Abstract
The aims of this study was to assessed the ability of a combination treatment of bone marrow stromal cell (BMSC) and atorvastatin in a rat model of spinal cord injury (SCI) as an appropriate substitute for current SCI treatments. In the present study, the female Wistar rats were divided into five groups (n = 20) after SCI by New York University Device: SCI, sham, atorvastatin, graft BMSC and graft BMSC plus atorvastatin. Locomotion was assessed using Basso, Beattie and Bresnahan (BBB) test and walking test after SCI. In addition, microvessel density (MVD) was calculated by immunohistochemistry after SCI. We also investigate the vascular endothelial growth factor (VEGF) and brain-derived neurotrophic factor (BDNF) expression level by western blot after drug treatment. The results showed that BBB scores and walking test were increased in atorvastatin plus BMSC group compared to single atorvastatin and BMSC groups (P < 0.05). In addition, MVD also significantly increased in combination group compared to single treatment group. Compared to sole drug, VEGF and BDNF expression were significantly up-regulated in atorvastatin combination with BMSC group (P < 0.05). These results imply that the combined use of atorvastatin and BMSC treatment may represent a promising strategy for clinically applicable pharmacological therapy for initiation of neuroprotection after SCI.
Keywords: Spinal cord injury, bone marrow stromal cells, atorvastatin, neuroprotective
Introduction
Spinal cord injury (SCI) is one of the most disabling diseases, which cause death of neural cells, severance and demyelination of descending and ascending axons, consequently, loss of motor and sensory function [1]. A larger number of endogenous repair efforts fail to repair the spinal cord and, as a result, the functional improvements are permanent [2,3]. Although potential treatments for SCI are being tested in the clinic, none of these have emerged as one that reverses the devastating functional consequences of SCI. Yet, several studied demonstrated that transplantation of stem cells like bone marrow stromal cells (BMSCs) [4], Schwann cells (SCs) [5], neural stem cells (NSCs) [6], or Olfactory bulb ensheathing cells (OEC) [7] into the injured spinal cord improves locomotor recovery.
Recently studies have shown that transplantation of NSCs improves functional recovery but induces neuropathic pain (mechanical Allodynia) [8]. However, several studied demonstrated that bone marrow stromal cells (BMSCs) may have therapeutic promise for SCI [9,10]. Survival, migration and differentiation of BMSCs after transplantation have been widely researched in recent years [9-11]. Thus, BMSCs are widely used in cell therapy studies [12]. Although results of clinical studies based on cell transplantation have been successful, these cells have a decreased survival rate and are mostly affected by apoptosis [14]. Studies have shown that the production of free radicals, lack of trophic factor support and apoptosis leads to poor survival of grafted cells [14]. To promote survival of transplanted stem cells, anti-apoptotic agents [15], trophic factor support [16] and antioxidants [17] were used.
Statins, a cholesterol-lowering drug, have been widely used in anti-inflammatory, antioxidant, and neuroprotective effects for many years [18]. Many studies have reported that following a stroke, traumatic brain injury (TBI), statin therapy has a synergetic effect with BMSCs, and can mobilize the engrafted BMSCs to the lesion area and promote its repair [16,19,20]. However, BMSC combination with statins treatment spinal cord injury had little been reported, thus, in this study we assessed the effectiveness of BMSC transplantation combined with administration atorvastatin of in a rat model of SCI as an acceptable substitute for current SCI treatments.
Materials and methods
Animals and atorvastatin administration
In this study, adult female Wistar rats (n = 100) weighting (250-300 g) were purchased from the Center for Experimental Animals of Jilin University (Changchun, China). All animal experiments were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals. All procedures in this study, including the use of animals, were approved by the Research Council of Jilin University (Changchun, China) Ethics Committee on Animal Experiments. Atorvastatin was dissolved in methanol (20 mg atorvastatin dissolved in 20 ml saline with 10 μL methanol) and injected subcutaneously (s.c.) at 24 hours after SCI. An atorvastatin dose of 10 mg/kg was selected based on pretreatment studies of in mice [21].
