vivo.
Materials and methods
Soybean lecithin consisting of 90-95% phosphatidylcholine and mPEG2000-DSPE and Mal-PEG2000-DSPE were purchased from Avanti lipid (USA). Cholesterol (CHO) was purchased from Chengdu Kelong Chemical Company (Chengdu, China). FITC-PE was purchased from Avanti lipid (USA). T7 peptide with terminal cysteine (Cys-HAIYPRH) was synthesized according to the standard solid phase peptide synthesis by Shanghai Dacheng Bio-pharmaceutical Co., Ltd. (Shanghai, China). Cell culture plates were purchased from corning Biotechnology Co. Ltd. (USA). Other chemicals and reagents were of analytical grade and obtained commercially.
BALB/c female athymic mice (about 20 g) were purchased from the Experiment Animal Center of Zhengzhou University (P. R. China). All of the animal experiments adhered to the principles of care and use of laboratory animals and were approved by the Experiment Animal Administrative committee of Zhengzhou University.
Synthesis of T7-PEG2000-DSPE
The T7-PEG2000-DSPE was synthesized according to literature with a modest modification [18]. T7 was conjugated with DSPE-PEG2000-BTC in 0.01 M isotonic HEPES buffer (pH 7.5) under the conditions of reaction (4 h at 4°C, gentle stirring and 1:2 molar ratio of peptides to DSPE-PEG2000-BTC). The reaction was traced by TLC till the peptide was completely consumed. The mixture was then dialyzed against water, and lyophilized. The resulting conjugate DSPE-PEG2000-T7 was then used for preparing liposomes without further purification.
Preparation of liposomes
T7 modified PTX loaded liposomes (T7-LP-PTX) were prepared by thin film hydration methods [19]. Briefly, SPC, cholesterol, PTX (10% of the SPC + cholesterol weight), DSPE-PEG2000 and DSPE-PEG2000-T7 were dissolved in chloroform (total molar ratio of phospholipid and cholesterol derivatives was 5:3, molar ratio of DSPE-PEG2000 and DSPE-PEG2000-T7 was 9.5:0.5). Chloroform was then evaporated by rotary evaporation and residual organic solvent was removed in vacuum overnight. Then the thin film was hydrated in phosphate-buffered saline (PBS, pH 7.4) for 1 h at 37°C, followed by an intermittently probe sonication for 50 s at 100 W.
FITC-labeled liposomes were prepared as the T7-LP-PTX with the SPC being replaced by the FITC-PE. The final concentration of FITC-PE was 10 μg/ml. DIR loaded liposomes were prepared as the T7-LP-PTX with the PTX being replaced by the DIR.
Characterization of liposomes
Size and zeta potential measurements
The size and zeta potential of the liposomes were measured by a dynamic light scattering detector (zetasizer Nano-ZS90, Malvern, UK).
Drug encapsulation efficiency (EE)
The free PTX was removed by passing through a Sephadex G-50 column. The amount of PTX encapsulated in the liposomes was measured by high performance liquid chromatography (HPLC, Agilent LC1200). A reversed phase Inertsil®ODS-3 column (150-4.6 mm, pore size 5 mm, GL Science Inc., Tokyo, Japan) was used. Liposomes were dissolved in 1 ml DCM. After evaporating DCM, 3 ml mobile phase (50:50 v/v acetonitrile/water solutions) was added to dissolve the drugs. The solution was then filtered by 0.45 mm PVDF syringe filter for HPLC analysis. The column effluent was detected at 227 nm with a UV/VIS detector.
EE (%) = (amount of drug encapsulated in LP/initial amount of drug used in the fabrication of LP) × 100%.
In vitro stability of liposomes in serum
In order to demonstrate the serum stability of liposomes, particle sizes and turbidity variations were monitored in the presence of fetal bovine serum (FBS) [20]. Briefly, liposomes were mixed with equal volume of FBS under 37°C with gentle shaking at 30 rpm. At predetermined time points (1 h, 2 h, 4 h, 8 h and 24 h), 200 mL of the sample was pipetted out and onto a 96-well plate to measure the transmittance at 750 nm by a microplate reader (Thermo Scientific Vari- oskan Flash, USA).
