Abstract
Essential oil has performed a variety of indirect services used as insect/pest repellent. The present study investigated the acute and subchronic toxicity of eucalyptus oil emulsion in water (EOE). In addition, we conduct safety pharmacology evaluation of EOE to supplement the toxicity tests and provide a basis for a comprehensive understanding of the toxicity of EOE. Acute administration of EOE was done as single dose from 2772 mg to 5742 mg of EOE per kg/bodyweight (b.wt.) and subchronic toxicity study for thirty days was done by daily oral administration of EOE at doses of 396, 792 and 1188 mg/kg b.wt. In SPF SD rats. The acute toxicity study showed the LD50 of EOE was 3811.5 mg/kg. The subchronic toxicity study suggested the high-dose and middle-dose EOE slowed down the growth of male rats. The clinical pathology showed the high-dose and middle-dose EOE could cause damage to liver and kidney. The safety pharmacology indicated that EOE had no side effects on rats. These results suggest that EOE is a safe veterinary medicine for external use.
Keywords: Essential oil, acute toxicity, subchronic toxicity, safety pharmacology
Introduction
In recent years, there were increased risks of pesticide resistance, enhanced pest resurgence, toxicological implications to human health and increased environmental pollution due to the excessive use of synthetic pesticides in the croplands, urban environment, and water bodies to get rid of noxious pests [1]. For the past few years, it is clear that essential oils are easily extractable, ecofriendly being biodegradable easily catabolized in the environment [2], hardly persistence in soil and water [3-5]. Moreover, they possess low or no toxicity against vertebrates [6] and play an important role in plant protection against pests [4,7,8]. Therefore, it is urgent to find safe essential oil from natural plants to replace these synthetic chemicals.
Eucalyptus oil is a kind of essential oil extracted from the plant named Cinnamomum longepaniculatum (Gamble) N. Chao which is a rare species of Lauraceae family [9]. It mainly consists of 1,8-cineole [10] and α-pinene [11] and has multiple applications in perfume and pharmaceutical industry [12-14]. Eucalyptus oil can act as an insect repellent to provide protection against parasites, such as human head lice [15], mosquito bites [16,17] and so on. It also possesses antifungal, antimicrobial [18], analgesic, anti-inflammatory [19,20], antioxidant [21] and anticancer activity [22]. In the present study, we describe a range of toxicological and safety pharmacology evaluations of eucalyptus oil emulsion in water for the purpose of development of a safety formulation of eucalyptus oil.
Materials and methods
Eucalyptus oil
Eucalyptus oil (70% content) extracted from Eucalyptus globulus Labill was supplied by the Xinran biotechnology Co., Ltd. (Shanghai, P. R. China). The eucalyptus oil emulsion in water (EOE) with a variety of concentrations including 4%, 6%, 8%, 12%, 18% and 30% was prepared by using the tween-80 and span-80 as the emulsifier.
Animals
Young adult male (average weight 100 ± 5 g) and female (average weight 100 ± 5 g) SPF Sprague-Dawley (SD) rats were purchased from Chengdu Dossy Experimental Animals Co., Ltd. [License No. SCXK (Sichuan) 2008-24]. They were kept in the animal houses of Sichuan Agriculture University (Ya’an, China), and in well ventilated sterile polypropylene cages. Each cage contained five rats of the same sex. Based on the Guidelines of the International Com- mittee on Laboratory Animals, they were maintained at a controlled temperature of 20-25°C and relative humidity of 55 ± 5% and 12 h light/dark cycle with the lights off at 7 p.m. Experiments were started after acclimating the rats for one week. They were treated with a started diet from Nuvital Nutrients (Colombol, PR, Brazil) and allowed to access to distilled water ad arbitrium.
Oral acute toxicity
An oral study for calculating LD50 was performed according to the Organization for Economic Co-operation and Development (OECD) Guideline 425 “Up and Down procedure” [23-25]. In this test, animals were dosed once at a time. If the animal survived, the dose of the next animal was increased; if the animal died, the dose for the next animal was decreased. Five experimental groups with 10 rats each, containing an equal number of both male and female, were formed. The five groups were treated with EOE at dose of 2772, 3267, 3960, 4752 and 5742 mg/kg. In each case, the product volume administered by gavage was 1 mL/100 g body weight (b.w.). The animals were observed for gross behavioral neurologic, autonomic and toxic effects for 24h and then daily for 14 days. The toxicological effect was assessed on the basis of mortality, which was expressed as an LD50 value. The LD50 was estimated according to the method described by Keplinger et al. [26].
Thirty-day subchronic oral toxicity
Four groups of 10 rats, each containing 5 females and 5 males, consumed a daily dose of 0 (Group II, emulsifier and distilled water), 396 (Group III, 4% EOE), 792 (Group IV, 8% EOE), 1188 (Group V, 12% EOE) mg/kg b.w. for 30 consecutive days. The animals were monitored for clinical and behavioral symptoms such as diarrhea, immobility and mortality during the 30 days. Each rat was marked with a unique identification number by using trinitrophenol and body weight was measured once a week.
Clinical examination
In the period of the test, all animals were observed once daily for the clinical signs of toxicity. The change of animals’ hair, eyes, mucous membrane, respiratory system, nervous system, physical activity and behavior were recorded. To reduce the residual interaction between the animals and postmortem tissue autolysis, the dead animals and endangered animals were dissected timely [27].
Body weight and food consumption
The quantity of food and water consumed was recorded for each group of animals every week. The weight of each rat was recorded on the first day and once a week thereafter (with intervals of 7 ± 1 days) throughout the course of the study and mean body weights were calculated. The weight of the heart, liver, spleen, lung, kidney, testis, uterus and ovary were recorded and expressed in relation to the final body weight.