Culture and identification of BMSCs
Bone marrow was isolated in sterile conditions from 8 weeks-old male Sprague Dawley rats weighting (250-300 g) as described in detail by Azizi et al. [13]. BMSCs were isolated from rat bone marrow and cultured as described by previous literature [14]. Briefly, rats were killed with an overdose of pentobarbital and the tibia and femur were dissected out, both ends of the bones were cut off and marrow was flushed out with 5 ml α-MEM (Sigma, Germany) with a 25-gauge needle. The suspension was centrifuged at 800 rpm for 5 minutes and the supernatant was removed. The cells were placed in a 25 cm2 plastic flask for cell culture with 5 ml DMEM-low glucose medium containing 10% fetal bovine serum (FBS). The cells were incubated at 37°C in 95% humidity and 5% CO2 for 24 h. At this time, non-adherent cells were removed by replacing the medium. After 48 hours, the non-adherent cells were removed by replacing the medium, when the adherent cells had grown to 80%-90% confluency they were removed by incubation in a solution containing 0.25% trypsin and 1 mM EDTA (Sigma, Germany) at 37°C for 5 minutes and passaged. BMSCs were sub-cultured 4 times in this way.
The cells were labeled with bromodeoxyuridine (Brdu) at a concentration of 3 µg/ml which was added to the incubation medium 3 days prior to transplantation. Cultured BMSCs were identified with antibodies against CD44 according to previous literature [14].
Spinal cord injury model preparation
The animals were anesthetized using intraperitoneal ketamine (80 mg/ kg) and xylazine (10 mg/kg). Rats were placed prone on an operating table covered with a warming blanket. Laminectomies were performed at the T8-T10 level. Spinal cords were injured using the weight drop technique according to Allen’s method [15] with a minor modification. A plastic iatorvastatinounder (2-mm diameter) was placed gently on the exposed dura and a 10-giron weight was dropped from a height of a 10 cm onto the iatorvastatinounder. The weight and iatorvastatinounder were immediately removed after iatorvastatinact and paravertebral muscle and skin were closed.
Transplantation procedure
The rats were randomly divided into five groups: 1. Sham group (n = 20) in which only a laminectomy was performed; 2. SCI group (n = 20) in which serum was administered by intraspinal injection; 3. BMSC group (n = 20) which received (3 × 105 BMSCs) by in the same way. BMSCs were transplanted by intraspinal injection using Hamilton syringe described as Bagher’s method [16]. 4. Atorvastatin group (n = 20) which received atorvastatin (10 mg/kg) daily for 28 days. 5. Atorvastatin plus BMSC group (n = 50) received BMSC and atorvastatin according to BMSC group and atorvastatin group method, respectively.
Myeloperoxidase (MPO) activity
Myeloperoxidase activity, an indicator of polymorphonuclear leukocyte (PMN) accumulation, was determined in the spinal cord tissues according to previous study [21]. MPO activity was defined as the quantity of enzyme degrading 1 μmol of peroxide min-1 at 37°C and was expressed in unit g-1 of wet tissue.
Behavioral assessments
Locomotor activity was evaluated over a period of 5 minutes by an open-field walking test. One animal at a time was allowed to move freely inside a circular plastic tray (90 cm diameter × 24 cm wall height). Two independent examiners observed the hind limb movements of the rat and scored the locomotor function according to the Basso Beattie and Bresnahan scale (BBB scale) that ranges from 0 (paralysis) to 21 points (normal gait). The final score for each animal was the mean value of both examiners. During the open field activity the animals were also video monitored with a digital camera. Functional tests were performed before the injury and transplantation and weekly for 5 weeks after transplantation.
To assess all group locomotion, the ability of rats to walk on an irregularly horizontal wire grid was determined as described by previous study [22]. Rats were allowed to walk on the grid weekly and tested at 5 week after the contusive SCI. Each rat was allowed to walk freely around for 4 min. If the hind paw of one side protruded entirely through the grid, with all toes and heel extended below the wire surface, it was counted as a misstep. Moreover, the total number of steps taken with the hindlimb of the same side was also counted. The result was showed as the percentage of missteps.
Immunohistochemistry and microvessel density (MVD)
Specific immunochemical staining of endothelial cells was performed using a monoclonal antibody to CD34. Mouse-anti-rabbit-CD34 monoclonal antibody was purchased from Santa Cruz Biotechnology (USA). In each spinal (T9-T10), the total number of CD34 positive blood vessels was counted in ten randomly chosen high power fields, which were distributed evenly inside the spinal. The blinded operator performed the quantification of angiogenesis using Image J software on 10 high power fields (200×) from each sample acquired.
