Abstract
Objective: This study is to investigate the antitumor effects and possible mechanisms of chlorin e6-mediated photodynamic therapy (Ce6-PDT) on human colon cancer SW480 cells. Methods: SW480 cells were treated with Ce6, followed by photodynamic irradiation. Subcellular localization of Ce6 in SW480 cells was observed with confocal laser scanning microscopy (LSCM). Reactive oxygen species (ROS) levels were monitored with fluorescence microscopy. Cell proliferation and apoptosis were detected by the MTT assay and flow cytometry, respectively. Scratch test and colony formation assay were employed to analyze the cell migration ability and colony formation ability. Results: LSCM showed that, in SW480 cells, Ce6 was evenly distributed within the ER and lysosomes, with nearly no distribution in the mitochondria and nuclei. When SW480 cells were subjected to Ce6-PDT, the ROS levels would be elevated, in a dose-dependent manner. Moreover, Ce6-PDT treatment could inhibit the cell proliferation and enhance the apoptotic process, in SW480 cells. However, Ce6 treatment alone without photodynamic irradiation could not induce any significant differences in the cell proliferation and apoptosis. In addition, the migration ability and colony formation ability of SW480 cells were decreased by Ce6-PDT treatment at appropriate dosages. Conclusion: Ce6-PDT treatment could enhance ROS production and apoptosis, inhibit cell proliferation, decrease migration ability and colony formation ability, in SW480 cells, in a dose-dependent manner. These findings might provide experimental evidence for the application of Ce6-PDT in clinical treatment of colorectal cancer.
Keywords: Colorectal cancer, chlorin e6, photodynamic therapy, SW480 cells
Introduction
Colorectal cancer (CRC) is the third most common cancer, and ranks the fourth leading cause of cancer-related mortality, throughout the world. There are more than 1.2 million new cases and 0.6 million deaths due to CRC each year [1]. At present, surgical resection is the most common curative therapy for CRC. The patient survival rate is related to the clinical stage of the cancer at diagnosis, and the 5-year relative survival is about 65% [2]. However, the relative survival rate of patients with unresectable metastatic lesions drops to less than 5% [3]. Moreover, treatment options are extremely limited in cases of patients with chemotherapy resistance, peritoneal carcinomatosis, and other poor conditions. Therefore, novel therapeutic strategies are still needed for refractory CRC cases.
In recently years, photodynamic therapy (PDT) has been considered as a promising anticancer modality in addition to traditional surgery and chemotherapy treatments. With PDT, photosensitizers are first systematically or topically administrated, and then excited by specific light wavelengths. The energy is transferred to oxygen to induce reactive oxygen species (ROS) production. When photosensitizers accumulate, subcellular damages would occur, which might result in apoptosis and/or necrosis [4,5]. Based on the preferential accumulation of photosensitizers, PDT provides improved selectivity in targeting tumors [6]. Moreover, since PDT causes essentially no effects on connective tissues, the anatomical integrity of hollow organs, including the colon, could be preserved in patients undergoing PDT [7]. Chlorin e6 (Ce6) is a second-generation photosensitizer with a strong absorption in the red spectrum of light, which could be readily synthesized from chlorophyll. Remarkable clinical benefits have been obtained with C6-mediated PDT (Ce6-PDT) in the treatment of various cancers, including melanoma, bladder cancer, and nasopharyngeal cancer [8-11].
In the present study, effects of Ce6-PDT on the ROS level, cell proliferation and apoptosis, migration ability, and clone formation ability in human colon cancer SW480 cells were investigated. Our findings might provide theoretical and experimental basis to support Ce6-PDT in treating CRC.
Materials and methods
Cell line and cell culture
SW480 colon cancer cells were purchased from Nanjing KGI Biotechnology Co., Ltd, Nanjing, Jiangsu, China. These cells were cultured with Leibovitz’s L-15 medium (Gibco, GrandIsland, NY, USA) containing 10% fetal bovine serum (FBS; Gibco), 2 mmol/L glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (all from Thermo Scientific, Waltham, MA, USA), in a 37°C, 5% CO2 humidified incubator. Cells were passaged every 2-3 d, and cells in the logarithmic growth phase were used for the experiments.
Photosensitizer preparation and PDT procedure
Chlorin e6 (Ce6; Frontier Scientific Inc., Logan, UT, USA) was dissolved in PBS (pH 7.4) in dark, and stored at -20°C. SW480 cells were incubated with Ce6 at indicated concentrations (e.g., 0, 0.125, 0.25, 0.5, 1.0, 2.0, 4.0, and 8.0 μg/ml), and then subjected to the photodynamic irradiation from a semiconductor laser (at the Key Laboratory of Optoelectronic Technology, Northwestern University, Xi’an, Shaanxi, China), with 32 mW output power at the light wavelength of 650 nm, giving an irradiation dosage of 6 J/cm2. After the treatments, these cells were cultured until further experiments.