Clinical pathology
Chemical pathological analysis was performed on all animals for the blood chemistry and hematology of the terminal sacrifice animals once toward the end of the in-life phase of the study. The blood samples which were about 0.5 mL each for hematology assessments were collected in a pre-calibrated tube containing heparin sodium. Approximately 1 mL blood samples were collected into a tube containing no preservative for clinical chemistry assessments. These samples were centrifuged in a refrigerated centrifuge, and the serum was transferred to a labeled tube.
Hematology
The hematological parameters included white blood count (WBC), red blood cell count (RBC), hemoglobin concentration (HGB), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), MCH concentration (MCHC), platelet count (PLT), and leukocyte differential count (lymphocytes, neutrophils, and monocytes).
Clinical chemistry
The clinical chemistry parameters measured included Albumin (ALB), total protein (TP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), urea nitrogen (BU), creatinine (CRE), glucose (GLU), triglycerides (TG), total cholesterol (TCH), potassium (K), sodium (Na), and chlorine (Cl).
Terminal necropsy
In the end of the course, all animals were euthanized under ether anesthesia. All animals in this study were subjected to a full necropsy. Kidney, spleen, liver, heart, lung, testis and ovaries were weighed wet immediately after dissection to avoid drying. The tissues and organs were procured, preserved in 10% neutral buffered formalin, and processed for histopathological assessment.
Histopathological examination
The preserved organs and tissues of rats from the control group (Group I) and the treated groups (from Group II to Group V) were subjected to histological examination. They were pressed in a fixation medium of 10% solution of buffered formalin (pH 7.4) and enclose in pa- raffin-intended subsequent histopathological examination. A 5 μm section of each organ tissue was stained with hematoxylin and eosin. Each section was examined under an optical microscope.
Safety pharmacology assay
Four groups of 10 rats, each containing 5 females and 5 males, consumed a daily dose of 0 (Group II, emulsifier and distilled water), 3% EOE (Group III), 6% EOE (Group IV), 12% EOE (Group V). Each rat was smeared in the skin of its back with the dose of 0.3 mL for 5 days.
Central nervous system assay
At the last day of the course, the general behavior, posture, gait change and pupil changes of every rat were closely observed within 4 hours after the last [28,29]. And rats with or without salivation, muscle trembling were also recoded. The rats’ situations were observed daily for 7 days after the last administration. The independent activities of rats were observed and recorded by the versatile recorder of rats’ locomotor activity at 0.5 h after the last administration. Each rat was also tested by the pole test at 0.5 h after the last administration. The rats were placed in the tip of the rod which was fixed on the base. Then, each rat was crawling down and rated according to the following criteria: 0: Step by step to climb down; 1: Downward side; 2: Unable to grasp the stick; 3: Loss of righting reflex.
Heart rate assay
The heart rate of each group of rats were measured by using BL-420F to record the electrocardiogram when the rats were anesthetized by ether before administration, at the end of the second administration and a week after the last administration [28,29].
Respiratory rate assay
The respiratory of each rat was measured by using BL-420F to record the electrocardiogram when the rats were anesthetized by ether before administration, at the end of the second administration and a week after the last administration [28,29].
Statistical analysis
Means and standard deviations were calculated for measurement data in each group, which contained b.w., food consumption, clinical pathological data, organ weights, heart rate and respiratory rates. The statistical significance was compared between control and experimental groups by one way analysis of variance (ANOVA) followed by the Student-Newman-Keuls test.
Results
Acute toxicity study
After treated for 50 min, the rats in the top-dose group (Group V) appear to move slowly, gather together, extreme sensitivity to noise and convulsion. The rats in the rest groups showed mild symptom and less death. Death necropsy showed a lot of undigested food and EOE in stomach and no tissue damage except for lung and liver.
In the end of the study (Day 14), the mortality of rats was shown in Table 1. The LD50 value of EOE by oral administration was 3811.5 mg/kg determined by Karber’s method, and the LD50 value within 95% confidence level was 3326.4 mg/kg-4306.5 mg/kg.
Table 1.
Results of oral acute toxicity
| Groups | Dosage (mg/kg) | Dose logarithmic | Rat number | Death number | Mortallity rat (%) |
|---|---|---|---|---|---|
| Groups I | 2772 | 3.4428 | 10 | 1 | 10% |
| Groups II | 3267 | 3.5141 | 10 | 3 | 30% |
| Groups III | 3960 | 3.5977 | 10 | 6 | 60% |
| Groups IV | 4752 | 3.6769 | 10 | 8 | 80% |
| Groups V | 5742 | 3.7591 | 10 | 9 | 90% |
Clinical observations for subchronic toxicity study
The behaviors of rats were not adversely affected by the EOE at the dose of 0, 396, 792, 1188 mg/kg b.wt. (Group II-Group V). The rats were healthy and no signs of toxicity observed during the experimental period.
Body weight and feed consumption
During the experimental period, the body weight of all the rats was in sustained growth. The results were shown in Figures 1 and 2. In male rats, compared with the control group (Group I), the body weight of solvent control group (Group II) was lower (P > 0.05), the low-dose group (Group III) was higher (P > 0.05), the middle-dose group (Group IV) and high-dose group (Group V) were significantly lower (P < 0.05). In female rats, compared with the control group (Group I), the body weight of the experimental groups (Group II -Group V) were all lower (P > 0.05).
Figure 1.
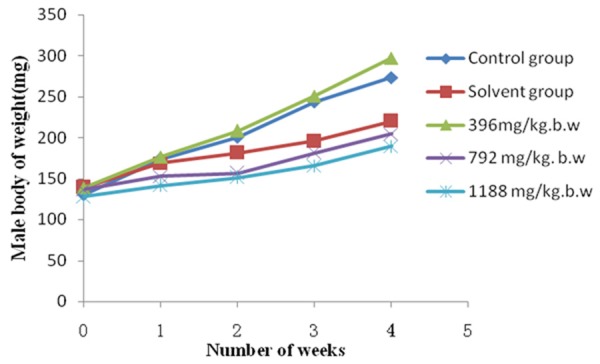
Effect of subchronic administration of EOE on body weight of male rats. Average body weights for male rats during the 30-day oral (gavage) toxicity study. The values are presented as means ± standard deviation (5 rats/sex/group).