Western blot
In order to detect protein expression level of VEGF, BDNF, western blot was performed as described in the online supplement. The spinal cord tissues were obtained from the peri-lesion region at days 28 post-surgery. Protein homogenates of spinal cord samples were prepared by rapid homogenization in 10 volumes of lysis buffer (2 mM EDTA, 10 mM EGTA, 0.4% NaF, 20 mM Tris-HCl, pH7.5). Tissue homogenate were centrifuged at 17,000 g for 1 h at 4°C and the protein concentration in the supernatant was determined by the Coomassie (G250) binding method. Equal amounts of protein (20 μg) from each sample were loaded for separated into a 8% gradient SDS-PAGE under denaturing conditions. Electroblotting proteins were transferred onto nitrocellulose membranes (Santa Cruz Biotechnology, Inc). After blocking with 5% nonfat dry milk overnight at 4°C, membranes were incubated for 2 h at room temperature in agitation with the following antibodies: rabbit polyclonal anti-VEGF (dilution 1:500; Santa Cruz Biotechnology, Inc), rabbit polyclonal anti-BDNF (dilution 1:1000; Santa Cruz Biotechnology, Inc), and rabbit polyclonal anti-β-actin (dilution 1:800; Santa Cruz Biotechnology, Inc). Secondary horseradish peroxidase conjugated rabbit anti-goat/goat anti-rabbit antibodies (Santa Cruz Biotechnology, Inc) were used at 1:3000 dilution for 2 h at room temperature in agitation. Immunoreactive bands were visualized using the enhanced chemiluminescence (ECL kit, Santa Cruz Biotechnology, Inc) and scanned using Chemi Imager 5500 V2.03 software. The integrated densities value (IDV) was analyzed with computerized image analysis system (Fluor Chen 2.0) and normalized with that of β-actin.
Statistics analysis
The statistical package SPSS13.0 (SPSS Incorporated, Chicago) was used for all analysis. One-way ANOVA followed by Bonferroni’s post hoc test were utilized to determine the significant difference among multiple groups. All values were expressed as mean ± SD. In general, p values less than 0.05 were considered statistically significant.
Results
BMSC cell culture and detection
Morphology of the primary cultured BMSCs were multiplicity. Most cells suspended in culture medium, non-adherent cells were removed by replacing the medium. Adherent cells began to proliferate and form cell colony at about 72 h latter. Cell surrounded the cell colony center to distribute toward circumference. Cell morphology was homogeneous, which appeared spindle-shaped with serial sub-cultivation (Figure 1A). Most of cultured adherent passage 3 cells expressed CD44 (Figure 1B).
Figure 1.
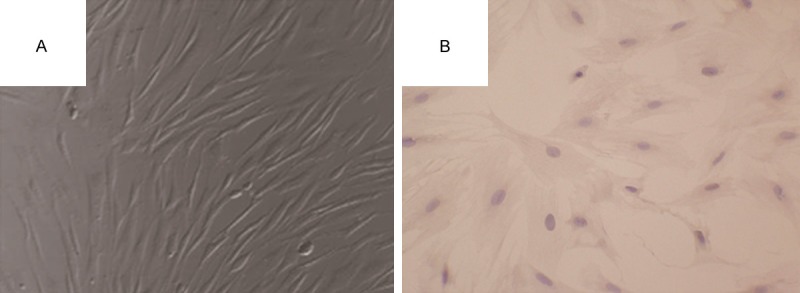
Bone marrow stromal cells culture and detection. A: Third passage of bone marrow mesenchymal stem cells (BMSCs) were observed using inverted phase contrast microscope. B: The expression of CD44 in BMSCs was observed by phase contrast the inverted microscope.
Effect of combination therapy on neutrophil infiltration
We investigated the effect of combination therapy with BMSC and atorvastatin on the neutrophil infiltration by measuring tissue MPO activity. MPO activity was significantly elevated in the spinal cord after injury in rats subjected to SCI when compared with sham-operated group (Figure 2). The MPO activity was significantly reduced by the combination therapy with BMSC and atorvastatin (Figure 2, P < 0.05). However, atorvastatin group or BMSC group as a single treatment group did not reduce the neutrophil infiltration in the injured spinal cord (Figure 2).
Figure 2.