In vitro drug release
In vitro PTX release study was conducted using dialysis method under sink conditions [21]. An aliquot of each PTX-loaded liposome (0.1 mL) or free PTX was placed into dialysis tube (MWCO 8000) and tightly sealed. Then the dialysis tubes were immersed into 100 mL PBS (pH 7.4) containing 0.1% (v/v) Tween 80 and were incubated in an incubator under 37°C for 24 h with mild oscillating at 50 rpm. At predetermined time points, 0.1 mL release medium was sampled and replaced with equal volume of fresh release medium. Then the samples were diluted with acetonitrile and the concentrations of PTX were determined by HPLC.
In vitro cellular uptake
A2780 cells were grown in RPMI-1640 medium (GIBCO) contains 10% FBS, 100 μg/mL streptomycin, and 100 U/mL penicillin. The cells were maintained at 37°C in a humidified incubator with 5% CO2.
For quantitative study, A2780 cells a (AmericanType Culture Collection) were harvested with 0.125% Gibcotrypsin-EDTA solution (Invitrogen) and seeded into 24 well assay plates (Corning Incorporated) at 105 viable cells/well. After the cells reached confluence, the cells were incubated with 100 uL of 10 μg/mL FITC-labeled liposomes (all three types) in the 1640 supplemented with 10% Hyclone fetal bovine serum (FBS, Thermo Scientific) and 1% Gibco penicillin-streptomycin (Invitrogen) at 37°C for 2 h and 4 h. At designated time period, the suspension was removed and the wells were washed three times with 1000 μL cold PBS, and were trypsinized and resuspended in 0.4 mL PBS. The fluorescent intensity of cells was measured by a flow cytometer (CytomicsTM FC 500, Beckman Coulter, Miami, FL, USA) with the excitation wavelength at 465 nm and the emission wavelength at 502 nm. Ten thousand events were recorded for each sample. Competitive effect of endocytosis was also studied by pre-incubating the cells with excess free T7 (200 mg/ml) before incubating with T7-LP.
For the qualitative study, A2780 cells were harvested with 0.125% Gibco trypsin-EDTA solution (Invitrogen) and seeded in LABTEK cover glass chambers (Nagle Nunc) having RPMI-1640 at a concentration of 5 × 103 viable cells/chamber. The cells were incubated overnight and were subsequently incubated with FITC -labeled liposomes in the RPMI-1640 (concentration of 10 μg/mL) at 37°C. After 4 h, the cells were washed 3 times with cold PBS and fixed by 4% paraformaldehyde for 20 min. Then, the cells were washed twice with cold PBS and observed by confocal laser scanning microscopy (CLSM) (Leica, Germany).
In vitro cytotoxicity and anti-proliferation assay
The cytotoxicity of PTX-loaded liposome was measured with MTT assay. A2780 cells were plated in 96-well plates at a density of 2 × 103 cells per-well and cultured for 24 h. PTX-loaded liposome and free-PTX were diluted to predetermined concentrations with PBS, and added into each well for 24 h and 48 h incubation. The final concentrations of PTX were in the range of 0.25~25 μg/ml. Then 20 ml MTT (5 mg/ml in PBS) was added into each well and incubated for 4 h under 37°C. Finally the medium was removed and replaced by 150 ml dimethyl sulfoxide. Then the absorbance was measured by a microplate reader (Thermo Scientific Varioskan Flash, USA) at 570 nm. The cells treated with PBS were evaluated as controls. Cell viability was calculated by the following formula: cell viability (%) = Atreated/Acontrol × 100, in which Atreated and Acontrol represented the absorbance of treated cells and control cells, respectively.
Evaluation of tumor spheroid penetration
To prepare the three-dimensional tumor spheroids, A2780 cells were seeded at a density of 2 × 103 cells/200 μL per well in 96-well plates coated by 80 μL of a 2% low-melting-temperature agarose. Seven days after the cells were seeded; tumors spheroids were treated with 10 μg/mL FITC-labeled liposome. After 4 h of incubation, the spheroids were rinsed three times with ice-cold PBS and fixed by 4% paraformaldehyde for 30 min. Then, the spheroids were transferred to glass slides and covered by glycerophosphate. Fluorescent intensity was observed by laser scanning confocal microscopy (Leica, Germany).
Growth inhibition of tumor spheroid
Tumor spheroids were prepared as described above. Seven days later, wells containing spheroids were treated with 0.25 μg/mL of PTX solution, and PTX-loaded liposomes. The length and width of each spheroid were measured every day for 7 days. The volume was calculated. A volume curve was drawn to compare the effect of each treatment by the different formulations.
