Abstract
Objective: Emerging evidence suggest that the acquisition of epithelial mesenchymal transition (EMT) and induction of cancer stem cell (CSC) or cancer stem-like cell phenotype are interrelated and contribute to tumor recurrence and drug resistance. The aim of this study is to shed light on the relationship between EMT and CSCs by using renal cell carcinoma (RCC) cell line, ACHN and 786-0. Methods: RCC cells were treated with 50 ng/ml of TNF-α for 14 days. To evaluate EMT, morphological changes were assessed by light microscopy. RT-PCR and Western blot for EMT-related markers. On TNF-α treated and untreated RCC cells, we performed stemness tests and stemness markers expression. Results: TNF-α treated ACHN cell lost its epithelial morphology assuming a fibroblast-like appearance. The same results were obtained for the 786-0 cells. RT-PCR and Western blot showed up-regulation of Vimentin and down-regulation of E-cadherin in TNF-α treated ACHN and 786-0 cells. Slug and ZEB1 mRNA transcripts were up-regulated in TNF-α treated RCC cells confirming EMT. This two cell line also showed overexpression of Oct4, Nanog, and Bmi-1, all genes of stemness. In addition, in TNF-α treated RCC cell, an increased tumorsphere-forming capacity was detectable. Conclusions: The induction of EMT by TNF-α exposure, in RCC cell results in the acquisition of mesenchymal profile and in the expression of stemness markers.
Keywords: Renal cell carcinoma, epithelial mesenchymal transition, cancer stem cell, TNF-α, stemness
Introduction
Evidence has recently been accumulating to support the hypothesis that tumors contain a subpopulation of tumor cells called cancer stem cells (CSCs), also known as tumor initiating cells or tumorigenic cells, which exhibit stem-like cell properties to self-renew, form tumorspheres, differentiate into heterogeneous populations of cancer cells, and seed new tumors in a xenotransplant system [1,2].
Epithelial mesenchymal transition (EMT) is often activated during cancer invasion and metastasis. The acquisition of EMT is associated with tumor progression [3]. Both the EMT and CSCs play a critical role in tumor metastasis, therapeutic resistance and recurrence [4,5]; however, each alone cannot explain the sum of the cellular events in tumor progression and the significance of EMT in regulating the stemness of CSCs remains unknown until recently [6]. Balancing these two concepts has led researchers to investigate a possible link between EMT and the CSC phenotype.
In this context, it is important to identify which factors could induce EMT and how the EMT cells could become a resource for cancer stem-like cells, developing novel and targeted therapies for malignance. Therefore, the aim of this study is to shed light on the possible relationship between EMT and CSCs by using RCC cell line ACHN and 786-0.
Materials and methods
Cell culture and reagent
Human RCC cell lines (ACHN, 786-0) were obtained from American Type Culture Collection (Manassas, VA), which were maintained in DMEM medium (Invitrogen) containing 10% fetal bovine serum (FBS; Invitrogen). Cells were plated at the density of 1 × 106 cells per 100-mm dish.
TNF-α treatment
In order to induce EMT process, ACHN and 786-0 cells were treated with 50 ng/ml TNF-α (Invitrogen, CA, USA) for 7 and 14 days, respectively. TNF-α has been added twice a week in the medium. To test the possible growth increase due to TNF-α treatment, 10000 cells were plated in 24-well plates and TNF-α untreated and treated cells were detached every 24 hours for 4 days. The number of cells for each experimental condition was counted and represented on a linear graph.
Relative level of mRNA by real time RT-PCR
Total cellular RNA was extracted with RNeasy mini kit (Qiagen, Gaithersburg, MD) and 1 μg RNA was subjected to a cDNA synthesis kit (Bio-Rad, Hercules, CA). One-tenth of the cDNA was subjected to a 25-μL PCR carried out in an iCycler thermal cycler (Bio-Rad) using iQ SYBR Green Supermix (Bio-Rad). The forward and reverse primers for E-cadherin, Vimentin, Slug, ZEB1, Oct 4, Nanog, Bmi-1 and 18S were synthesized by Invitrogen (Table 1). qRT-PCR was performed as described previously [7].
Table 1.