Figure 2.
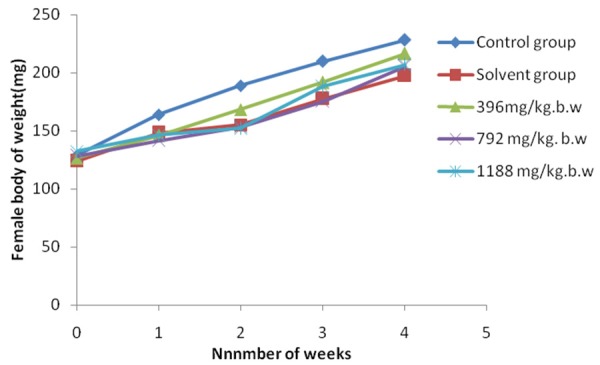
Effect of subchronic administration of EOE on body weight of female rats. Average body weights for female rats during the 30-day oral (gavage) toxicity study. The values represented as means ± standard deviation (5 rats/sex/group).
Hematology parameters
The changes of hematology parameters including hemoglobin (HGB), red blood cell (RBC), white blood cell (WBC), neutrophils (GRA), lymphocytes (LYM), monocytes (MID), blood platelet (PLT) were shown in Table 2. The results indicated that no significant difference (P > 0.05) was noted between the control group (Group I) and the experimental groups (Group II-Group V). The values are presented as means ± standard deviation (10 rats/group). HGB: hemoglobin; RBC: red Blood Cell; WBC: white Blood Cell; GRA: neutrophils; LYM: lymphocytes; MID: monocytes; PLT: blood platelet. There was no significant difference in test groups and the control.
Table 2.
Effect of subchronic administration of EOE on hematological parameters
| Parameters | Group I | Group II | Group III | Group IV | Group V |
|---|---|---|---|---|---|
| HGB, g/L | 161.67 ± 28.33 | 164.83 ± 28.63 | 175.50 ± 28.72 | 177.00 ± 22.60 | 172.17 ± 11.02 |
| RBC, 1012/L | 7.47 ± 1.49 | 7.75 ± 1.36 | 7.95 ± 1.36 | 8.35 ± 1.06 | 8.22 ± 0.61 |
| WBC, 109/L | 9.10 ± 2.32 | 7.74 ± 1.92 | 9.97 ± 2.50 | 10.22 ± 1.56 | 9.94 ± 2.58 |
| GRA, 109/L | 1.68 ± 0.54 | 1.29 ± 0.35 | 1.65 ± 0.57 | 1.79 ± 0.24 | 1.86 ± 0.46 |
| LYM, 109/L | 7.28 ± 1.86 | 6.19 ± 1.53 | 7.97 ± 2.00 | 8.17 ± 1.24 | 7.95 ± 2.06 |
| MID, 109/L | 0.15 ± 0.15 | 0.26 ± 0.23 | 0.35 ± 0.19 | 0.26 ± 0.11 | 0.13 ± 0.11 |
| PLT, 109/L | 841.50 ± 275.07 | 765.33 ± 266.21 | 765.33 ± 266.21 | 911.50 ± 213.12 | 1084.00 ± 234.79 |
The values are presented as means ± standard deviation (10 rats/group). HGB: hemoglobin; RBC: red Blood Cell; WBC: white Blood Cell; GRA: neutrophils; LYM: lymphocytes; MID: monocytes; PLT: blood platelet. There was no significant difference in test groups and the control (P > 0.05).
Serum biochemical parameters
The changes of serum biochemical parameters including albumin (Alb); alanine aminotransferase (ALT); aspartate transaminase (AST); total protein (TP); blood urea nitrogen (BUN); glucose (GLU); Creatinine (CRE); total cholesterol (TCH); triglycerides (TG); sodium (Na); potassium (K); chlorine (Cl) were shown in Table 3. The data including ALT, TCH, TP, BUN, K and Alb showed no significant difference (P > 0.05) between the control group (Group I) and the experimental groups (Group II-Group V). The data of AST, CRE and GLU of the rats in the medium group (Group IV) and the high dose group (Group V) showed significant difference compared with the control group (Group I) (P < 0.05). The data of TG of the high dose group (Group V) showed significant difference compared with the control group (Group I) (P < 0.05). The data of Na of the low dose group (Group III) showed significant difference compared with the control group (Group I) (P < 0.05).
Table 3.