In vivo tumor growth inhibition study
The ovarian carcinoma nude mice xenograft models were established by injecting A2780 cells (1 × 107 cells per animal, subcutaneous injection) into the back of 4-6 week-old BALB/c female athymic nude mice. 40 nude mice with ovarian carcinoma xenograft models were divided into four groups. When the tumors reached 100-200 mm3, the mice were administrated with saline, free PTX, LP-PTX and T7-LP-PTX, respectively. The drugs were administrated once every other day (totally 10 mg/kg) were measured. The tumor inhibition rate was calculated by the formula as fellow.
Tumor inhibition rate = (1 - VX/Vsaline) × 100%. Vx was the tumor volume of mice treated and Vsaline was the tumor volume of saline group.
In vivo imaging
The DIR-loaded liposomes were utilized, as previously described, to investigate the distribution of liposomes in nude mice bearing A2780 cells. The nude mice xenograft models were established as described in 2.9. The DiR-loaded liposomes were injected into nude mice bearing A2780 cells via intravenously administration, and then the in vivo fluorescence imaging was performed by IVIS Spectrum system (Caliper, Hopkington, MA).
Statistical analysis
Analysis of variance (ANOVA) was used to check the variance of the whole values in each group. Statistical significance was evaluated by using Student’s t-test for the comparisons of experimental groups.
Results
Characterization of the liposomes
Particle size, size distribution and drug encapsulation efficiency
As shown in Table 1, the average diameter of the conventional PTX-loaded liposomes was around 120 nm with a PDI of 0.20. The drug encapsulation efficiency (EE) of the liposome is crucial to justify their clinical applications. The encapsulation efficiency of LP-PTX and T7-LP-PTX were above 87%. Table 1 show the EE of the two types of liposomes formulations. Obviously, such a formulation system demonstrates the prospect for a practically useful drug delivery carrier with appropriate size, stability and drug loading capacity.
Table 1.
Characteristics of PTX-loaded LP and T7-LP (n = 3)
| Group | Particle Size (nm) | Polydispersity | Zeta-potential (mV) | Encapsulation Efficiency (%) |
|---|---|---|---|---|
| LP-PTX | 111±8.8 | 0.157 | -4.53±1.26 | 88.66±4.25 |
| T7-LP-PTX | 120±9.7 | 0.202 | 3.59±1.55 | 87.15±3.57 |
Stability of PTX-loaded liposomes in the presence of fetal bovine serum
Liposomal particle stability against physiological condition is prerequisite for the further application in vivo, so 50% FBS was employed to mimic the in vivo situation. Particle sizes and transmittance variations as important parameters were monitored in our study to explore the serum stability of liposomes. As can been seen in Figure 1A, the particle sizes and transmittance have hardly changed for the liposomes over 24 h, indicating that there was no aggregation in the presence of serum.
Figure 1.

A. The variations in turbidity (represented by transmittance) of liposomes in 50% FBS (n = 3, mean ± SD). B. The PTX release profiles of free PTX, LP-PTX and T7-LP-PTX in PBS over 24 h (n = 3, mean ± SD).
Herein the in vitro release of PTX from the liposomes was investigated. Figure 1B shows the release profile of these four groups. Compared with the rapid release of free PTX, all the four liposome groups exhibited similar and sustained release manners and no burst initial release was observed.
Cellular uptake
To investigate the selectivity and internalization of liposomes, the cellular uptake of different liposomes in A2780 cells was examined, as shown in Figure 2. In A2780 cells, T7-LP uptake was much higher than that of LP, about 3.7-fold higher, respectively. That was the result of the targeting capacity of transferrin receptors expression in A2780 cells. The cell uptake efficiency of T7-LP at 4 h was about 3.1-fold higher than that at 2 h. The cellular uptake results were consistent with the transferrin expression levels on cell surface, indicating that the T7 motif had the ability to recognize and target transferrin receptors expressed on the surface of cells. The fluorescence intensity of the LP was the lowest observed in A2780 cells. In The fluorescence intensity of T7-LP was much stronger than LP, and the quantitative results indicated almost the same results as the fluorescence imaging (Figure 3). For the T7-LP, the receptor-mediated endocytosis (RME) can further facilitates the cellular uptake, resulting in higher uptake efficiency than LP.
Figure 2.

Measurement of in vitro uptake of CFPE-labeled liposomes by A2780 cells at 2 h and 4 h. Data represented the mean ± SD, n = 3. Compare to LP. ***, P < 0.001.