Sequences of the primer pairs used in reverse transcription polymerase chain reactions
| Genes | Forward primer/reverse primer |
|---|---|
| E-cadherin | 5’-AGAGGGTCACCGCGTCTATG-3’ |
| 5’-CTCACAGGTGCTTTGCAGTT-3’ | |
| Vimentin | 5’-TCCAGCAGCTTCCTGTAGGT-3’ |
| 5’-CCCTCACCTGTGAAGTGGAT-3’ | |
| Slug | 5’-TGTTGCAGTGAGGGCAAGAA-3’ |
| 5’-GACCCTGGTTGCTTCAAGGA-3’ | |
| ZEB1 | 5’-GCCAATAAGCAAACGATTCTG-3’ |
| 5’-TTTGGCTGGATCACTTTCAAG-3’ | |
| Oct 4 | 5’-GTCCGAGTGTGGTTCTGTA-3’ |
| 5’-CTCAGTTTGAATGCATGGGA-3’ | |
| Nanog | 5’-CAAAGGCAAACAACCCACTT-3’ |
| 5’-TCTGCTGGAGGCTGAGGTAT-3’ | |
| Bmi-1 | 5’-CTGGTTGCCCATTGACAGC-3’ |
| 5’-CAGAAAATGAATGCGAGCCA-3’ | |
| 18S | 5’-GGAATTGACGGAAGGGCACCACC-3’ |
| 5’-GTGCAGCCCCGGACATCTAAGG-3’ |
Western blot analysis
Equal amounts of protein were separated in 10% SDS-PAGE and transferred to nitrocellulose membrane. After blocking with 5% non-fat dry milk in PBS containing 0.2% Tween-20, membranes were incubated at 4°C overnight with primary antibody, including primary antibodies against E-cadherin (1:400), Vimentin (1:1000), Slug (1:400), ZEB1 (1:350), Oct 4 (1:400), Nanog (1:400), Bmi-1 (1:400) and β-actin (1:1000). All primary antibodies were purchased from Santa Cruz Biotechnology. Membranes were then incubated with horseradish peroxidase-conjugated secondary antibodies for 2 hrs. ECL reagent (GE Healthcare) was used for protein detection.
Clonogenic assay
Clonogenic assay was conducted to evaluate the clonogenicity due to TNF-α treatment, as described previously [8]. Briefly, treated and untreated RCC cells were seeded in 6-well plates (Corning) at a clonal density (i.e., 2,000 cells per well) and cultured for 14 days prior to fixing in 4% paraformaldehyde and staining with crystal violet. The number of clones containing more than 20 cells was counted. Representative fields were photographed. Each assay was performed three times.
Tumorsphere formation assay
In order to evaluate the effect of TNF-α on tumorspheres growth and formation, the RCC cells was plated at a density of 4000 cells per well in 24-well ultra-low attachment plates (Corning Inc., Corning, NY, USA) in serum-free DMEM/F12 supplemented with 20 ng/ml human EGF, 10 ng/ml human bFGF, 2% B27 and 1% N2 supplement (Invitrogen). Cells were incubated in a humidified atmosphere at 37°C with 5% CO2. Fresh aliquots of TNF-α, were added twice a week. Two weeks later, plates were analyzed for tumorsphere formation and were quantified using an inverted microscope.
Statistical methods
All data were reported as means ± standard error of the mean (SEM). Statistical significance of differences between mean values was assessed by Student’s t test for unpaired data. Comparisons of data between multiple groups were performed with analysis of variance (ANOVA). P < 0.05 was considered statistically significant.
Results
TNF-α treatment induces morphologic changes in RCC cells
In order to investigate the effect of TNF-α on ACHN and 786-0 cells, we treated them with 50 ng/ml of TNF-α. The results show that ACHN cells treated with TNF-α lost epithelial cell morphology after 4 days of treatment; they were dispersed and grew as fibroblast-like or long spindle-shaped, and the nucleus was still central (Figure 1A). Similar results were obtained with 786-0 cells, which lost the epithelial morphology and acquired mesenchymal appearance starting from 4 days of TNF-α treatment. The cells became spindle-shaped, fibroblast-like with central nucleus (Figure 1B). This mesenchymal morphology was maintained throughout all time of treatment.