Effect of subchronic administration of EOE on serum biochemistry parameters
| Parameters | Group I | Group II | Group III | Group IV | Group V |
|---|---|---|---|---|---|
| Liver function test | |||||
| ALT (U/L) | 47.17 ± 8.47 | 53.17 ± 15.97 | 52.33 ± 12.56 | 60.00 ± 11.05 | 62.83 ± 11.58 |
| AST (U/L) | 156.83 ± 22.19 | 161.83 ± 22.07 | 179.00 ± 39.27 | 197.33 ± 39.27* | 208.83 ± 35.67* |
| TCH (mmol/L) | 2.09 ± 0.33 | 2.19 ± 0.37 | 2.21 ± 0.39 | 2.27 ± 0.38 | 2.08 ± 0.27 |
| TG (mmol/L) | 0.57 ± 0.07 | 0.47 ± 0.11 | 0.83 ± 0.26 | 0.76 ± 0.21 | 0.57 ± 0.74* |
| TP (g/L) | 75.73 ± 4.10 | 71.10 ± 4.44 | 73.7 ± 5.80 | 71.28 ± 1.47 | 71.47 ± 6.70 |
| Renal function test | |||||
| BUN (mmol/L) | 10.14 ± 2.46 | 11.57 ± 3.70 | 8.70 ± 0.94 | 10.68 ± 0.94 | 9.39 ± 2.26 |
| CRE (µmol/L) | 47.67 ± 7.79 | 48.83 ± 4.40 | 45.17 ± 6.62 | 56.67 ± 6.77* | 57.00 ± 8.17* |
| Serum electrolytes | |||||
| Na (mmol/L) | 140.33 ± 3.27 | 141.67 ± 2.07 | 132.67 ± 8.96* | 137.00 ± 1.67 | 135.00 ± 4.00 |
| K (mmol/L) | 5.70 ± 0.35 | 5.48 ± 0.57 | 5.63 ± 0.23 | 5.87 ± 0.34 | 5.56 ± 0.27 |
| Cl (mmol/L) | 104.83 ± 3.60 | 105.50 ± 1.52 | 98.67 ± 7.37* | 100.33 ± 2.07 | 97.83 ± 3.43* |
| Other test | |||||
| GLU (mmol/L) | 6.64 ± 0.73 | 7.04 ± 0.59 | 5.80 ± 0.62 | 5.19 ± 1.19* | 4.75 ± 0.48* |
| Alb (g/L) | 35.85 ± 4.08 | 34.75 ± 0.79 | 35.03 ± 3.34 | 34.35 ± 1.49 | 34.32 ± 0.95 |
The values are presented as means ± standard deviation (10 rats/sex/group). Alb, albumin; ALT, alanine aminotransferase; AST, aspartate transaminase; TP, total protein; BUN, blood urea nitrogen; GLU, glucose; CRE, Creatinine; TCH, total cholesterol; TG, triglycerides; Na, sodium; K, potassium; Cl, chlorine.
P < 0.05 shown there was significantly difference from control.
Organ coefficient
The results of organ coefficient were shown in Table 4. The organ coefficient of heart, liver, spleen, lung, kidney, testis (in male) and ovary (in female) in test groups (Group II-Group V) had no statistical difference (P > 0.05) compared with those in the control group (Group I).
Table 4.
Effect of subchronic administration of EOE on terminal body weight and organic coefficient (g/100 g) in grams of male and female rats
| Group | Group I | Group II | Group III | Group IV | Group V |
|---|---|---|---|---|---|
| Male | |||||
| Heart (g/100 g) | 0.36 ± 0.04 | 0.34 ± 0.03 | 0.38 ± 0.05 | 0.43 ± 0.02 | 0.44 ± 0.03 |
| Liver (g/100 g) | 3.47 ± 0.11 | 3.19 ± 0.45 | 3.70 ± 0.49 | 3.73 ± 0.25 | 4.17 ± 0.10 |
| Spleen (g/100 g) | 0.23 ± 0.02 | 0.21 ± 0.03 | 0.22 ± 0.03 | 0.25 ± 0.03 | 0.20 ± 0.04 |
| Lung (g/100 g) | 0.62 ± 0.14 | 0.62 ± 0.05 | 0.55 ± 0.03 | 0.76 ± 0.05 | 0.71 ± 0.05 |
| Kidney (g/100 g) | 0.83 ± 0.07 | 0.78 ± 0.03 | 0.78 ± 0.10 | 0.91 ± 0.09 | 0.84 ± 0.04 |
| Testis (g/100 g) | 1.26 ± 0.13 | 1.85 ± 0.34 | 1.26 ± 0.16 | 1.84 ± 0.25 | 1.34 ± 0.23 |
| Female | |||||
| Heart (g/100 g) | 0.39 ± 0.33 | 0.44 ± 0.02 | 0.44 ± 0.03 | 0.40 ± 0.03 | 0.47 ± 0.09 |
| Liver (g/100 g) | 3.75 ± 0.15 | 3.74 ± 0.38 | 3.54 ± 0.07 | 4.12 ± 0.38 | 4.32 ± 0.16 |
| Spleen (g/100 g) | 0.26 ± 0.05 | 0.29 ± 0.05 | 0.29 ± 0.05 | 0.25 ± 0.03 | 0.25 ± 0.04 |
| Lung (g/100 g) | 0.68 ± 0.13 | 0.67 ± 0.05 | 0.85 ± 0.18 | 0.83 ± 0.02 | 0.70 ± 0.08 |
| Kidney (g/100 g) | 0.84 ± 0.07 | 0.88 ± 0.10 | 0.82 ± 0.04 | 0.80 ± 0.14 | 0.82 ± 0.04 |
| Ovary (g/100 g) | 0.06 ± 0.00 | 0.06 ± 0.01 | 0.05 ± 0.01 | 0.06 ± 0.02 | 0.05 ± 0.01 |
The values are presented as means ± standard deviation (10 rats/group). There was no significant difference in test groups and the control (P > 0.05).
Histopathological analysis
In the liver of the control group (Group I) and the solvent group (Group II), the cross-section showed the normal appearance of liver, sinusoids, and hepatocytes in a clearly conserved form (Figure 3A). Central venous extended with hyperemia and varying degrees of vacuolar degeneration of hepatocytes were found in the liver of the experimental groups (Group III-Group V) (Figure 3B).
Figure 3.
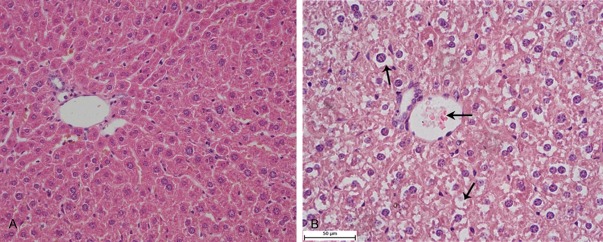
Histological changes of the liver. A. The liver of rats in the control group (HE, 400×). B. The liver of rats in the experiment group, vesicular degeneration (↑↓) and central venous hyperemia (←) (HE, 400×).