Figure 3.

Confocal laser scanning microscopy (CLSM) images show the internalization of fluorescent liposome in cells (4 h incubation).
In vitro cytotoxicity and anti-proliferation assay
It is shown in Figure 4 that for the cancer cells incubated with free PTX at 25, 10, 2.5 and 0.25 μg/ml drug concentration, the cell viability (the survival rate) after 24 h treatment was measured to be 61.37%, 63.86%, 68.18% and 71.12%, respectively. Instead after 48 h treatment at the four designated drug concentrations, the cell viability decreased to 43.68%, 47.22%, 53.70% and 57.65%, respectively. It is straight forward to understand that higher drug concentration and longer incubation time will cause lower cell viability, or equivalently higher mortality of the cells. For the cytotoxicity of the liposome formulations, the same concentration 25, 10, 2.5 and 0.25 μg/ml of the drug, which is encapsulated in the liposomes, were applied. After 24 h treatment, the cell viability were found to be 67.43%, 69.88%, 73.49% and 78.75% for the LP-PTX, and 41.33%, 46.19%, 53.81% and 56.48% for T7-LP-PTX, respectively. Instead, after 48 h treatment, the cell viability was found decreased to 51.53%, 55.48%, 58.66% and 61.22% for the LP-PTX, and 18.43%, 21.36%, 26.55% and 29.04% for the T7-LP-PTX, respectively. It showed that the anti-proliferative effect of the drug-loaded liposomes was markedly elevated by the modification with T7, and the increased myotoxicity of PTX incorporated in T7-LP may be related to the fast internationalization of this formulation and the successive drug release from T7-LP to reach therapeutic concentration range inside the cells.
Figure 4.

The diagrams of cell viability at various concentrations of the drug under 24 h (A) and 48 h (B) treatment. The data are presented as the mean ± SD (n = 6). Compare to free/LP. #, P < 0.01. Compare to 24 h . **, P < 0.01.
Tumor spheroid penetration and growth inhibition of tumor spheroid
In many solid tumors, there are hypoxic and avascular tumor regions. Due to the poor permeation of delivery systems, the amount of drug accessing inside the solid tumors is low. We prepared the tumor spheroid which could imitate the in vivo status because the tumor spheroids are free of blood vessels [20,21]. The tumor spheroid can serve as an invaluable tool to evaluate the solid tumor penetration effect of nanoparticles. Figure 5A shows confocal laser scanning microscopic images of 3D tumor spheroids 4 h.
Figure 5.
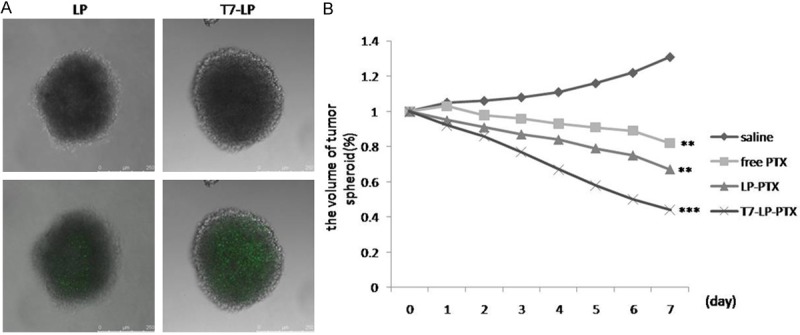
A. Penetration of FITC-labeled LP and T7-LP throughout A2780 tumor spheroids at 4 h. B. Change ratios of tumor spheroid volume (%) after applying various PTX formulations and blank control. Compare to LP. ***, P < 0.001, **, P < 0.01.
The influence of various treatments on the growth of tumor spheroids was also studied. Figure 5B represents the in vitro tumor spheroid volume ratios after treatment with saline, free PTX, LP-PTX and T7-LP-PTX at the final PTX concentration of 0.25 μg/ml, respectively. It was observed that tumor spheroids continued to grow in size and volume in the absence of any drug (131% of the primary volume after 7 days). The obvious reduction in volume of tumor spheroids was observed for all PTX formulations after 7 days treatment, indicating that tumor spheroids were sensitive to PTX. The final tumor spheroid volumes were nearly 82%, 67% and 44% for free PTX, LP-PTX and T7-LP-PTX of the initial tumor spheroid volumes on day 7 respectively. The result indicated that T7-LP-PTX significantly improved the inhibitory effects on the 3D tumor spheroids. For solid tumors, there are regions with high pressure and few vessels. Since the tumor spheroids could imitate the in vivo status because the tumor spheroids are free of blood vessels, the higher inhibitory effect suggests that T7-LP-PTX may improve therapeutic effect in vivo.