Laser scanning confocal microscopy (LSCM)
Subcellular localization of Ce6 in SW480 cells was detected with LSCM. Briefly, SW480 cells were planted onto the confocal laser dish at a density of 5 × 104 cells/mL, and cultured for 24 h. After treated with 0.5 μg/ml Ce6 for 8 h, these cells were washed with PBS, and then incubated with 1 μmol/L ER-Tracker™ Green, 50 nmol/L LysoTracker Deep Red, and 100 nmol/L MitoTracker Green FM (all from Life Technologies, Gaithersburg, MD, USA), respectively, in dark at 37°C for 30 min, to label ER, lysosomes, and mitochondria. After washed with PBS, the cells were observed under LSCM (Leica, Heidelberg, Germany) with emission wavelengths of 514 nm, 488 nm, and 647 nm.
ROS level measurement
ROS levels were assessed with the kit from Beyotime (Haimen, Jiangsu, China), according to the manufacturer’s instructions. SW480 cells were seeded onto a 6-well plate as a density of 5 × 104 cells/ml, and cultured for 24 h. After the Ce6-PDT treatment, these cells were treated with 2’, 7’-dichlorofluorescin diacetate (DCFH-DA; 1:1000 diluted in serum-free culture medium) at 37°C for 20 min. SW480 cells free from intervention were used as the blank control, cells treated with 0.5 μg/ml Ce6 without photodynamic irradiation were used as the negative control, and cells treated with ROS inducer were used as the positive control. The fluorescence was observed under an inverted fluorescence microscope (IX71; Olympus, Tokyo, Japan), and Image J software was used for the fluorescence intensity analysis.
MTT assay
SW480 cells were seeded onto a 96-well plate in 200 μL medium, at a density of 3 × 104 cells/ml, and cultured for 24 h. After the Ce6-PDT treatment, these cells were incubated with 5 mg/ml MTT (Sigma, St. Louis, MO, USA) for 4 h. Then 100 μL DMSO (Sigma) was added, and the cells were shaken for 10 min. Absorbance (OD) at 570 nm was read on a microplate reader (BioRad, Hercules, CA, USA), and the cell survival rate was calculated according to the following equation: Cell survival rate (%) = 1-(ODcontrol-ODtreatment)/(ODcontrol-ODmedium) × 100%.
Annexin-V/PI staining and flow cytometry
SW480 cells were seeded onto a 6-well plate at a density of 4 × 105 cells/well. After the Ce6-PDT treatment, cells were collected and re-suspended in 100 μl binding buffer. Cells were stained with 20 μL annexin V/PI (Roche, Basel, Switzerland) in dark for 20 min, and then cell apoptosis was detected by the flow cytometer (Beckman Coulter, Miami, FL, USA).
Scratch test
The cell migration ability was assessed by the scratch test. SW480 cells were seeded onto a 24-well plate at a density of 2 × 105 cells/ml, and cultured for 24 h. The linear scratch was made with a 10-μl sterile pipette tip, and then the cells were subjected to the Ce6-PDT treatment. After 48 h, scratch wound healing was observed under microscope.
Colony formation assessment
SW480 cells were seeded onto a 24-well plate at a density of 4 × 102 cells/ml. Following the Ce6-PDT treatment, these cells were further cultured for 1-2 w. After washing with PBS, cells were subsequently fixed with 5 ml methanol for 20 min and stained with Giemsa staining solution for 30 min. The stained cells were washed with running water and dried up in air, and then observed under microscope. Colony with more than 50 cells were counted, and the colony formation rate was calculated according to the following equation: Colony formation rate (%) = (Number of colony formation /Number of seeded cells) × 100%.
Statistical analysis
Data were expressed as mean ± SD. SPSS19.0 software was used for statistical analysis. The t-test was used for the pairwise comparisons, and ANOVA was used for the multiple comparisons. The LSD test was performed for the intergroup comparison, and the Kruskal-Wallis H test was applied in case of non-normal distribution. P < 0.05 was considered statistically significant.