In the spleen of the control group (Group I) and the solvent group (Group II), the cross-section showed the normal appearance of spleen, white pulp, red pulp and spleen trabecula (Figure 4A). Red pulp extended with hyperemia and a large number of macrophages and Langhans cells (LC) infiltration were found in the liver of the experimental groups (Group III-Group V) (Figure 4B).
Figure 4.
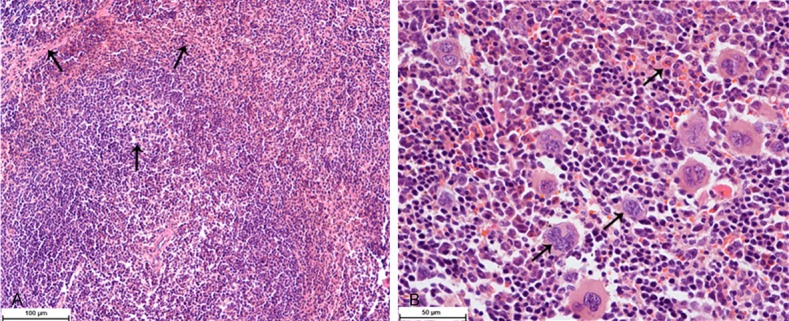
Histological changes of the spleen. A. The spleen of rats in the control group, white pulp, red pulp and spleen trabecula (↑) (HE, 200×). B. The spleen of rats in the experiment group, red pulp hyperemia, macrophages and Langhans cells (↑) (HE, 400×).
In the kidney of the control group (Group I) and the solvent group (Group II), the cross-section showed the normal appearance of kidney, glomerulus, renal capsule and renal tubular epithelial cell (RTEC) (Figure 5A). Glomerulus with varying degrees of hyperemia, RETC with varying degrees of granular degeneration and the narrowed renal tubular were found in the kidney of the experimental groups (Group III-Group V) (Figure 5B).
Figure 5.
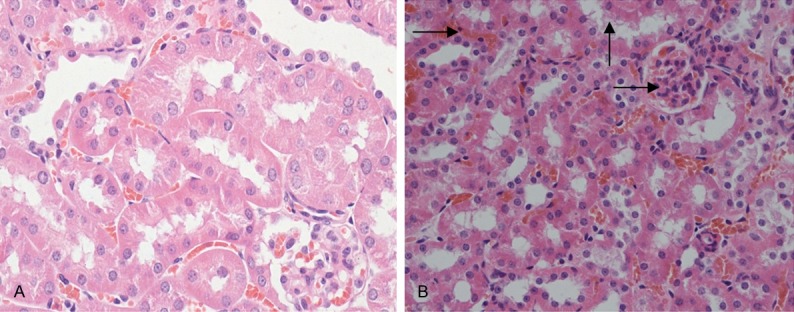
Histological changes of kidney. A. The kidney of rats in the control group, glomerulus, renal capsule and RTEC (↑) (HE, 400×). B. The kidney of rats in the experiment group, glomerulus hyperemia (→), RETC with granular degeneration (↑) (HE, 400×).
In the heart, lung, stomach, intestines, testicles and ovaries, the cross-section showed no significant lesions between the control groups (Group I and Group II) and the experiment groups (Groups III-V).
Changes of central nervous system
There were no abnormalities observed on the behavior performance, posture, gait and pupil change; no bizarre behaviors such as salivation and muscle trembling of all the rats were observed. In the climbing pole test, there were no changes between the experimental groups (Group II to Group V) and the control group (Group I). The level of all the groups was “0”.
Changes of cardiovascular system
The result was shown in the Table 5. By measuring heart rate of all the rats, there were no significant changes (P > 0.05) found in the cardiovascular system between the experimental group (Group II to Group V) and the control group (Group I).
Table 5.
Effects on the heart rate of rats
| The time of measuring | Group I (Beats/min) | Group II (Beats/min) | Group III (Beats/min) | Group IV (Beats/min) | Group V (Beats/min) |
|---|---|---|---|---|---|
| Before dosing | 433.50 ± 8.17 | 433.83 ± 8.13 | 433.00 ± 9.47 | 435.33 ± 5.99 | 434.00 ± 7.95 |
| The second day after dosing | 443.17 ± 30.10 | 443.00 ± 1.55 | 450.17 ± 13.93 | 446.83 ± 18.00 | 443.00 ± 23.97 |
| One week after dosing | 425.50 ± 38.91 | 451.83 ± 12.14 | 448.17 ± 40.39 | 448.17 ± 30.10 | 437.00 ± 25.98 |
The values are presented as means ± standard deviation (10 rats/group). There was no significant difference in test groups and the control (P > 0.05).
Changes of respiratory system
The result was shown in the Table 6. By measuring respiratory rate of all the rats, there were no significant changes (P > 0.05) observed in the respiratory system between the experimental group (Group II to Group V) and the control group (Group I).
Table 6.
Effect on the respiratory rates of rats
| The time of measuring | Group I (Beats/min) | Group II (Beats/min) | Group III (Beats/min) | Group IV (Beats/min) | Group V (Beats/min) |
|---|---|---|---|---|---|
| Before dosing | 91.50 ± 5.89 | 93.00 ± 3.58 | 85.83 ± 4.45 | 91.00 ± 10.20 | 91.17 ± 12.27 |
| The second day after dosing | 92.83 ± 7.17 | 93.50 ± 3.39 | 86.67 ± 5.57 | 88.17 ± 7.91 | 91.67 ± 4.13 |
| One week after dosing | 86.67 ± 5.24 | 89.33 ± 4.93 | 86.33 ± 6.59 | 83.67 ± 1.97 | 84.17 ± 3.66 |
The values are presented as means ± standard deviation (10 rats/group).There was no significant difference in test groups and the control (P > 0.05).