In vivo tumor growth inhibition study
For defining the efficacy of the functionalized liposomes in animals, the ovarian carcinoma nude mice xenograft models were established. As shown in Table 2, Compared to physiological saline and PTX group, LP-PTX and T7-LP-PTX groups significantly inhibit the growth of tumor, such superiority of the liposomal formulations in vivo compared to PTX may be attributed to the sustained release profiles, prolonged blood circulation time and EPR effect of liposome. A significant enhanced tumor inhibition effect was observed in T7-LP-PTX over the other groups.
Table 2.
In vivo anti-tumor effect of different PTX formulations in A2780-bearing mice
| group | Weight of tumor (g) | Inhibition rate (%) |
|---|---|---|
| saline | 4.32±0.35 | - |
| Free PTX | 3.40±0.28 | 21.2±3.3 |
| LP-PTX | 2.94±0.27 | 32.1±2.2 |
| T7-LP-PTX | 1.47±0.12 | 66.4±5.4 |
NIR imaging
The in vivo biodistribution and tumor accumulation profiles of DIR-loaded liposomes were clearly visualized by monitoring the whole body NIRF intensity in subcutaneous xenograft bearing nude mice model (Figure 6). Obviously, the tumor accumulation was the highest for T7-LP. The results implied that the T7-LP could efficiently target to solid tumors and decrease nonspecific accumulation in normal organs such as livers, lungs and kidneys. Control animals injected with PBS produced no fluorescence signals, which confirmed that the observed fluorescence signal was truly from the liposomes. Preliminary studies have demonstrated that the passive accumulation of liposomes reached maximum in tumor tissue between 24 and 48 h, which provided sufficient time for liposomes to accumulate at the tumor site. The results of serum stability and biodistribution together suggested that the liposome was capable of increasing the stability of liposomes and the T7-LP obtained remarkable accumulation in the tumor region.
Figure 6.

The in vivo image of the mice that were anesthetized at 24 hours after intravenous injection of different kind of DIR-loaded liposomes.
Discussion
Ovarian carcinoma is a serious cancer, thus, to improve the therapeutic efficiency of ovarian carcinoma in the clinic. Receptor‑modified nanocarriers were developed for ovarian carcinoma targeting and therapy. The ability of carriers to specifically targeting delivery cargoes to tumors is important to effective cancer therapy [18]. The active tumor targeted liposomes, which were modified with some specific ligands such as transferrin, folic acid, peptides or antibodies, could selectively recognize and bind to the specific receptor over-expressed on tumor cells, then result in increased targeting efficiency and less toxicity. In this liposomal formulation, the receptor‑targeting properties of T7 were combined with the enhanced cell uptake effect to improve the transport of desired cargo to the tumor.
Particle size plays a critical role in their clearance by the sinusoidal spleens of human and rats. Particles must be small enough to avoid the splenic filtration process at the interendothelial cell slits in the walls of venous sinuses. It has been reported that the particle size of nanocarrier less than 200 nm can effectively reach the tumor tissue by enhanced permeability and retention (EPR) effect [14]. In the current study, the sizes of the prepared T7-LP-PTX were about 130 nm, which provided a favorable size condition for tumor targeting by enhanced permeability and retention (EPR) effect.
In this study, we developed transferrin receptor ligand T7 peptide modified liposomes, the cellular uptake of T7-LP and LP was characterized using A2780 cells. The results (Figure 2) showed that the uptake of T7-LP was great than LP, demonstrating that T7 effectively mediated LP uptake by A2780 cells.
In the cytotoxicity experiment, the PTX-loaded LP and T7-LP demonstrated time- and dose-dependent cytotoxic activity towards A2780 cells. Notably, the T7-LP-PTX formulation achieved the lowest cell viability among the PTX formulations in all equivalent drug concentration levels applied. This further confirmed the advantages for cellular uptake shown in the previous experiments, which resulted from the coactivation of the TF receptor, thus contributing to an additional pathway through which the drug could be delivered into the cell cytoplasm to induce cell apoptosis.