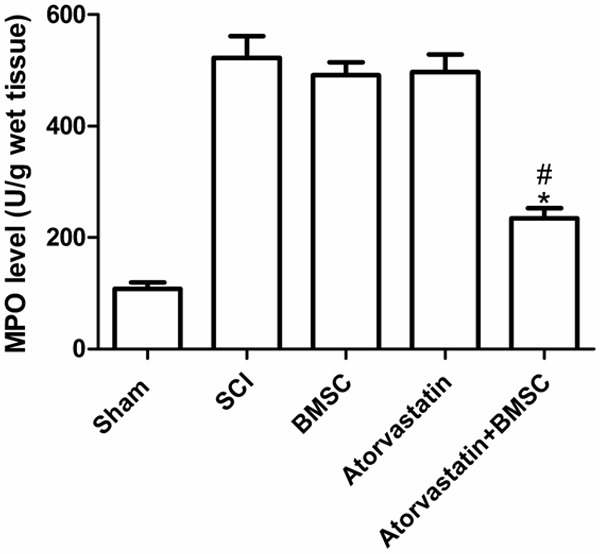
Effects of BMSC combination with atorvastatin on MPO activity. Following the injury, MPO activity in spinal cord from SCI mice was significantly increased at 24 h after the damage in comparison to sham mice. Treatment with BMSC and atorvastatin significantly reduced the SCI-induced increase in MPO activity. A single treatment did not reduce the neutrophil infiltration in the injured spinal cord. *P < 0.05 versus SCI group, #P < 0.05 versus BMSC group.
Combination treatment of atorvastatin and BMSC improve functional recovery after SCI
To determine whether and atorvastatin combination treatment mediated tissue protection and repair also had an effect on functional recovery, the BBB locomotor test was performed at 1 d, 3 d and weekly up to 5 weeks after SCI (Figure 3A). One day after SCI, the contused rats demonstrated considerable loss of hind limb locomotor function with no movement and BBB scores of 0-1 points. In the following days, the locomotor performance substantially improved and reached a relative plateau at the 3rd week. A minus but not statistically significant increase of BBB scores can be found in BMSC and atorvastatin group from 5th week after SCI. The scores in BMSC and atorvastatin combination treatment group were consistently higher than those in the other groups and the differences between the combination group and the other three groups were statistically significant starting from the 3 days and continued until the 5th week. The sham rats in BBB test all achieved maximal 21 scores (Data not show).
Figure 3.
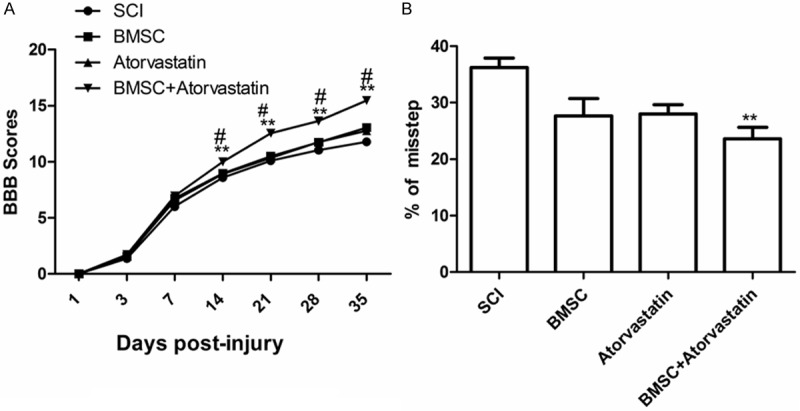
Combination treatment of BMSC and atorvastatin improved functional recovery after SCI. A: BBB tests were performed in different groups at different time after treatment; B: Grid walking test was evaluated in different groups 35 days after treatment; showed that the percentages of missteps in combining treated group were significantly lower than the control group.**P < 0.05 as compared to SCI group, #P < 0.05 as compared to BMSC group.
Results from the grid walking test also showed that the percent of missteps of hind paws were dramatically reduced in BMSC combination with atorvastatin treated rats compared to other group (Figure 3B).
Microvessel density (MVD) assay
MVD within spinal samples was measured by specific immunochemical staining of endothelial cells (CD34+). In each spinal (T9-T10), the total number of CD34 positive blood vessels was counted in ten randomly chosen high power fields, which were distributed evenly inside the spinal. The counts from the five fields (200×) were averaged and used for comparisons. The MVD in the BMSC combination with atorvastatin group (53.96 ± 4.88) was much higher than that of BMSC group (37.34 ± 3.04) and atorvastatin group (34.18 ± 3.15) 2 weeks after treatment (Figure 4) (P < 0.05). In addition, the MVD in single drug group was higher than that of SCI group (21.01 ± 2.03) (Figure 4) (P < 0.05).
Figure 4.