Discussion
As regards the toxicity of eucalyptus oil, not much is known; however, they have been categorized as GRAS by USEPA [1]. Considering the potential health risk, EOE requires further evaluation for their efficacy and safety due to its growing demand on medicinal use. Currently, we conducted a comprehensive toxicological evaluation and a necessary safety pharmacology evaluation on EOE by performing acute and 30-day subchronic oral toxicity studies and a safety experiment.
In this study, the acute toxicity showed that the LD50 value of EOE was 3811.5 mg/kg by oral route (Table 1). The data is of high reliability because the value is in the 95% confidence interval [30]. According to the acute toxicity grading standards [29], if the LD50 = 501-5000 mg/kg, the drug is of low-toxicity. Therefore, EOE belongs to the low-toxicity drug.
The result found in this study was compatible to the report [31] that the oral and acute LD50 of eucalyptus oil and 1,8-cineole to rat is 4440 mg/kg BW and 2480 mg/kg BW. It is also a complement to the report [32] that the oral and acute LD50 of eucalyptus oil to mice is 1824.01 mg/kg.
To assess the long term hazard, subchronic toxicity studies are always valuable in evaluating the safety of xenobiotics [33]. Changes in body weight have been used as an indicator of adverse effects of drugs and chemical [34]. In this study, significant changes (P < 0.05) were found in the medium dose (Group IV) and the high dose (Group V) compared with the control group in male rats. It indicated that high dose of EOE may be able to slow down the weight gain of male rats (Figures 1, 2). The result was similar with Wolff’s report [35]. Moreover, there were no significant changes in the general behavior. It can be conclude that the low-dose of EOE has no effect on the growth and functions of rats.
The hematopoietic system is one of the most sensitive parameters to assess toxicity of drugs in humans and animals [36]. This study indicated that there was no significant difference in HGB, RBA, WBC, GRA, LYM, MID and PLT between the treated groups and the control group, indicating that EOE had no effects on the circulating blood cells (Table 2).
Liver is the main site of the synthesis of plasma proteins, and any damage to the liver results in elevations of both ALT and AST in the blood [36]. Moreover, ALT found in the serum is taken as a first sign of cell and liver damage [37,38]. GLU, TG, TCH are also important biochemical indicators related to the liver [39]. The changes of the three indicators indicate inflammation, necrosis, poisoning and biliary disease of liver. The preset study indicated that ALT and AST were elevated slightly compared with the control group (Table 3). The result was in accord with that of Wang and Arise [30,40]. In addition, TG and TCH of high-dose group (Group V) and middle-dose group (Group IV) were also elevated compared with the control group (Group I). These results indicated that the liver was the target organ of EOE toxicity.
CRE is known as a good indicator for renal function. Rises in CRE mean obvious damage to kidney [36,41]. Present study indicated that CRE of high dose group (Group V) and medium dose group (Group IV) were higher than the control group (Group I) indicating EOE could cause damages to the kidney of rats. Moreover, the results showed that the damage of EOE to the rats is lower than the mice [30].
Organ index is the radio of organs to body [42]. It is an important indicator of the functional status of the animals. The increase of Organ coefficient indicated organ congestion, edema, or hypertrophy, etc. while the decrease of organ coefficient indicated organs atrophy and other degenerative changes [39,43,44]. In this study, the result suggested that the index of livers and kidneys of high-dose group (Group V) and middle-dose group (Group IV) were higher than the control group (Group I), indicating the livers and kidneys were oncotic slightly (P > 0.05) (Table 4).
To supplement the toxicity tests and provide a basis for a comprehensive understanding of the toxicity of EOE, we conducted the safety pharmacology study. Safety pharmacology evaluation is the part of extensively pharmacological effect research expect for the main pharmacological activity [28]. Therefore, it is helpful to investigate adverse reactions and find new uses and mechanism of action the drug. According to the technical research guideline of veterinary medicine and natural medicine on safety pharmacology [28,29,45], this study measured the impact of EOE on the cardiovascular system in anesthetized rats by non-invasive way for the first time. This method effectively reduced the interference of related physiological indicator which due to the stress response and surgery interference, and ensured that the various physiological indictors could reflect the true state of the rats. The results suggested that EOE had no effect on the nervous system, respiratory system and cardiovascular system (Tables 5, 6).
The histopathological changes were mainly occurred in the liver, spleen and kidney. LC derived from bone marrow possesses Ia antigen, C3 and Fc-IgG receptors and has the function of antigen presentation and allergenic stimulation which is similar to macrophage [46,47]. Macrophages and Langhans cells found in the spleen suggested that EOE could stimulate the body’s immune system and boost immunity (Figure 4). The lesions found in the spleen and kidney suggests that the target organs of EOE are the liver and kidney, which are also consistent with the hematology and biochemical findings (Figures 3, 5).
In conclusion, the LD50 value of EOE by the oral route was 3811.5 mg/kg indicating that EOE is a low-toxicity drug. Results in the subchronic oral toxicity showed that the dose over 792 mg/kg b.wt. of EOE may slow down the growth of male rats. The target organs of the toxic effects of EOE were the liver, kidney and spleen. In the safety pharmacology study, EOE administration didn’t produce any side effects to rats in nervous system, cardiovascular system and respiratory system. The results of this study indicate that EOE is a safe veterinary medicine for external use.