Figure 1.
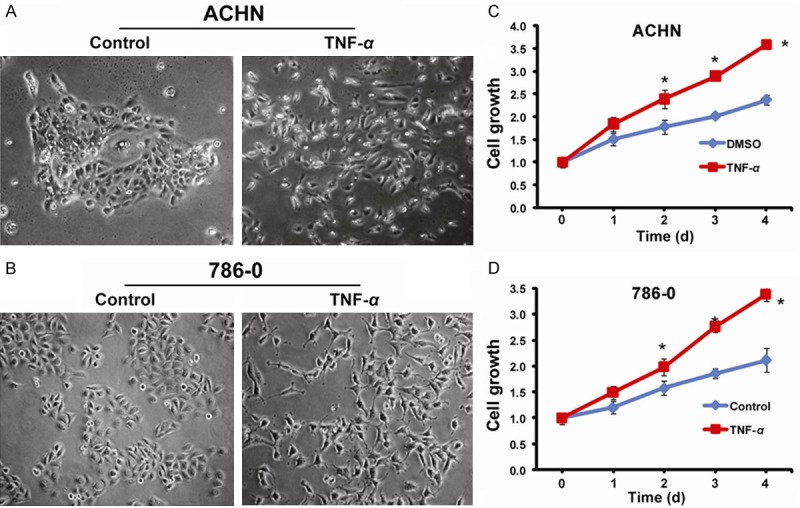
Morphological changes and growth curves after TNF-α treatment. A: Untreated ACHN and treated ACHN, at 4 days 50 ng/ml TNF-α treatment; B: Untreated 786-0 and treated 786-0, at 4 days 50 ng/ml TNF-α treatment; C: Growth curves of untreated and treated ACHN; D: Growth curves of untreated and treated 786-0.
TNF-α treatment promotes EMT of RCC cells
The morphological changes induced by TNF-α treatment in ACHN and 786-0 cells suggested that TNF-α promoted an EMT. The morphological changes of cells undergoing EMT are accompanied by a shift in gene expression from an epithelial to a mesenchymal phenotype. To determine whether TNF-α induces such a shift, we used real time RT-PCR and Western blot to examine the expression of E-cadherin, Vimentin, Slug and ZEB1 in TNF-α-treated RCC cells (ACHN and 786-0 cells). The RT-PCR data showed that there was marked shift of gene expression from a pattern characteristic of epithelial cells to that of mesenchymal cells in ACHN cells with a considerable increase in the expression of EMT-inducing transcription factors, specifically Slug (9.21 fold), and ZEB1 (7.25 fold) indicating their EMT phenotype. (Figure 2A). Also, Western blot results showed that TNF-α treatment led to increased expression of Vimentin, Slug, ZEB1 and decreased expression of E-cadherin in ACHN (Figure 2C). Similar results were obtained with 786-0 cells (Figure 2B, 2D). We conclude that TNF-α led to the acquisition of EMT phenotype in RCC cells.
Figure 2.