Due to the poor permeation of delivery systems, the level of drug that is able to access the inner area of solid tumors is low. As a consequence, these chemotherapy ‘blind areas’ eventually and ineluctably induce the recurrence of cancer, and the overall chemotherapeutic efficacy of anticancer agents is compromised. For a cancer treatment to be curative, the delivery system must efficiently penetrate the tumor tissue to reach all of the viable cells [21]. Thereby, three‑dimesnional multicellular modeling, which represents the avascular regions found in numerous solid tumor tissues, can serve as an invaluable tool to evaluate the solid tumor penetration effect of a drug delivery system. In the present study, the results (Figure 5A and 5B) demonstrated the penetration capabilities of LP and T7-LP. The T7 could enhance the transport and increase the uptake and penetration of lipsomes by the A2780 spheroids. These findings indicated that the T7 was able to effectively transport the liposome across the spheroids. Also, the tumor spheroid was used to imitate the in vivo status of the solid tumor and to evaluate the antitumor efficiency of the different PTX formulations. The results showed that PTX-loaded T7-LP possessed the greatest antitumor activity, which may benefit from its increased penetration and uptake by tumors.
In vivo imaging and antitumor experiment further demonstrated that T7-LP-PTX could effectively target to the tumor and inhibit the growth of tumor in vitro. In conclusion, we successfully developed the targeting liposomes modified with the specific ligand T7. This liposomal delivery system possessed increased cellular uptake efficiency and targeting specificity in A2780 cells whose transferrin expression levels were high, and achieved an efficient targeted delivery of payload into tumor cells in A2780 tumor bearing nude mice and ultimately achieved excellent therapeutic efficacy on tumor-bearing mice. Based on all the studies in this report, we claimed the T7 modified liposomes as a potential anti-tumor drug delivery system.
Disclosure of conflict of interest
None.
References
- 1.Shahzad MK, Shin YH. Biological significance of HORMA domain containing protein 1 (HORMAD1) in epithelial ovarian carcinoma. Cancer Lett. 2012;18:76–85. doi: 10.1016/j.canlet.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chao HT, Wang L. Low dose histone deacetylase inhibitor, LBH589, potentiates anticancer effect of paclitaxel in epithelial ovarian cancer via PI3K/Akt pathway. Cancer Lett. 2012;2:29–38. doi: 10.1016/j.canlet.2012.08.035. [DOI] [PubMed] [Google Scholar]
- 3.Gould N, Sill MW, Mannel RS, Thaker PH, Disilvestro P, Waggoner S, Yamada SD, Armstrong DK, Wenzel L, Huang H, Fracasso PM, Walker JL. A phase I study with an expanded cohort to assess the feasibility of intravenous paclitaxel, intra peritoneal carboplatin and intra peritoneal paclitaxel in patients with untreated ovarian, fallopian tube or primary peritoneal carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2012;125:54–8. doi: 10.1016/j.ygyno.2011.12.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Despierre E, Lambrechts D. The molecular genetic basis of ovarian cancer and its roadmap towards a better treatment. Gynecol Oncol. 2010;117:358–365. doi: 10.1016/j.ygyno.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Curtin JP, Blessing JA. Paclitaxel, an active agent in nonsquamous carcinomas of the uterine cervix: a gynecologic oncology group study. J. Clin. Oncol. 2001;19:1275–1278. doi: 10.1200/JCO.2001.19.5.1275. [DOI] [PubMed] [Google Scholar]
- 6.McGuire WP, Blessing JA, Moore D, Lentz SS, Photopulos G. Paclitaxel has moderate activity in squamous cervix cancer. A Gynecologic Oncology Group study. J. Clin. Oncol. 1996;14:792–795. doi: 10.1200/JCO.1996.14.3.792. [DOI] [PubMed] [Google Scholar]