Acknowledgements
This research was financially supported by National Natural Science Foundation of China (Grant No. 31272612, 31372477); the Doctoral Program of Higher Education Research Fund (Instructor Dr. Class 20105103110001); the Sichuan Youth Science and Technology Innovation Research Team for waterfowl disease prevention and control (2013TD0015); the Sichuan International Cooperation Projects (2014HH0058); A Project Supported by Scientific Research Fund of Sichuan Provincial Education Department (No. 14TD0031).
Disclosure of conflict of interest
None.
References
- 1.Batish DR, Singh HP, Kohli RK, et al. Eucalyptus essential oil as a natural pesticide. Forest Ecology and Management. 2008;256:2166–2174. [Google Scholar]
- 2.Zygadlo JA, Grosso NR. Comparative study of the antifungal activity of essential oils from aromatic plants growing wild in the central region of Argentina. Flavour Frag J. 1995;10:113–118. [Google Scholar]
- 3.Misra G, Pavlostathis SG. Biodegradation kinetics of monoterpenes in liquid and in soil-slurry system. Appl Microbiol Biotechnol. 1997;47:572–577. [Google Scholar]
- 4.Isman MB. Plant essential oils for pest and disease management. Crop Prot. 2000;19:603–608. [Google Scholar]
- 5.Isman MB. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu Rev Entomol. 2006;51:45–66. doi: 10.1146/annurev.ento.51.110104.151146. [DOI] [PubMed] [Google Scholar]
- 6.Enan E, Beigler M, Kende A. Proceedings of the International Symposium on Plant Protection. Gent, Belgium: 1998. Insecticidal action of terpenes and phenols to cockroaches: effect on octopamine receptors. [Google Scholar]
- 7.Isman MB, Machial CM. Pesticides based on plant essential oils: from traditional practice to commercialization. In: Rai M, Carpinella MC, editors. Naturally Occurring Bioactive Compounds. Advances in Phytomedicine. vol. 3. Elsevier; 2006. pp. 29–44. [Google Scholar]
- 8.Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils--a review. Food Chem Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 9.Chen BQ, Yu XJ, Ding JK, et al. Cinnamomum plant resources of China and its aromatic ingredients. Kunming: Yunnan Science and Technology Press; 1997. p. 3435. [Google Scholar]
- 10.Yang YC, Choi HC, Choi WS, Clark JM, Ahn YJ. Ovicidal and adulticidal activity of Eucalyptus globulus leaf oil terpenoids against Pediculus humanus capitis (Anoplura: Pediculidae) J Agric Food Chem. 2004;52:2507–2511. doi: 10.1021/jf0354803. [DOI] [PubMed] [Google Scholar]
- 11.Sartorelli P, Marquioreto AD, Amaral-Baroli A, Lima ME, Moreno PR. Chemical composition and antimicrobial activity of the essential oils from two species of Eucalyptus. Phytother Res. 2007;21:231–233. doi: 10.1002/ptr.2051. [DOI] [PubMed] [Google Scholar]
- 12.Boland DJ, Brophy JJ, House APN. Eucalyptus leaf oils: use, chemistry, distillation and marketing. Inkata Press; 1991. [Google Scholar]
- 13.FAO. Chapter 5. Flavour and Fragrances of Plant Origin. 1995. Eucalyptus oil. [Google Scholar]
- 14.Brooker MIH, Kleinig DA. Field Guide to Eucalyptus. Third edition. vol.1. Melbourne, South-eastern Australia: Bloomings; 2006. [Google Scholar]
- 15.Yang YC, Choi HC, Choi WS, Clark JM, Ahn YJ. Ovicidal and adulticidal activity of Eucalyptus globulus leaf oil terpenoids against Pediculus humanus capitis (Anoplura: Pediculidae) J Agric Food Chem. 2004;52:2507–2511. doi: 10.1021/jf0354803. [DOI] [PubMed] [Google Scholar]
- 16.Seyoum A, Killeen GF, Kabiru EW, Knols BG, Hassanali A. Field efficacy of thermally expelled or live potted repellent plants against African malaria vectors in western Kenya. Trop Med Int Health. 2003;8:1005–1011. doi: 10.1046/j.1360-2276.2003.01125.x. [DOI] [PubMed] [Google Scholar]
- 17.Kuehn BM. CDC: new repellents for West Nile fight. JAMA. 2005;293:2583. doi: 10.1001/jama.293.21.2583. [DOI] [PubMed] [Google Scholar]
- 18.Yeh RY, Shiu YL, Shei SC, Cheng SC, Huang SY, Lin JC, Liu CH. Evaluation of the antibacterial activity of leaf and twig extracts of stout camphor tree, Cinnamomum kanehirae, and the effects on immunity and disease resisitance of white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2009;27:26–32. doi: 10.1016/j.fsi.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Shylaja M, Ravindran PN, Babu KN. Cinnamon and cassia: the genus Cinnamomum. 2003. 15 Other Useful Species of Cinnamomum; p. 330. [Google Scholar]
- 20.Li H, Huang L, Zhou A, Li X, Sun J. Study on anti-inflammatory effect of different chemotype of Cinnamomum camphora on rat arthritis model induced by Freund’s adjuvant. Zhongguo Zhong Yao Za Zhi. 2009;34:3251–3254. [PubMed] [Google Scholar]
- 21.Siurin SA. Effect of essential oil on lipid peroxidation and lipid metabolism in patients with chronic bronchitis. Klin Med (Mosk) 1997;75:43–45. [PubMed] [Google Scholar]
