Abstract
In this study, we investigated the anti-allodynia effect of safranal, the main volatile constitute of saffron, in spinal nerve transection model of rats. Meanwhile, to elucidate the mechanism, we determined the dynamic expression changes of glial activation markers (GFAP and OX-42) and inflammatory cytokines (TNF-α and IL-1β) in ipsilateral dorsal horn of lumbar enlargement post surgery. Results showed significant increase of these markers along with development of mechanical allodynia. Safranal (0.1 mg/kg, i.p.) attenuated the pain sensitivity and inhibited the expression of these markers. The results demonstrate that the antiallodynia effect of safranal after nerve injury might be attributed to its inhibiting effect on glial activation and inflammatory cytokine production in central nervous system.
Keywords: Safranal, anti-allodynia, glial activation, pro-inflammatory cytokines, spinal nerve transection
Introduction
Safranal (Figure 1) is the main volatile fraction of Crocus sativus L. (commonly known as saffron) [1], which has been widely used for pain relief and other ailments including bronchospasm, asthma and cardiovascular diseases in traditional medicine of Iran, India, Greece and other countries [2-4]. The main active constituents of saffron include carotenoid pigments called tricrocin, bicrocin and crocin, a bitter glycoside called picocrocin, and safranal [5]. Ethanolic and aqueous extract of saffron and crocin showed anti-allodynia and anti-inflammatory effects in different animal models [6-10]. As the main volatile constitution of saffron, safranal has also been demonstrated antiallodynia effect in the formalin, acetic acid or carrageenan induced acute pain, and sciatic chronic constriction induced neuropathic pain [11,12]. Besides the antiallodynia effect, pharmacological studies have suggested the anti-oxidative, anti-inflammatory, anti-cancer and tissue protective effects of safranal [10,13].
Figure 1.
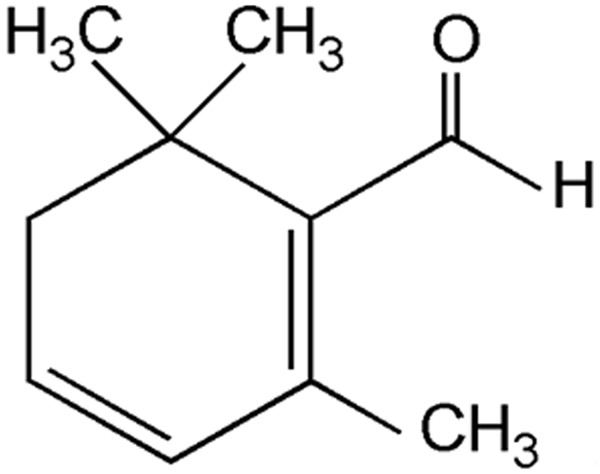
Chemical structure of Safranal.
Though some study suggested the role of its anti-inflammatory and anti-oxidative activities [14,15], the anti-allodynia mechanism of safranal has yet been elucidated. Moreover, no study has been reported to reveal the underlying mechanism of the antiallodynia mechanism of safranal under neuropathic pain conditions.
It has been suggested that inflammatory and immune mechanisms play key roles in neuropathic pain [16]. In response to nerve damage, infiltration of inflammatory cells and activation of resident immune cells may lead to the production and secretion of inflammatory cytokines, which may in turn activate the neuroimmune response and sensitize the primary sensory neurons and contribute to neuropathic pain [17,18]. Inflammatory cytokines such as IL-1, TNF-α and IL-6 [16], as well as immune-like glia cells including microglia and astrocytes [19,20] have been implicated in the development of neuropathic pain.
In this study, we investigated the antiallodynia effect of safranal in a model of neuropathic pain induced by spinal nerve transection (SNT) in rat. Meanwhile, we detected the expression changes of glial activation markers (GFAP and OX-42) and inflammatory cytokines (TNF-α and IL-1β) in the ipsilateral dorsal horn of lumbar enlargement. The results suggested that the antiallodynia effect of safranal may, at least in part, be attributed to its inhibition of glial activation and inflammatory cytokine production in the nervous system.
Materials and methods
Animals and drugs
Male SD rats with initial weight of 160-180 g were purchased from Animal center of Beijing institute of pharmacology and toxicology. Rats were housed in an environment of 12 h light/dark cycle with freedom to food and water.
Safranal was obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA) and diluted in saline before use [7]. The purity of safranal was confirmed to be > 99% by 1H-NMR analysis. Propentofylline (purity > 98%) was purchased from Carbone Scientific Co., LTD (London, United Kingdom) and diluted in saline before use.