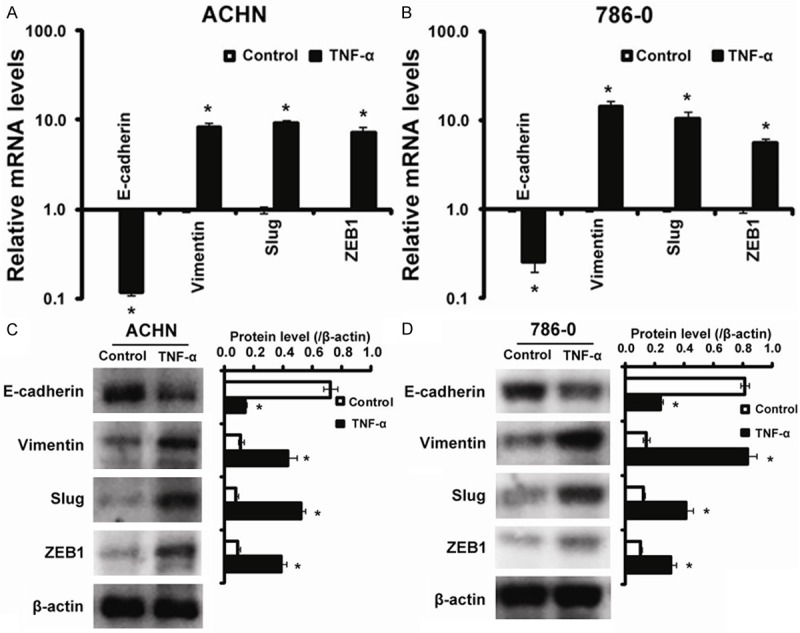
The expression of EMT phenotype marker induced by TNF-α treatment. TNF-α upregulates mesenchymal markers expression and downregulates epithelial markers expression. A: mRNA levels for E-cadherin, Vimentin, Slug and ZEB1 in untreated (control) and TNF-α treated in ACHN cell line after 4 days of treatment; B: mRNA levels for E-cadherin, Vimentin, Slug and ZEB1 in untreated (control) and TNF-α treated in 786-0 cell line after 4 days of treatment; C: Western blotting analysis for E-cadherin, Vimentin, Slug and ZEB1 in untreated (control) and TNF-α treated in ACHN cell line after 4 days of treatment; D: Western blotting analysis for E-cadherin, Vimentin, Slug and ZEB1 in untreated (control) and TNF-α treated in 786-0 cell after 4 days of treatment. *P < 0.05 compared to Control.
Gene expression profiling of stemness markers in RCC cells treated with TNF-α
To determine the genes regulating and maintaining the stem cell phenotype of RCC cells having EMT signatures (termed EMT-ACHN, EMT-786-0 cells), we performed RT-PCR and Western blot for Oct 4, Nanog and Bmi-1. Interestingly, “stemness” genes Oct 4, Nanog and Bmi-1, known to be sufficient to reprogram somatic cells to undifferentiated, pluripotent stem cells, were significantly increased in EMT-ACHN cells with an increase of 6.8 fold, 5.2 fold, and 4.6 fold for Oct 4, Nanog and Bmi-1, respectively more than parental cells (Figure 3A). To further investigate the stemness properties of EMT-ACHN cells, by using western blot analyses, we confirmed preferential expression of these stemness genes. Our results showed that the expression of Oct 4, Nanog and Bmi-1 were significantly increased in EMT-ACHN cells compared with that of ACHN. Similar results were obtained in 786-0 cells. Therefore, the increased expression of these stemness genes indicate the acquisition of stem cell phenotype in the EMT RCC cells (Figure 3).
Figure 3.
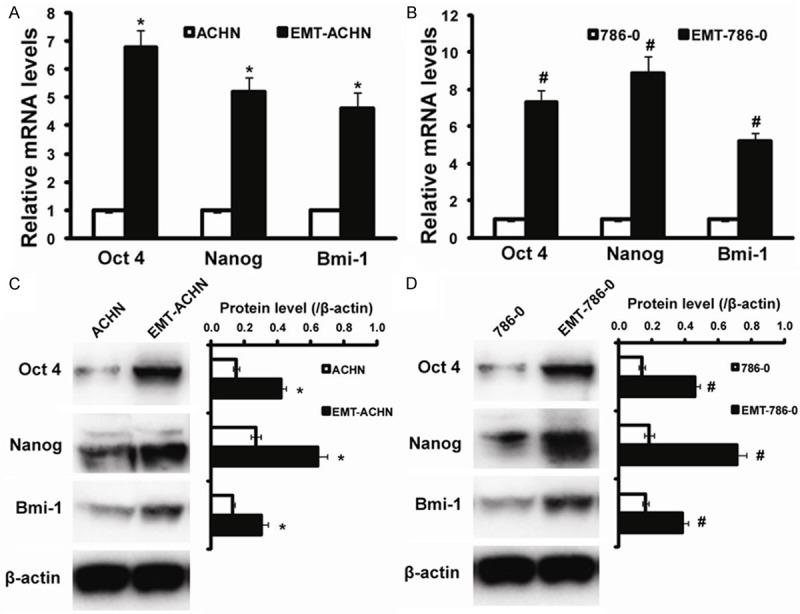
Stemness markers distribution on EMT-ACHN and EMT-786-0 cells. A: Real time RT-PCR evaluation for Oct 4, Nanog and Bmi-1 genes on ACHN and EMT-ACHN cells; B: Real time RT-PCR evaluation for Oct 4, Nanog and Bmi-1 genes on 786-0 and EMT-786-0 cells; C: Western blot for Oct 4, Nanog and Bmi-1 genes on ACHN and EMT-ACHN cells; D: Western blot for Oct 4, Nanog and Bmi-1 genes on 786-0 and EMT-786-0 cells. *P < 0.05 compared to ACHN, #P < 0.05 compared to 786-0.