Results
Subcellular localization of Ce6 in human colon cancer SW480 cells
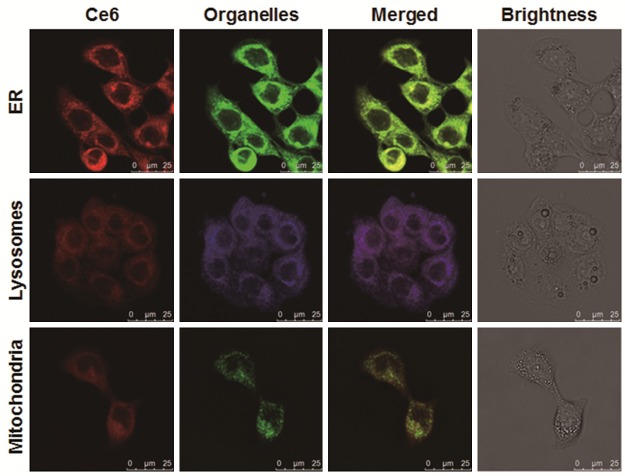
The subcellular localization of Ce6 in human colon cancer SW480 cells was first investigated. SW480 cells were incubated with 0.5 μg/ml Ce6 for 8 h. LSCM showed that Ce6 was evenly distributed within the ER and lysosomes. In contrast, Ce6 was hardly located in the mitochondria and nuclei (Figure 1). In the following experiments, the effects of Ce6-mediated photodynamic therapy (Ce6-PDT) on the ROS level, cell proliferation and apoptosis, migration ability, colony formation ability, in human colon cancer SW480 cells were investigated.
Figure 1.
Subcellular localization of Ce6 in human colon cancer SW480 cells. SW480 cells were cultured incubated with 0.5 μg/ml Ce6 for 8 h, and then stained with 1 μmol/L ER-Tracker™ Green, 50 nmol/L LysoTracker Deep Red, and 100 nmol/L MitoTracker Green FM, respectively, in dark for 30 min. The subcellular distribution of Ce6 (red), ER (green in the upper panel), lysosomes (blue in the middle panel), and mitochondria (green in the lower panel) were detected with LSCM. Scale bar, 25 μm.
Ce6-PDT increases ROS level in human colon cancer SW480 cells
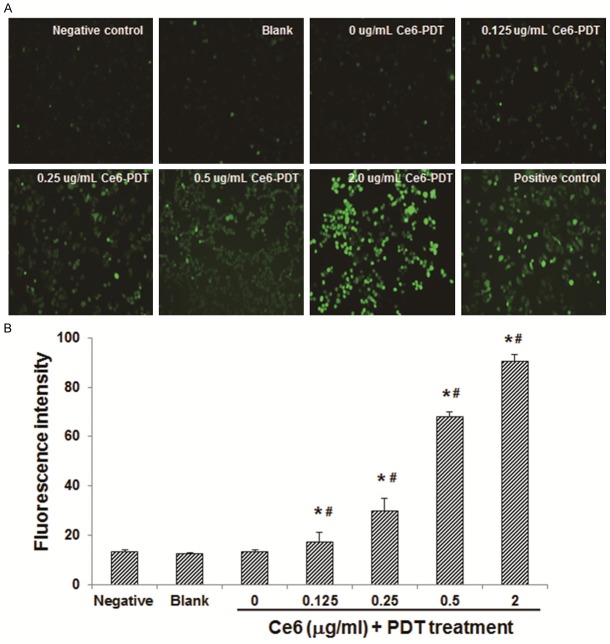
The effects of Ce6-PDT on the ROS level in human colon cancer SW480 cells were next investigated. These SW480 cells were incubated with Ce6 at the concentrations of 0, 0.125, 0.25, 0.5, and 2.0 μg/ml, respectively, and then subjected to the photodynamic irradiation at 650 nm, with an irradiation dose of 6 J/cm2. After the treatment, the ROS levels in SW480 cells were measured. SW480 cells free from intervention were used as the blank control, cells treated with 0.5 μg/ml Ce6 without photodynamic irradiation were used as the negative control, and cells treated with ROS inducer were used as the positive control. Our results indicated that, compared with the blank control group, the ROS levels were significantly increased in the Ce6-PDT groups (Figure 2; P < 0.05). Moreover, within the Ce6-PDT-treated groups, the ROS level was elevated along with the increasing treatment concentrations of Ce6 (Figure 2B), with statistical significance (P < 0.05). These results suggest that the Ce6-PDT treatment could significantly increase the ROS level in human colon cancer SW480 cells, in a dose-dependent manner.
Figure 2.