- 7.Rose PG, Blessing JA, Gershenson DM, McGehee R. Paclitaxel and cisplatin as first-line therapy in recurrent or advanced squamous cell carcinoma of the cervix: a gynecologic oncology group study. J. Clin. Oncol. 1999;17:2676–2680. doi: 10.1200/JCO.1999.17.9.2676. [DOI] [PubMed] [Google Scholar]
- 8.Chua DT, Sham JS, Au GK. A phase II study of docetaxel and cisplatin as first line chemotherapy in patients with metastatic nasopharyngeal carcinoma. Oral Oncol. 2005;41:589–595. doi: 10.1016/j.oraloncology.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 9.McCarthy JS, Tannock IF, Degendorfer P, Panzarella T, Furlan M, Siu LL. A phase II trial of docetaxel and cisplatin in patients with recurrent or metastaticnasopharyngeal carcinoma. Oral Oncol. 2002;38:686–690. doi: 10.1016/s1368-8375(01)00134-8. [DOI] [PubMed] [Google Scholar]
- 10.Horwitz SB. Taxol (paclitaxel): mechanisms of action. Ann Oncol. 1994;5:3–6. [PubMed] [Google Scholar]
- 11.Liebmann J, Cook JA, Mitchell JB. Solvent for paclitaxel, and toxicity. Lancet. 1993;342:14–28. doi: 10.1016/0140-6736(93)92789-v. [DOI] [PubMed] [Google Scholar]
- 12.Bidros DS, Vogelbaum MA. Novel drug delivery strategies in neuro-oncology. Neurotherapeutics. 2009;3:539–546. doi: 10.1016/j.nurt.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pillai JJ, Thulasidasan AK, Anto RJ, Chithralekha DN, Narayanan A, Kumar GS. Folic acid conjugated cross-linked acrylic polymer (FA-CLAP) hydrogel for site specific delivery of hydrophobic drugs to cancer cells. J Nanobiotechnology. 2014;12:25. doi: 10.1186/1477-3155-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin Y, Chen H, Zhang Q, Wang X, Yuan W, Kuai R, Tang J, Zhang L, Zhang Z, Zhang Q, Liu J, He Q. Liposome formulated with TAT-modified cholesterol for improving brain delivery and therapeutic efficacy on brain glioma in animals. Int J Pharm. 2011;420:304–312. doi: 10.1016/j.ijpharm.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Gao H, Qian J, Cao S, Yang Z, Pang Z, Pan S, Fan L, Xi Z, Jiang X, Zhang Q. Precise glioma targeting of and penetration by aptamer and peptide dual-functioned nanoparticles. Biomaterials. 2012;33:5115–5123. doi: 10.1016/j.biomaterials.2012.03.058. [DOI] [PubMed] [Google Scholar]
- 16.Miyata S, Kawabata S, Hiramatsu R, Doi A, Ikeda N, Yamashita T, Kuroiwa T, Kasaoka S, Maruyama K, Miyatake S. Computed tomography imaging of transferrin targeting liposomes encapsulating both boron and iodine contrast agents by convection-enhanced delivery to F98 rat glioma for boron neutron capture therapy. Neurosurgery. 2011;68:1380–1387. doi: 10.1227/NEU.0b013e31820b52aa. [DOI] [PubMed] [Google Scholar]
- 17.Du W, Fan Y, Zheng N, He B, Yuan L, Zhang H, Wang X, Wang J, Zhang X, Zhang Q. Transferrin receptor specific nanocarriers conjugated with functional 7peptide for oral drug delivery. Biomaterials. 2013;34:794–806. doi: 10.1016/j.biomaterials.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Qin Y, Chen H, Yuan W, Kuai R, Zhang Q, Xie F, Zhang L, Zhang Z, Liu J, He Q. Liposome formulated with TAT-modified cholesterol for enhancing the brain delivery. Int J Pharm. 2011;419:85–95. doi: 10.1016/j.ijpharm.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 19.Yin Y, Wu X, Yang Z, Zhao J, Wang X, Zhang Q, Yuan M, Xie L, Liu H, He Q. The potential efficacy of R8-modified paclitaxel-loaded liposo-mes on pulmonary arterial hypertension. Ph-arm Res. 2013;30:2050–2062. doi: 10.1007/s11095-013-1058-8. [DOI] [PubMed] [Google Scholar]
- 20.Gao H, Yang Z, Cao S, Xiong Y, Zhang S, Pang Z, Jiang X. Tumor cells and neo-vasculature dual targeting delivery for glioblastoma treatment. Biomaterials. 2014;35:2374–2382. doi: 10.1016/j.biomaterials.2013.11.076. [DOI] [PubMed] [Google Scholar]
- 21.Huile G, Shuaiqi P, Zhi Y, Shijie C, Chen C, Xinguo J, Shun S, Zhiqing P, Yu H. A cascade targeting strategy for brain neuroglial cells employing nanoparticles modified with angiopep-2 peptide and EGFP-EGF1 protein. Biomaterials. 2011;32:8669–8675. doi: 10.1016/j.biomaterials.2011.07.069. [DOI] [PubMed] [Google Scholar]