Colony formation ability was increased in EMT-RCC cells
For determining the self-renewal capacity, colony-forming assays were employed. As shown in Figure 4, the cells were adherently plated and could form holoclone. The EMT-ACHN cells had more holoclone colonies than the ACHN cells (Figure 4A, 4B). Similarly, the data from clonogenic assay in 786-0 cells showed that EMT-786-0 cells generated more colonies than the 786-0 cells (Figure 4C, 4D). Therefore, the EMT RCC cells were capable of proliferating extensively, indicating that EMT RCC cells could play a critical role in maintaining tumor growth.
Figure 4.
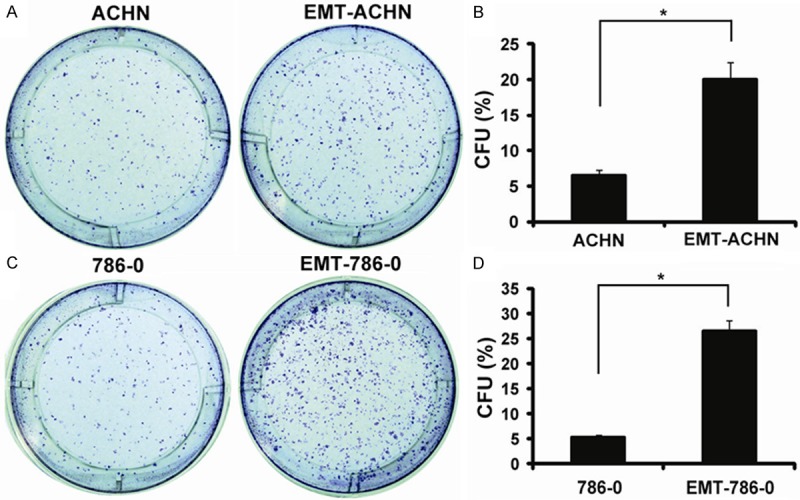
Colony efficiency analyses. A: Photographs of colonies from ACHN and EMT-ACHN cells; B: The colony number was counted and the data were presented as CFU (%) respect to cell number seeded; C: Photographs of colonies from 786-0 and EMT-786-0 cells; D: The colony number was counted and the data were presented as CFU (%) respect to cell number seeded. *P < 0.05.
Tumorspheres formation ability was increased in EMT-RCC cells
Tumorsphere formation assays have been widely used to measure the self-renewing ability of stem or progenitor cells. RCC cells were cultured in serum-free stem cell medium. After 14 days, tumorsphere cells of 50 to 100 µm in diameter were observed. The self-renewal capacity of RCC cells was assessed by dissociating them into single cell, and growing at a density of 2,000 cells/ml to form tumorsphere. After 14 days, the EMT-ACHN generated more tumorspheres with size larger than 100 µm diameter (Figure 5). The tumorsphere formation rate of EMT-ACHN cells was significantly higher than that of ACHN cells (Figure 5B). Moreover, we analyzed the size of tumorspheres and found that EMT-ACHN cells could generate larger size (diameter > 100 µm). The percentage of tumorspheres with a diameter of larger than 100 µm was significantly higher in EMT-ACHN cells than that in ACHN cells (Figure 5B). Similar results were obtained in 786-0 cells (Figure 5C, 5D). Based on this functional assay, we concluded that the cells generated by an EMT acquired yet another attribute of renal cancer stem cells.
Figure 5.