Effects of Ce6-PDT on the ROS level in human colon cancer SW480 cells. A. SW480 cells were treated with Ce6 at 0, 0.125, 0.25, 0.5, and 2.0 μg/ml, respectively, followed by photodynamic irradiation at 650 nm (6 J/cm2). SW480 cells free from intervention were used as the blank control, cells treated with 0.5 μg/ml Ce6 without photodynamic irradiation were used as the negative control, and cells treated with ROS inducer were used as the positive control. The ROS level was measured with fluorescence probe. B. Statistical analysis of the fluorescence intensities indicating ROS level in SW 480 cells. Compared with the blank control group, *P < 0.05; compared with the previous group, #P < 0.05.
Ce6-PDT inhibits proliferation of human colon cancer SW480 cells
To investigate the effects of Ce6-PDT treatment on SW480 cell proliferation, these cells were treated with Ce6 (0, 0.125, 0.25, 0.5, 1.0, 2.0, 4.0, and 8.0 μg/ml, respectively) and the subsequent photodynamic irradiation, and then MTT assay was performed. SW480 cells treated with Ce6 at corresponding concentrations without photodynamic irradiation were used as controls. Our results showed that, photodynamic irradiation alone could not influence the cell survival rate. On the other hand, without photodynamic irradiation, different treatment concentrations of Ce6 did not result in significant differences in the cell survival rate, either. Importantly, the survival rates of SW480 cells treated with Ce6-PDT were dramatically de- creased compared with corresponding controls (Figure 3A). Moreover, the cell survival rate exhibited a declining trend when Ce6 treatment concentration increased in the Ce6-PDT groups (Figure 3A). These results suggest that Ce6-PDT treatment could inhibit the proliferation of human colon cancer SW480 cells, in a dose-dependent manner.
Figure 3.
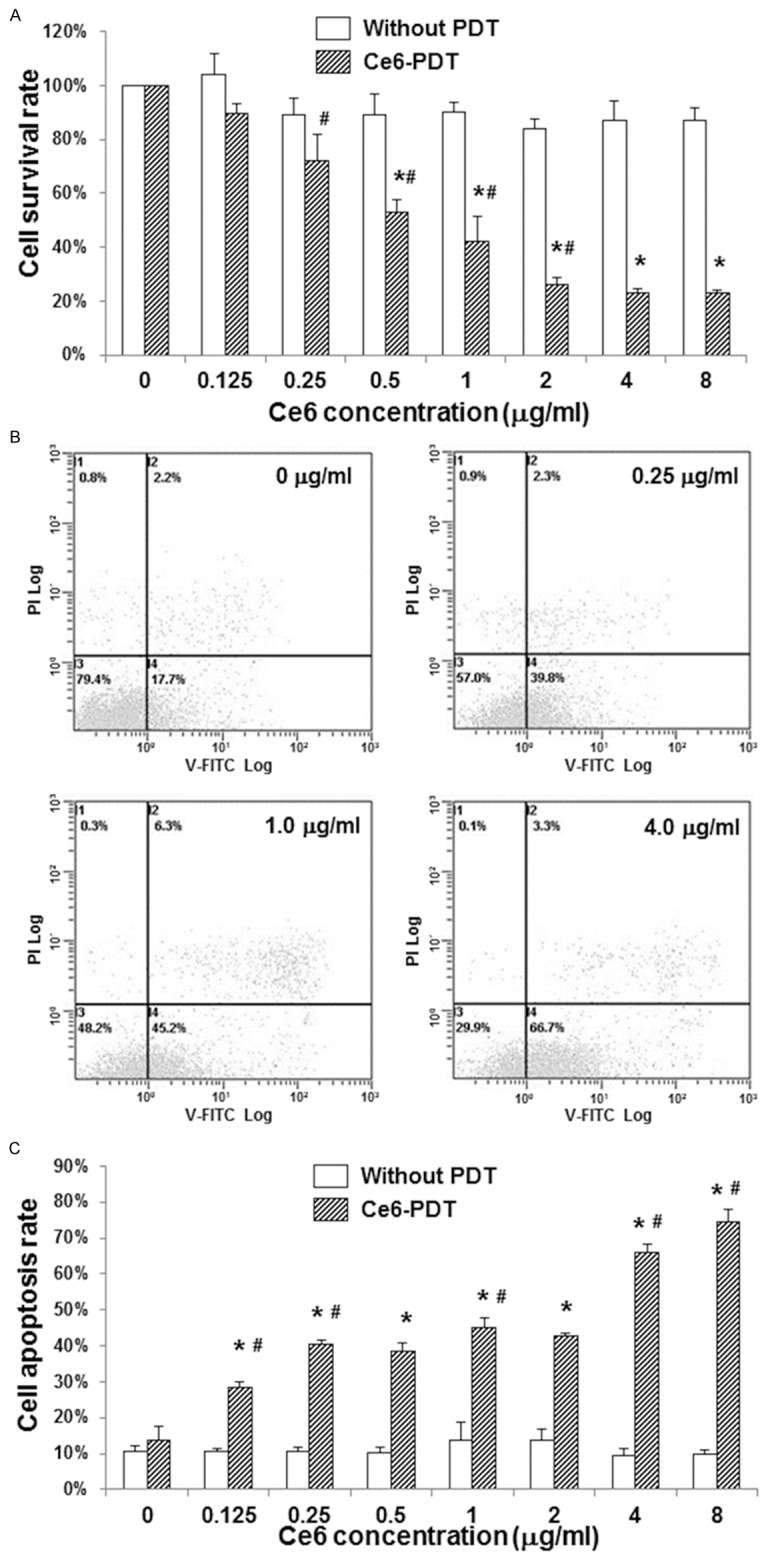
Effects of Ce6-PDT on cell proliferation and apoptosis of human colon cancer SW480 cells. SW480 cells were treated with Ce6 at 0, 0.125, 0.25, 0.5, 1.0, 2.0, 4.0, and 8.0 μg/ml, respectively, followed by photodynamic irradiation. SW480 cells treated with Ce6 at corresponding concentrations without photodynamic irradiation were used as controls. A. The cell survival rates of SW480 cells were assessed by the MTT assay. B-C. Cell apoptosis of SW480 cell was detected by the annexin-V/PI staining and flow