Spinal nerve transection and drug administration
Sixty male SD rats were randomly divided into four groups: the sham group, vehicle group (2 ml/kg, i.p.), propentofylline group (10 mg/kg, i.p.) and safranal (0.1 mg/kg, i.p.) group [7]. Rats were anesthetized with sodium pentobarbital (40 mg/kg, i.p.). A small incision to the skin overlying L5-S1 was made and the paravertebral musculature was retracted from the vertebral transverse processes. By partially removing the L6 transverse process, the L4 and L5 spinal nerves were exposed. The L5 spinal nerve was selectively transected leaving the L4 spinal nerve untouched. The wound was irrigated with saline and closed in two layers with 3-0 suture. Animals in the sham group underwent the sham operation without injuring the spinal nerve. After surgery, animals were administered drugs immediately. Drugs were administered daily for 21 consecutive days. Animals in sham group were administered with saline (2 ml/kg, i.p.).
Behavior study
Mechanical sensitivity of rats was tested with von Frey filaments on two consecutive days before surgery and 1, 7, 21 days post surgery. Animals were placed on an elevated steel mesh with a translucent plexiglass cover, and an adaptation of 30 min was permitted. The test procedure was followed the up-and-down method of Dixon [21]. A series of filament were presented to the sural nerve innervated area in the plantar surface of the injury-side hind paw. At least one robust paw lift occurred in every two stimuli was recorded as a positive reaction. When a positive reaction occurred, a higher filament was used; otherwise, a lower filament was used. After the appearance of positive/negative cross, another four stimuli were presented. The fifty percent threshold was calculated following the formula given by Dixon [21].
Tissue collection
After anesthesia with phenobarbital sodium, animals were sacrificed by decapitation. A segment of vertebral column including the lumbar enlargement was cut off. The spinal cord segment was pushed out by compressed saline from an injector manually. The dorsal horn of the lumbar enlargement was removed and the ipsilateral and contralateral sides were separated. The dorsal horn samples were stored at -70°C for real-time PCR and western blot analysis.
RNA extraction and real-time PCR
Total RNA was extracted from dorsal horn of lumbar enlargement with TRIzol regent following the protocol given by the producer (Invitrogen, Burlington, Canada). RNA integrity was checked in agarose gel electrophoresis. The reverse transcription was carried out with 1 μg of RNA sample under catalysis of M-MLV reverse transcriptase (Invitrogen, Burlington, Canada). Real-time PCR was carried out in a 20 μL reaction mixture containing 10 μL 2* SYBR Green Master Mix (PE, Biosystems), 2 μL cDNA solution, 4 μM forward and reverse primers in a ABI Prism 7500 system (PE, Applied Biosystems, Foster City, CA). The reaction procedure consists of a pre-incubation of 10 min at 95°C followed by 40 cycles at 95°C for 15 sec and 60°C for 1 min. The relative mRNA content of target genes were calculated by 2-ΔΔCt with β-actin as the inner control. The primers used were as follows: GFAP: forward, 5’-TGGCCACCAGTAACATGCAA-3’, reverse, 5’-CAGTTGGCGGCGATAGTCAT-3’; OX-42: forward, 5’-CTGCCTCAGGGATCCGTAAAG-3’, reverse, 5’-CCTCTGCCTCAGGAATGACATC-3’ [22]; TNF-α: forward, 5’-TGCCTCAGCCTCTTCTCATTC-3’, reverse, 5’-GCTCCTTCTGCTTGGTGGTTT-3’; IL-1β: forward, 5’-GCACCTTCTTTTCCTTCATCTTTG-3’, reverse, 5’-TTTGTCGTTGCTTGTCTCTCCTT-3’ [23].
Protein extraction and western blot
To prepare the protein from dorsal horn of the lumbar enlargement, the tissue samples were homogenized in 10 folds volume of RIPA buffer containing protease inhibitors (Thermo Scientific, Rockford, IL). Protein concentration was determined with Bradford method. 30 μg of total protein was loaded onto each well of a 5-10% Bis-Tris acrylamide gel. After electrophoresis, proteins were transferred onto NC membrane and blocked in 5% non-fat milk for 45 min at room temperature. Then the membrane was incubated at 4°C overnight with a rabbit polyclonal anti-OX42 (1:300, Novus Biologicals, Inc), or a rabbit polyclonal anti-GFAP antibody (1:5000, Novus Biologicals, Inc), or a mouse monoclonal anti-beta-actin antibody (1:3000, Sigma Aldrich). Then the membrane were washed with TBS containing 0.1% Tween-20 three times of 10 min and incubated in the secondary antibodies conjugated with horseradish peroxidase (1:2000, Vector) for 1 h at room temperature. After three times washes of 10 min, the immunoreactive proteins were detected with enhanced chemiluminescence (Amersham Biosciences, Arlington Heights, IL, USA), and the signal were captured by a Fujifilm LAS-1000 imager system. Quantification of the proteins was performed using the ImageJ software with beta-actin as the inner control.