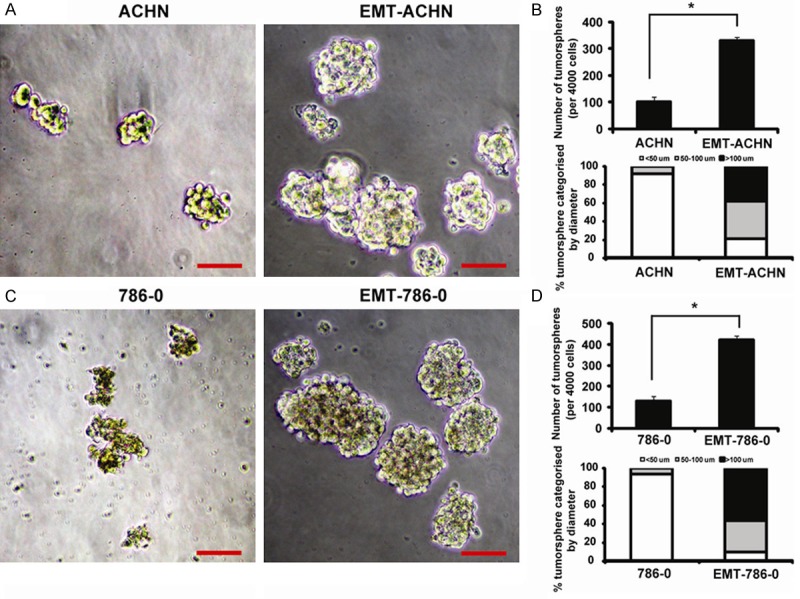
Tumorspheres formation ability evaluation on EMT-ACHN and EMT-786-0 cells. A: ACHN and EMT-ACHN tumorspheres, Bar = 50 μm; B: The tumorsphere number was counted and plotted, Percentage of tumorspheres with diameters < 50, 50-100, or > 100 μm were caculated and plotted; C: 786-0 and EMT-786-0 tumorspheres, Bar = 50 μm; D: The tumorsphere number was counted and plotted, Percentage of tumorspheres with diameters < 50, 50-100, or > 100 μm were calculated and plotted.
Discussion
The identification of cancer stem cells (CSCs) in hematologic malignancies has provoked intensive research in oncology in order to identify and characterize them in many different solid tumors [9,10]. CSCs represent a small proportion of tumor cells and possess morphologic markers and functional properties (i.e. self-renewal) associated with normal stem cells. Many studies demonstrated that CSCs are responsible for cancer relapses and metastasis [11,12].
EMT is a critical physiological process during development and wound healing [13]. Studies demonstrate that the EMT is activated in many types of cancers, and the acquisition of EMT is associated with tumor invasion and metastasis [3]. During the process of tumor progression, cancer cells acquire skills of self-renewal in order to spread the metastases, which similar to that exhibited by stem cells. Recently, studies have shown that EMT plays a vital role not only in tumor metastasis but also in tumor recurrence, which is believed to be strongly linked with the signatures of cancer stem-like cells [14-16]. Taken together, these evidences suggest a possible relationship between EMT and cancer stem cells.
In the current study, we aim to show that EMT acquisition is associated with an increase of stemness signatures in RCC cells. Because EMT plays a critical role in the tumor progression, we chose ACHN and 786-0 cells as model system of EMT. We investigate the effect of TNF-α on EMT and stemness. The results showed that TNF-α induces EMT in RCC cells by acquisition of mesenchymal cell morphology, increased expression of mesenchymal molecular markers such as Vimentin and decreased expression of epithelial molecular markers such as E-cadherin. We treated RCC cells with TNF-α 50 ng/ml for 14 days. Only after 4 days, we observe cell morphological changes with the acquisition of EMT phenotype as characterized by the loss of expression of epithelial molecular markers such as E-cadherin and the increased expression of mesenchymal molecular markers such as Vimentin. More importantly, RCC cells displayed an increased clonogenic and tumorsphere-forming capacity, the characteristics that are known to be associated with cancer stem-like cell characteristics. Our results suggest that EMT-type RCC cells show stem-like cells or cancer stem-like cell characteristics. Moreover, these primary data support the notion that EMT, a critical process of cancer invasion and metastasis, is associated with stemness property of cancer cells. At the same time, the identification of relationship between EMT and stemness property in RCC remains a high priority to develop new therapeutic strategies and to advance the comprehension of RCC biology.