cytometry. B. Representative results of flow cytometry indicating the apoptosis assessment of SW480 cells treated with Ce6-PDT at 0, 0.25, 1.0, and 4.0 μg/ml, respectively. C. Statistical analysis of apoptosis rates of SW480 cells treated with Ce6-PDT. Compared with the corresponding control group without photodynamic irradiation, *P < 0.05; compared with the previous group treated with Ce6-PDT, #P < 0.05.
Ce6-PDT promotes apoptosis of human colon cancer SW480 cells
To further investigate the effects of Ce6-PDT treatment on the apoptosis of SW480 cells, these cells were subjected to the Ce6-PDT treatment, and then the annexin-V/PI staining and flow cytometry analysis were performed. SW480 cells treated with Ce6 at corresponding concentrations without photodynamic irradiation were used as controls. Our results showed that, Ce6 treatment alone did not lead to significant differences in the apoptosis rate of SW480 cells. The apoptosis rates of SW480 cells treated with Ce6-PDT were dramatically increased compared with corresponding controls (Figure 3B, 3C; except for the groups treated with 0 μg/ml Ce6-PDT, P < 0.05). Besides, the apoptosis rates of SW480 cells were enhanced along with the increasing treatment concentrations of Ce6 in the Ce6-PDT groups (Figure 3C). These results suggest that the Ce6-PDT treatment could promote the apoptosis of human colon cancer SW480 cells, in a dose-dependent manner.
Ce6-PDT declines migration ability of human colon cancer SW480 cells
To investigate the effects of Ce6-PDT treatments on the migration ability of SW480 cells, the scratch test was conducted. These cells were treated with Ce6 (0, 0.125, 0.25, 0.5, 1.0, 2.0, 4.0, and 8.0 μg/ml, respectively) and the subsequent photodynamic irradiation. Our results showed that, higher Ce6 treatment concentration resulted in slower scratch wound healing process (Figure 4). These results suggest that the Ce6-PDT treatment could decline the migration ability of human colon cancer SW480 cells.
Figure 4.
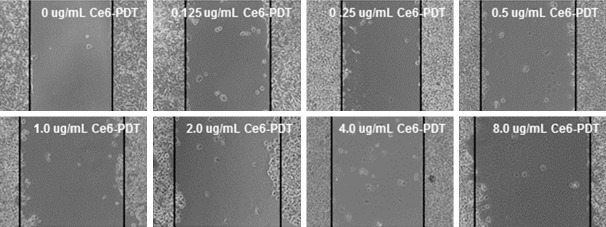
Effects of Ce6-PDT on the migration ability of human colon cancer SW480 cells. Cell migration ability was assessed by the scratch test. The linear scratch was made with a 10-μl sterile pipette tip, followed by the Ce6-PDT treatment (0, 0.125, 0.25, 0.5, 1.0, 2.0, 4.0, and 8.0 μg/ml, respectively). After 48 h, scratch wound healing was observed and analyzed.