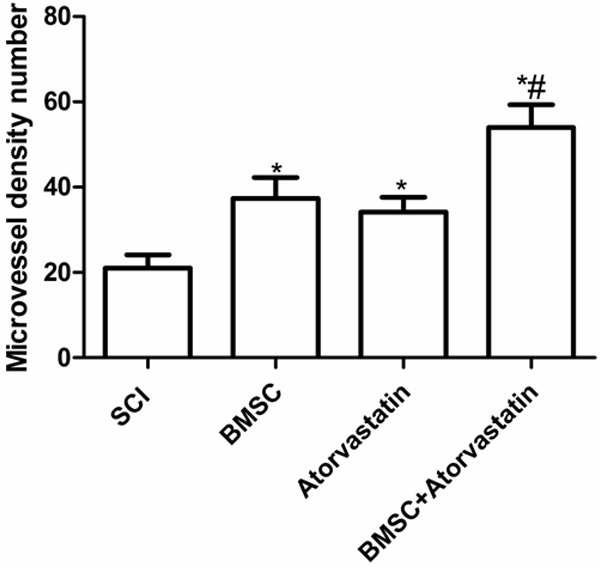
The microvessel density (MVD) level was measured in different groups 35 days after treatment using single BMSC and atorvastatin or both, *P < 0.05 as compared to SCI group, #P < 0.05 as compared to BMSC group.
Combination treatment of BMSC and atorvastatin increased VEGF and BDNF expression level after SCI
In order to detect the VEGF and BDNF in rat with SCI after BMSC combination with atorvastatin treatment, western blot was performed. As shown in Figure 5, VEGF and BDNF expression levels in treatment group were significantly increased after compared to SCI group (P < 0.05), moreover, BMSC combination with atorvastatin group were significantly higher than those of BMSC group and atorvastatin group (P < 0.05). These results showed that BMSC combination with atorvastatin could induce VEGF and BDNF expression.
Figure 5.
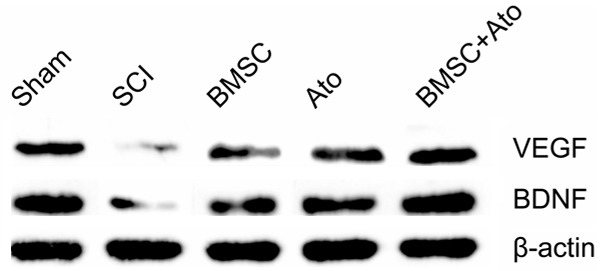
Effect of BMSC combination with atorvastatin on the expression of VEGF and BDNF in the injured spinal cord. The expression of BDNF and VEGF in the treatment group was significantly higher than SCI group; the expression of BDNF and VEGF in the BMSC combination with atorvastatin group was significantly higher than those of single BMSC group and atorvastatin group. *P < 0.05 as compared to SCI group, #P < 0.05 as compared to BMSC group.
Discussion
Bone marrow stromal cells (BMSCs) are non-hematopoietic multipotent stem cells capable of trans-differentiating into neurons, astrocytes or oligodendrocytes [23]. In addition, BMSCs have potential to differentiate into other kinds of cells such as osteoblasts, adipocytes, and chondrocytes, and produce a variety of neurotrophic factors, cytokines, cell adhesion molecules, and growth factor thereby providing the pathway for regenerating axons [23-25]. Extensive studies have shown that transplantation of BMSCs significantly improves hind limb function after SCI [23,26], which was agreement with our results. Our data demonstrates that the transplantation of BMSCs into the injured spinal cord of rats can improve functional recovery. Thus, BMSCs have the potential to restore injured spinal cord tissue and promote functional recovery.
It is now widely accepted that the adult contains plenty of endogenous stem cells, which are normally quiescent [17]. Therefore, it is an important strategy to stimulate the proliferation, migration and differentiation of endogenous stem cells including BMSCs in vivo for the treatment of SCI. A larger number of study showed that treatment with statin drug can promote the migration of BMSCs and improve functional recovery after stroke or ischemia [15,16,19,20]. Leone et al. reported that after myocardial infarction in humans, treatment with statin improved the spontaneous mobilization of endogenous BMSCs, which may have contributed to more favorable cardiac remodeling [15]. Importantly, Han et al study showed that treatment for SCI with single low dose of statin drug administered by subarachnoid injection that can significantly promote the migration of BMSCs to the injured spinal cord [27]. In addition, it has been proven that stain drug inhibited proliferation of BMSCs [28]. Therefore, in this study, we selected atorvastatin combination BMSC for treatment SCI. Our result showed that BMSC combination with atorvastatin could improve MVD and promote angiogenesis, enhances the migration of BMSCs after SCI, which further confirmed that statin could improve BMSC role in treatment nervous disease.