Acknowledgements
This study was supported by a grant from National Natural Science Foundation of China (No. 30801152, No. 81272811 to LL Zhang).
Disclosure of conflict of interest
None.
References
- 1.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 2.Pietras A. Cancer stem cells in tumor heterogeneity. Adv Cancer Res. 2011;112:255–281. doi: 10.1016/B978-0-12-387688-1.00009-0. [DOI] [PubMed] [Google Scholar]
- 3.Steinestel K, Eder S, Schrader AJ, Steinestel J. Clinical significance of epithelial-mesenchymal transition. Clin Transl Med. 2014;3:17. doi: 10.1186/2001-1326-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Y, Zhou BP. New insights of epithelial-mesenchymal transition in cancer metastasis. Acta Biochim Biophys Sin (Shanghai) 2008;40:643–650. doi: 10.1111/j.1745-7270.2008.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gil J, Stembalska A, Pesz KA, Sasiadek MM. Cancer stem cells: the theory and perspectives in cancer therapy. J Appl Genet. 2008;49:193–199. doi: 10.1007/BF03195612. [DOI] [PubMed] [Google Scholar]
- 6.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L, Li L, Jiao M, Wu D, Wu K, Li X, Zhu G, Yang L, Wang X, Hsieh JT, He D. Genistein inhibits the stemness properties of prostate cancer cells through targeting Hedgehog-Gli1 pathway. Cancer Lett. 2012;323:48–57. doi: 10.1016/j.canlet.2012.03.037. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L, Jiao M, Li L, Wu D, Wu K, Li X, Zhu G, Dang Q, Wang X, Hsieh JT, He D. Tumorspheres derived from prostate cancer cells possess chemoresistant and cancer stem cell properties. J Cancer Res Clin Oncol. 2012;138:675–686. doi: 10.1007/s00432-011-1146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan Y, Gerhard B, Hogge DE. Detection, isolation, and stimulation of quiescent primitive leukemic progenitor cells from patients with acute myeloid leukemia (AML) Blood. 2003;101:3142–3149. doi: 10.1182/blood-2002-10-3062. [DOI] [PubMed] [Google Scholar]
- 10.Frank NY, Schatton T, Frank MH. The therapeutic promise of the cancer stem cell concept. J Clin Invest. 2010;120:41–50. doi: 10.1172/JCI41004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hemmings C. The elaboration of a critical framework for understanding cancer: the cancer stem cell hypothesis. Pathology. 2010;42:105–112. doi: 10.3109/00313020903488773. [DOI] [PubMed] [Google Scholar]
- 12.Besançon R, Valsesia-Wittmann S, Puisieux A, Caron de Fromentel C, Maguer-Satta V. Cancer stem cells: the emerging challenge of drug targeting. Curr Med Chem. 2009;16:394–416. doi: 10.2174/092986709787315531. [DOI] [PubMed] [Google Scholar]
- 13.Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelialmesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119:1438–1449. doi: 10.1172/JCI38019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yasui K, Shimamura M, Mitsutake N, Nagayama Y. SNAIL induces epithelial-to-mesenchymal transition and cancer stem cell-like properties in aldehyde dehydroghenase-negative thyroid cancer cells. Thyroid. 2013;23:989–996. doi: 10.1089/thy.2012.0319. [DOI] [PubMed] [Google Scholar]
- 15.Devarajan E, Song YH, Krishnappa S, Alt E. Epithelial-mesenchymal transition in breast cancer lines is mediated through PDGF-D released by tissue-resident stem cells. Int J Cancer. 2012;131:1023–1031. doi: 10.1002/ijc.26493. [DOI] [PubMed] [Google Scholar]
- 16.Yi SY, Hao YB, Nan KJ, Fan TL. Cancer stem cells niche: a target for novel cancer therapeutics. Cancer Treat Rev. 2013;39:290–296. doi: 10.1016/j.ctrv.2012.10.004. [DOI] [PubMed] [Google Scholar]