- 22.Yuanbo SU, Qingbiao LI, Chuanyi YAO, et al. Antitumor action of ethanolic extractives from camphor leaves. Chemical Industry and Engineering Progress. 2006;25:200–204. [Google Scholar]
- 23.Bruce RD. An up-and-down procedure for acute toxicity testing. J Fund Appl Toxicol. 1985;5:151–157. doi: 10.1016/0272-0590(85)90059-4. [DOI] [PubMed] [Google Scholar]
- 24.Jung H, Choi SC. Sequential method of estimating the LD50 using a modified up-and-down rule. J Biopharm Stat. 1994;4:19–30. doi: 10.1080/10543409408835069. [DOI] [PubMed] [Google Scholar]
- 25.OECD. Test No. 425: Acute Oral Toxicity: Up-and-Down Procedure. OECD Publishing; 2008. [Google Scholar]
- 26.Keplinger ML, Deichmann WB. Acute toxicity of combinations of pesticides. Toxicol Appl Pharm. 1967;10:586–595. doi: 10.1016/0041-008x(67)90097-x. [DOI] [PubMed] [Google Scholar]
- 27.Schneeman BO. Dietary fiber and gastrointestinal function. Nutr Res. 1987;45:129–132. doi: 10.1111/j.1753-4887.1987.tb06343.x. [DOI] [PubMed] [Google Scholar]
- 28.China Institute of Veterinary Drugs Control. Test No. 1596: Safety pharmacology research technical guidelines of veterinary medicine, natural medicine. 2011. [Google Scholar]
- 29.Duan WL, Liang XM. Technical guidelines assembly of veterinary medicine research. Beijing: Chemical Industry Press; 2011. [Google Scholar]
- 30.Xu J, Hu ZQ, Wang C, Yin ZQ, Wei Q, Zhou LJ, Li L, Du YH, Jia RY, Li M, Fan QJ, Liang XX, He CL, Yin LZ. Acute and subacute toxicity study of 1,8-cineole in mice. Int J Clin Exp Pathol. 2014;7:1495. [PMC free article] [PubMed] [Google Scholar]
- 31.Regnault-Roger C. The potential of botanical essential oils for insect pest control. Integrated Pest Manage Rev. 1977;2:25–34. [Google Scholar]
- 32.Yu YL, Wang CT, Feng GW, et al. Acute toxicity and genetic toxicity of eucalyptus oil. J Toxicol. 2010;24:501–503. [Google Scholar]
- 33.Aniagu SO, Nwinyi FC, Akumka DD, Ajoku GA, Dzarma S, Izebe KS, Ditse M, Nwaneri P, Wambebe C, Gamaniel K. Toxicity studies in rats fed nature cure bitters. Afr J Biotechnol. 2005;4:72–78. [Google Scholar]
- 34.Tofovic SP, Jackson EK. Effects of long-term caffeine consumption on renal function in spontaneously hypertensive heart failure prone rats. J Cardiovasc Pharmacol. 1999;33:360–366. doi: 10.1097/00005344-199903000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Wolff GL. Twenty-eight day gavage and encapsulated feed study on 1,8-cineole in Fischer 344 rats. NTP chemical. 1987;50:02–06. [Google Scholar]
- 36.Rahman MF, Siddiqui MK, Jamil K. Effects of vepacide (Azadirachta indica) on aspartate and alanine aminotransferase profiles in a subchronic study with rats. Hum Exp Toxicol. 2001;20:243–249. doi: 10.1191/096032701678227730. [DOI] [PubMed] [Google Scholar]
- 37.El Hilaly J, Israili ZH, Lyouss B. Acute and chronic toxicological studies of Ajuga iva in experimental animals. J Ethnopharmacol. 2004;91:43–50. doi: 10.1016/j.jep.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 38.Auza NJ, Olson WG, Murphy MJ, Linn JG. Diagnosis and treatment of copper toxicosis in ruminants. J Am Vet Med Assoc. 1999;214:1624–1628. [PubMed] [Google Scholar]
- 39.Wang SL. Veterinary clinical diagnostics. Beijing: China Agriculture Press; 2006. [Google Scholar]
- 40.Arise RO, Malomo SO, Adebayo JO, et al. Effects of aqueous extract of Eucalyptus globulus on lipid peroxidation and selected enzymes of rat liver. Journal of Medicinal Plants Research. 2009;3:077–081. [Google Scholar]
- 41.Lameire N, Van Biesen W, Vanholder R. Acute renal failure. Lancet. 2005;365:417–430. doi: 10.1016/S0140-6736(05)17831-3. [DOI] [PubMed] [Google Scholar]
- 42.Xia JY, Lei PQ, Zeng XL, Li H. Determinations to the weight of main organs and biochemical indexes of SPF KM Mice. Sichuan Journal of Physiological Sciences. 2009;31:104–107. [Google Scholar]
- 43.Wei JZ, Wu XG, Lin HH. A further observation on the serum biochemical values of chickens infected with Eimeriatenella or E. acervulina and the discussion on the pathological lesions. Chinese Journal of Preventive Veterinary Medicine. 2002;32:25–27. [Google Scholar]
- 44.Kristiansen E, Madsen C. Induction of protein droplet (α2μ-globulin) nephropathy in male rats after short-term dosage with 1,8-cineole and l-limonene. Toxicol Lett. 1995;80:147–152. doi: 10.1016/0378-4274(95)03390-7. [DOI] [PubMed] [Google Scholar]
- 45.CFDA. Test No. GPT2-1: Acute toxicity studies technical guidelines of traditional Chinese medicine and natural medicine[z] CFDA Publishing; 2004. [Google Scholar]
- 46.Thorbecke GJ, Silberberg-Sinakin I, Flotte TJ. Langerhans cells as macrophages in skin and lymhphoid organs. J Invest Dermatol. 1980;75:32–43. doi: 10.1111/1523-1747.ep12521083. [DOI] [PubMed] [Google Scholar]
- 47.Stingl G, Katz SI, Clement L, Green I, Shevach EM. Immunologic functions of Ia-bearing epidermal Langerhans cells. J Immunol. 1978;121:2005–2013. [PubMed] [Google Scholar]