BDNF, a major source of neurotrophic factors, plays an important role in the survival and regeneration of neurons in SCI [29]. Previous study showed that increased production of BDNF in the injured spinal cord could improve functional recovery In addition, it has been proven that stain drug inhibited proliferation of BMSCs [30]. In addition, several studies showed that treatment with atorvastatin can improve the expression of BDNF, thereby improving neurological recovery after stroke [31]. In this study, we found that atorvastatin combination with BMSC can significantly improve the expression of BNDF after SCI.
Angiogenesis plays an important role in increasing blood flow at the lesion site, where hypoxia occurs after SCI and is an essential component of nerve regeneration across a gap of injured spinal cord [32]. VEGF is a potent stimulator of angiogenesis and affects blood permeability, which is important in wound healing after SCI [32]. Previous studied had shown that administration of VEGF could significantly increase the microvascular density in central nervous system [33,34], and improve the motor function of animals [35]. In addition, Geiger et al reported that VEGF producing bone marrow stromal cells (BMSC) enhance vascularization and resorption of a natural coral bone substitute. Chen et al studies showed that treatment with atorvastatin can improve the expression of VEGF after stroke [31]. Han et al reported that boosting the expression of BDNF and VEGF by statin drug could modulates the environment in the injured spinal cord, resulting in better survival, proliferation and differentiation into neural lineage of BMSCs that migrate to the lesion site [27]. In the present study, we found that atorvastatin combination with BMSC could improve microvascular density and the expression of VEGF in the SCI.
In conclusion, we report a treatment for SCI with atorvastatin combination with BMSC, which can significantly promote the expression of BDNF and VEGF and accelerate the recovery of hind limb function in rats. These results imply that the combined use of atorvastatin and BMSC treatment may represent a promising strategy for clinically applicable therapy for initiation of neuroprotection after SCI.
Acknowledgements
The authors gratefully acknowledge the financial support provided by health bureau of Jilin (2010Z025).
Disclosure of conflict of interest
None.
References
- 1.Lim PA, Tow AM. Recovery and regeneration after spinal cord injury: a review and summary of recent literature. Ann Acad Med Singapore. 2007;36:49–57. [PubMed] [Google Scholar]
- 2.Kambi N, Halder P, Rajan R, Arora V, Chand P, Arora M, Jain N. Large-scale reorganization of the somatosensory cortex following spinal cord injuries is due to brainstem plasticity. Nat Commun. 2014;5:3602. doi: 10.1038/ncomms4602. [DOI] [PubMed] [Google Scholar]
- 3.Wollaars MM, Post MW, van Asbeck FW, Brand N. Spinal cord injury pain: the influence of psychologic factors and impact on quality of life. Clin J Pain. 2007;23:383–391. doi: 10.1097/AJP.0b013e31804463e5. [DOI] [PubMed] [Google Scholar]
- 4.Himes BT, Neuhuber B, Coleman C, Kushner R, Swanger SA, Kopen GC, Wagner J, Shumsky JS, Fischer I. Recovery of function following grafting of human bone marrow-derived stromal cells into the injured spinal cord. Neurorehabil Neural Repair. 2006;20:278–296. doi: 10.1177/1545968306286976. [DOI] [PubMed] [Google Scholar]
- 5.Pearse DD, Sanchez AR, Pereira FC, Andrade CM, Puzis R, Pressman Y, Golden K, Kitay BM, Blits B, Wood PM, Bunge MB. Transplantation of Schwann cells and/or olfactory ensheathing glia into the contused spinal cord: Survival, migration, axon association, and functional recovery. Glia. 2007;55:976–1000. doi: 10.1002/glia.20490. [DOI] [PubMed] [Google Scholar]
- 6.Cummings BJ, Uchida N, Tamaki SJ, Anderson AJ. Human neural stem cell differentiation following transplantation into spinal cord injured mice: association with recovery of locomotor function. Neurol Res. 2006;28:474–481. doi: 10.1179/016164106X115116. [DOI] [PubMed] [Google Scholar]