Ce6-PDT decreases colony formation ability of human colon cancer SW480 cells
The effects of Ce6-PDT treatments on colony formation ability of SW480 cells were investigated. Following the Ce6-PDT treatment, SW480 cells were further cultured for 1-2 w, and then subjected to Giemsa staining and observation. SW480 cells treated with Ce6 at corresponding concentrations without photodynamic irradiation were used as controls. Our results showed that, in the Ce6-PDT groups, when the Ce6 treatment concentrations were higher than 0.5 μg/ml, the colony formation abilities of SW480 cells were significantly lower than the corresponding controls (Figure 5; P < 0.05). Moreover, the sizes and number of colony formation in the groups treated with higher Ce6 concentrations were dramatically smaller than the groups treated with lower Ce6 concentrations (Figure 5A). Furthermore, within the Ce6-PDT-treated groups, the colony formation ability was declined as the Ce6 treatment concentration was increased (Figure 5B; P < 0.05). These results suggest that the Ce6-PDT treatment could decrease the colony formation ability of human colon cancer SW480 cells, in a dose-dependent manner.
Figure 5.
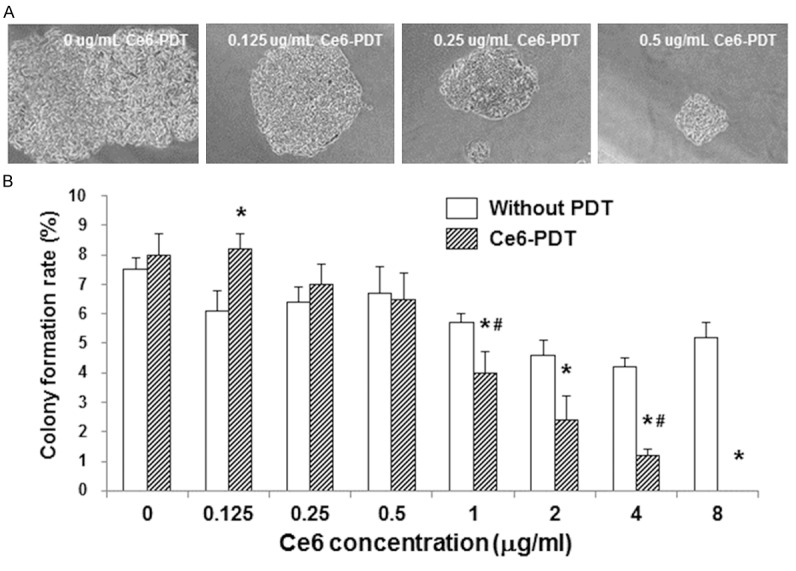
Effects of Ce6-PDT on the colony formation ability of human colon cancer SW480 cells. After the treatment of Ce6-PDT treatment (0, 0.125, 0.25, 0.5, 1.0, 2.0, 4.0, and 8.0 μg/ml, respectively), SW480 cells were further cultured for 1-2 w. Formed colonies with more than 50 cells were counted, and the colony formation rate was calculated. SW480 cells treated with Ce6 at corresponding concentrations without photodynamic irradiation were used as controls. A. Representative results from colony formation tests of SW480 cells treated with Ce6-PDT at 0, 0.125, 0.25, and 0.5 μg/ml, respectively. B. Statistical analysis of colony formation rates of SW480 cells treated with Ce6-PDT. Compared with the corresponding control group without photodynamic irradiation, *P < 0.05; compared with the previous group treated with Ce6-PDT, #P < 0.05.
Discussion
Since 1970s, photodynamic therapy (PDT) has been developed, which is a promising treatment method for cancers and other diseases [12,13]. During PDT, photosensitizers accumulate in tumor cells, and the subsequent photodynamic irradiation at specific wavelength stimulates the photosensitizers to produce ROS, through which those targeted cells would be killed [14,15]. Several organelles, including ER, lysosomes, mitochondria, Golgi, and plasma membrane, etc., are popular locations for photosensitizers [14,16,17]. However, a given photosensitizer might not usually bind to a specific intracellular structure and/or localization, which would lead to the diversity of cell death pathways involved in PDT [18,19].
PDT stimulates the production of ROS, which is an important cellular signaling molecule participating in cell proliferation, apoptosis, differentiation, immune response, and other physiological and/or pathological processes. ROS induced by PDT would cause toxic effects, in which the singlet oxygen is considered to be the major damaging species within cells. In fact, the life span of singlet oxygen in liquid environment is rather short, with the estimated maximum of 4 microseconds. During this time period, the singlet oxygen can diffuse only 10-20 nm from the generation spot. Even in the third half-life, the diffusion distance of singlet oxygen cannot exceed the whole cell or organelles [20]. Therefore, the investigation of the subcellular localization of photosensitizers is of great importance for the initial injury site, response type, and treatment outcome with PDT.