- 7.Hunanyan AS, Alessi V, Patel S, Pearse DD, Matthews G, Arvanian VL. Alterations of action potentials and the localization of Nav1.6 sodium channels in spared axons after hemisection injury of the spinal cord in adult rats. J Neurophysiol. 2011;105:1033–1044. doi: 10.1152/jn.00810.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Macias MY, Syring MB, Pizzi MA, Crowe MJ, Alexanian AR, Kurpad SN. Pain with no gain: allodynia following neural stem cell transplantation in spinal cord injury. Exp Neurol. 2006;201:335–348. doi: 10.1016/j.expneurol.2006.04.035. [DOI] [PubMed] [Google Scholar]
- 9.Wakao S, Hayashi T, Kitada M, Kohama M, Matsue D, Teramoto N, Ose T, Itokazu Y, Koshino K, Watabe H, Iida H, Takamoto T, Tabata Y, Dezawa M. Long-term observation of auto-cell transplantation in non-human primate reveals safety and efficiency of bone marrow stromal cell-derived Schwann cells in peripheral nerve regeneration. Exp Neurol. 2010;223:537–547. doi: 10.1016/j.expneurol.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 10.Bunge MB. Novel combination strategies to repair the injured mammalian spinal cord. J Spinal Cord Med. 2008;31:262–269. doi: 10.1080/10790268.2008.11760720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Someya Y, Koda M, Dezawa M, Kadota T, Hashimoto M, Kamada T, Nishio Y, Kadota R, Mannoji C, Miyashita T, Okawa A, Yoshinaga K, Yamazaki M. Reduction of cystic cavity, promotion of axonal regeneration and sparing, and functional recovery with transplanted bone marrow stromal cell-derived Schwann cells after contusion injury to the adult rat spinal cord. J Neurosurg Spine. 2008;9:600–610. doi: 10.3171/SPI.2008.9.08135. [DOI] [PubMed] [Google Scholar]
- 12.Khalatbary AR, Tiraihi T. A comparative study of therapeutic benefits of intraspinal and intravenous bone marrow stromal cell administration to spinal cord injuries. Iran Biomed J. 2009;13:43–48. [PubMed] [Google Scholar]
- 13.Hellal F, Hurtado A, Ruschel J, Flynn KC, Laskowski CJ, Umlauf M, Kapitein LC, Strikis D, Lemmon V, Bixby J, Hoogenraad CC, Bradke F. Microtubule stabilization reduces scarring and causes axon regeneration after spinal cord injury. Science. 2011;331:928–931. doi: 10.1126/science.1201148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koda M, Nishio Y, Kamada T, Someya Y, Okawa A, Mori C, Yoshinaga K, Okada S, Moriya H, Yamazaki M. Granulocyte colony-stimulating factor (G-CSF) mobilizes bone marrow-derived cells into injured spinal cord and promotes functional recovery after compression-induced spinal cord injury in mice. Brain Res. 2007;1149:223–231. doi: 10.1016/j.brainres.2007.02.058. [DOI] [PubMed] [Google Scholar]
- 15.Leone AM, Rutella S, Bonanno G, Abbate A, Rebuzzi AG, Giovannini S, Lombardi M, Galiuto L, Liuzzo G, Andreotti F, Lanza GA, Contemi AM, Leone G, Crea F. Mobilization of bone marrow-derived stem cells after myocardial infarction and left ventricular function. Eur Heart J. 2005;26:1196–1204. doi: 10.1093/eurheartj/ehi164. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Zhang D, Zhang Y, He G, Zhang F. Augmentation of neovascularization in murine hindlimb ischemia by combined therapy with simvastatin and bone marrow-derived mesenchymal stem cells transplantation. J Biomed Sci. 2010;17:75. doi: 10.1186/1423-0127-17-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okano H, Sakaguchi M, Ohki K, Suzuki N, Sawamoto K. Regeneration of the central nervous system using endogenous repair mechanisms. J Neurochem. 2007;102:1459–1465. doi: 10.1111/j.1471-4159.2007.04674.x. [DOI] [PubMed] [Google Scholar]
- 18.Patel CB, Cohen DM, Ahobila-Vajjula P, Sundberg LM, Chacko T, Narayana PA. Effect of VEGF treatment on the blood-spinal cord barrier permeability in experimental spinal cord injury: dynamic contrast-enhanced magnetic resonance imaging. J Neurotrauma. 2009;26:1005–1016. doi: 10.1089/neu.2008.0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui X, Chopp M, Zacharek A, Roberts C, Lu M, Savant-Bhonsale S, Chen J. Chemokine, vascular and therapeutic effects of combination Simvastatin and BMSC treatment of stroke. Neurobiol Dis. 2009;36:35–41. doi: 10.1016/j.nbd.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang YJ, Qian HY, Huang J, Li JJ, Gao RL, Dou KF, Yang GS, Willerson JT, Geng YJ. Combined therapy with simvastatin and bone marrow-derived mesenchymal stem cells increases benefits in infarcted swine hearts. Arterioscler Thromb Vasc Biol. 2009;29:2076–2082. doi: 10.1161/ATVBAHA.109.189662. [DOI] [PubMed] [Google Scholar]
- 21.Gertz K, Laufs U, Lindauer U, Nickenig G, Bohm M, Dirnagl U, Endres M. Withdrawal of statin treatment abrogates stroke protection in mice. Stroke. 2003;34:551–557. doi: 10.1161/01.str.0000054055.28435.bf. [DOI] [PubMed] [Google Scholar]
- 22.Yu P, Huang L, Zou J, Yu Z, Wang Y, Wang X, Xu L, Liu X, Xu XM, Lu PH. Immunization with recombinant Nogo-66 receptor (NgR) promotes axonal regeneration and recovery of function after spinal cord injury in rats. Neurobiol Dis. 2008;32:535–542. doi: 10.1016/j.nbd.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Tohill M, Mantovani C, Wiberg M, Terenghi G. Rat bone marrow mesenchymal stem cells express glial markers and stimulate nerve regeneration. Neurosci Lett. 2004;362:200–203. doi: 10.1016/j.neulet.2004.03.077. [DOI] [PubMed] [Google Scholar]
- 24.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 25.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 26.Ide C, Nakai Y, Nakano N, Seo TB, Yamada Y, Endo K, Noda T, Saito F, Suzuki Y, Fukushima M, Nakatani T. Bone marrow stromal cell transplantation for treatment of sub-acute spinal cord injury in the rat. Brain Res. 2010;1332:32–47. doi: 10.1016/j.brainres.2010.03.043. [DOI] [PubMed] [Google Scholar]
- 27.Han X, Yang N, Cui Y, Xu Y, Dang G, Song C. Simvastatin mobilizes bone marrow stromal cells migrating to injured areas and promotes functional recovery after spinal cord injury in the rat. Neurosci Lett. 2012;521:136–141. doi: 10.1016/j.neulet.2012.05.071. [DOI] [PubMed] [Google Scholar]
- 28.Baek KH, Lee WY, Oh KW, Tae HJ, Lee JM, Lee EJ, Han JH, Kang MI, Cha BY, Lee KW, Son HY, Kang SK. The effect of simvastatin on the proliferation and differentiation of human bone marrow stromal cells. J Korean Med Sci. 2005;20:438–444. doi: 10.3346/jkms.2005.20.3.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tolwani RJ, Cosgaya JM, Varma S, Jacob R, Kuo LE, Shooter EM. BDNF overexpression produces a long-term increase in myelin formation in the peripheral nervous system. J Neurosci Res. 2004;77:662–669. doi: 10.1002/jnr.20181. [DOI] [PubMed] [Google Scholar]
- 30.Ying Z, Roy RR, Zhong H, Zdunowski S, Edgerton VR, Gomez-Pinilla F. BDNF-exercise interactions in the recovery of symmetrical stepping after a cervical hemisection in rats. Neuroscience. 2008;155:1070–1078. doi: 10.1016/j.neuroscience.2008.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, Katakowski M, Lu M, Chopp M. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005;25:281–290. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carmeliet P, Storkebaum E. Vascular and neuronal effects of VEGF in the nervous system: implications for neurological disorders. Semin Cell Dev Biol. 2002;13:39–53. doi: 10.1006/scdb.2001.0290. [DOI] [PubMed] [Google Scholar]
- 33.Blight AR. Morphometric analysis of blood vessels in chronic experimental spinal cord injury: hypervascularity and recovery of function. J Neurol Sci. 1991;106:158–174. doi: 10.1016/0022-510x(91)90253-4. [DOI] [PubMed] [Google Scholar]
- 34.Widenfalk J, Lipson A, Jubran M, Hofstetter C, Ebendal T, Cao Y, Olson L. Vascular endothelial growth factor improves functional outcome and decreases secondary degeneration in experimental spinal cord contusion injury. Neuroscience. 2003;120:951–960. doi: 10.1016/s0306-4522(03)00399-3. [DOI] [PubMed] [Google Scholar]
- 35.Patel CB, Cohen DM, Ahobila-Vajjula P, Sundberg LM, Chacko T, Narayana PA. Effect of VEGF treatment on the blood-spinal cord barrier permeability in experimental spinal cord injury: dynamic contrast-enhanced magnetic resonance imaging. J Neurotrauma. 2009;26:1005–1016. doi: 10.1089/neu.2008.0860. [DOI] [PMC free article] [PubMed] [Google Scholar]