In the present study, the subcellular localization of Ce6 in SW480 cells was detected with LSCM. Our results indicated that Ce6 was mainly located within the ER and lysosomes, without any distribution in the mitochondria and nuclei. In another research, we investigated the subcellular localization of Ce6 in human colon cancer SW620 cells, which is of the same origin with SW480 cells, but with differential metastatic potentials. The results indicated that, in SW620 cells, Ce6 was mainly located in the ER, followed by the mitochondria, with hardly any distribution in the lysosomes and nuclei (unpublished data). Moreover, it has been reported that lysosomes are the major locating sites for Ce6 in human fibroblasts [21]. These results indicate that even the same photosensitizer will related to different intracellular structures and/or locations, depending on the cell type, indicating that various cell death pathways might be induced by PDT.
Interestingly, we found that in SW480 cells treated with low concentrations of Ce6 (0.125 and 0.25 μg/ml), the cell proliferation rate and colony formation ability were slightly increased. It has been reported that the ROS stimulated by PDT could simultaneously induce cell apoptosis and autophagy [22,23]. Moreover, some photosensitizers at low doses can induce apoptosis and autophagy, while at high dosages, they mainly induce apoptosis [24]. Based on these results, we presume that Ce6 treatment at low dosage might mainly induce autophagy in cells, which may actually protect the tumor cells, while high dosages of Ce6 would lead to cell deaths. Of course, further studies are still needed to clarify the detailed mechanisms.
In conclusion, our results showed that Ce6 was evenly distributed within the ER and lysosomes in SW480 cells. Ce6-PDT treatment could enhance the ROS production and apoptosis, inhibit the cell proliferation, decrease the migration ability and colony formation ability, in SW480 cells, in a dose-dependent manner. These findings might provide experimental evidence for the application of Ce6-PDT in clinical treatment of CRC.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 81060186) and the Xinjiang Uygur Autonomous Region Natural Science Foundation (No. 2014211C003).
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Nitsche U, Maak M, Schuster T, Künzli B, Langer R, Slotta-Huspenina J, Janssen KP, Friess H, Rosenberg R. Prediction of prognosis is not improved by the seventh and latest edition of the TNM classification for colorectal cancer in a single-center collective. Ann Surg. 2011;254:793–800. doi: 10.1097/SLA.0b013e3182369101. discussion 800-801. [DOI] [PubMed] [Google Scholar]
- 3.Mulsow J, Merkel S, Agaimy A, Hohenberger W. Outcomes following surgery for colorectal cancer with synchronous peritoneal metastases. Br J Surg. 2011;98:1785–1791. doi: 10.1002/bjs.7653. [DOI] [PubMed] [Google Scholar]
- 4.Moan J, Berg K. Photochemotherapy of cancer: experimental research. Photochem Photobiol. 1992;55:931–948. doi: 10.1111/j.1751-1097.1992.tb08541.x. [DOI] [PubMed] [Google Scholar]
- 5.Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel D, Korbelik M, Moan J, Mroz P, Nowis D, Piette J, Wilson BC, Golab J. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011;61:250–281. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garg AD, Nowis D, Golab J, Agostinis P. Photodynamic therapy: illuminating the road from cell death towards anti-tumour immunity. Apoptosis. 2010;15:1050–1071. doi: 10.1007/s10495-010-0479-7. [DOI] [PubMed] [Google Scholar]
- 7.Wei MF, Chen MW, Chen KC, Lou PJ, Lin SY, Hung SC, Hsiao M, Yao CJ, Shieh MJ. Autophagy promotes resistance to photodynamic therapy-induced apoptosis selectively in colorectal cancer stem-like cells. Autophagy. 2014;10:1179–1192. doi: 10.4161/auto.28679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orenstein A, Kostenich G, Roitman L, Shechtman Y, Kopolovic Y, Ehrenberg B, Malik Z. A comparative study of tissue distribution and photodynamic therapy selectivity of chlorin e6, Photofrin II and ALA-induced protoporphyrin IX in a colon carcinoma model. Br J Cancer. 1996;73:937–944. doi: 10.1038/bjc.1996.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheleg SV, Zhavrid EA, Khodina TV, Kochubeev GA, Istomin YP, Chalov VN, Zhuravkin IN. Photodynamic therapy with chlorin e(6) for skin metastases of melanoma. Photodermatol Photoimmunol Photomed. 2004;20:21–26. doi: 10.1111/j.1600-0781.2004.00078.x. [DOI] [PubMed] [Google Scholar]
- 10.Lee LS, Thong PS, Olivo M, Chin WW, Ramaswamy B, Kho KW, Lim PL, Lau WK. Chlorin e6-polyvinylpyrrolidone mediated photodynamic therapy--A potential bladder sparing option for high risk non-muscle invasive bladder cancer. Photodiagnosis Photodyn Ther. 2010;7:213–220. doi: 10.1016/j.pdpdt.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Kostenich GA, Zhuravkin IN, Zhavrid EA. Experimental grounds for using chlorin e6 in the photodynamic therapy of malignant tumors. J Photochem Photobiol B. 1994;22:211–217. doi: 10.1016/1011-1344(93)06974-8. [DOI] [PubMed] [Google Scholar]
- 12.Wang JB, Liu LX. Use of photodynamic therapy in malignant lesions of stomach, bile duct, pancreas, colon and rectum. Hepatogastroenterology. 2007;54:718–724. [PubMed] [Google Scholar]
- 13.Geltzer A, Turalba A, Vedula SS. Surgical implantation of steroids with antiangiogenic characteristics for treating neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2013;1:CD005022. doi: 10.1002/14651858.CD005022.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piette J, Volanti C, Vantieghem A, Matroule JY, Habraken Y, Agostinis P. Cell death and growth arrest in response to photodynamic therapy with membrane-bound photosensitizers. Biochem Pharmacol. 2003;66:1651–1659. doi: 10.1016/s0006-2952(03)00539-2. [DOI] [PubMed] [Google Scholar]
- 15.Peng Q, Moan J, Nesland JM. Correlation of subcellular and intratumoral photosensitizer localization with ultrastructural features after photodynamic therapy. Ultrastruct Pathol. 1996;20:109–129. doi: 10.3109/01913129609016306. [DOI] [PubMed] [Google Scholar]
- 16.Kessel D, Luo Y, Deng Y, Chang CK. The role of subcellular localization in initiation of apoptosis by photodynamic therapy. Photochem Photobiol. 1997;65:422–426. doi: 10.1111/j.1751-1097.1997.tb08581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buytaert E, Callewaert G, Hendrickx N, Scorrano L, Hartmann D, Missiaen L, Vandenheede JR, Heirman I, Grooten J, Agostinis P. Role of endoplasmic reticulum depletion and multidomain proapoptotic BAX and BAK proteins in shaping cell death after hypericin-mediated photodynamic therapy. FASEB J. 2006;20:756–758. doi: 10.1096/fj.05-4305fje. [DOI] [PubMed] [Google Scholar]
- 18.Galanou MC, Theodossiou TA, Tsiourvas D, Sideratou Z, Paleos CM. Interactive transport, subcellular relocation and enhanced phototoxicity of hypericin encapsulated in guanidinylated liposomes via molecular recognition. Photochem Photobiol. 2008;84:1073–1083. doi: 10.1111/j.1751-1097.2008.00392.x. [DOI] [PubMed] [Google Scholar]
- 19.Yoo JO, Ha KS. New insights into the mechanisms for photodynamic therapy-induced cancer cell death. Int Rev Cell Mol Biol. 2012;295:139–174. doi: 10.1016/B978-0-12-394306-4.00010-1. [DOI] [PubMed] [Google Scholar]
- 20.Oliveira CS, Turchiello R, Kowaltowski AJ, Indig GL, Baptista MS. Major determinants of photoinduced cell death: Subcellular localization versus photosensitization efficiency. Free Radic Biol Med. 2011;51:824–833. doi: 10.1016/j.freeradbiomed.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 21.Mojzisova H, Bonneau S, Vever-Bizet C, Brault D. Cellular uptake and subcellular distribution of chlorin e6 as functions of pH and interactions with membranes and lipoproteins. Biochim Biophys Acta. 2007;1768:2748–2756. doi: 10.1016/j.bbamem.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Sasnauskiene A, Kadziauskas J, Vezelyte N, Jonusiene V, Kirveliene V. Damage targeted to the mitochondrial interior induces autophagy, cell cycle arrest and, only at high doses, apoptosis. Autophagy. 2009;5:743–744. doi: 10.4161/auto.5.5.8701. [DOI] [PubMed] [Google Scholar]
- 23.Dewaele M, Martinet W, Rubio N, Verfaillie T, de Witte PA, Piette J, Agostinis P. Autophagy pathways activated in response to PDT contribute to cell resistance against ROS damage. J Cell Mol Med. 2011;15:1402–1414. doi: 10.1111/j.1582-4934.2010.01118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kessel D, Reiners JJ. Apoptosis and autophagy after mitochondrial or endoplasmic reticulum photodamage. Photochem Photobiol. 2007;83:1024–1028. doi: 10.1111/j.1751-1097.2007.00088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]