Statistical analysis
All data was expressed as mean ± SEM. Data were analyzed with two-way analysis of variance, followed by Bonferroni post hoc test. Difference with P value lower than 0.05 was accepted as significant.
Results
Effects of safranal and propentofylline on mechanical allodynia induced by SNT
SNI surgery induced significant mechanical allodynia of the injured paw. Across the observation period, the mechanical withdrawal threshold of the model group was significantly lower than the sham group, while both propentofylline and safranal significantly attenuated the reduction of the mechanical withdrawal threshold at 7 and 21 days post surgery (Figure 2).
Figure 2.
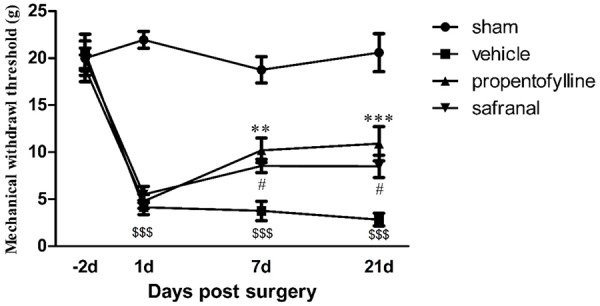
Development of mechanical allodynia post SNT surgery. Significant decrease of mechanical withdrawal threshold of model animals in vehicle group was observed throughout the observation period. Both propentofylline (10 mg/kg, i.p.) and safranal (0.1 mg/kg, i.p.) inhibited the development of mechanical allodynia at 7 and 21 d post surgery. $$$P < 0.001, compared to sham group; **P < 0.01, ***P < 0.001, #P < 0.05, compared to vehicle group.
Effects of safranal and propentofylline on protein levels of OX-42 and GFAP expression in dorsal horn after SNT
Protein levels of GFAP and OX-42 in ipsilateral dorsal horn of SNI animals increased significantly at day 1, 7 and 21 post surgery. Propentofylline inhibited the up-regulation of GFAP and OX-42 expression at 7 and 21 d post surgery. Safranal inhibited the up-regulation of OX-42 expression at 7 and 21 d post surgery and inhibited the up-regulation of GFAP expression at 7 d post surgery (Figure 3).
Figure 3.
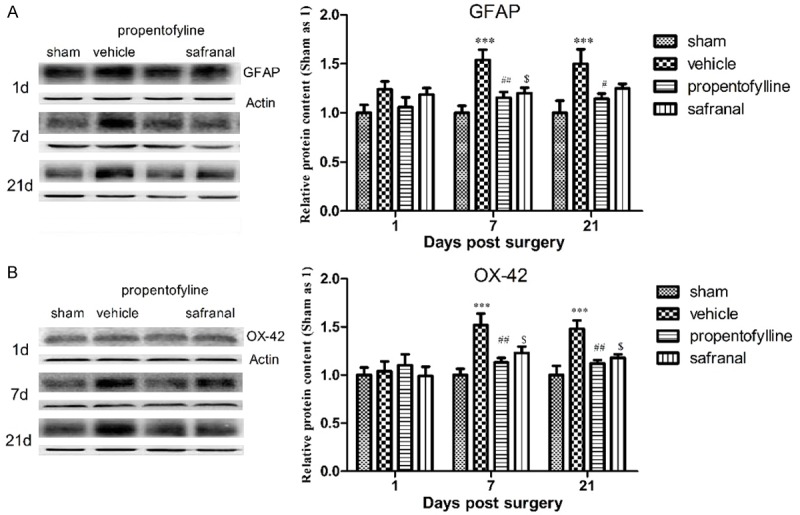
Protein levels of GFAP (A) and OX-42 (B) in ipsilateral dorsal horn of lumbar enlargement of rats post SNT surgery. Protein levels of GFAP and OX-42 increased significantly at 7 and 21 d post surgery. Propentofylline significantly inhibited the up-regulation of GFAP and OX-42 at 7 and 21 d post surgery. Safranal inhibited the up-regulation of GFAP at 7 d post surgery and inhibited the up-regulation of OX-42 at 7 and 21 d post surgery. ***P < 0.001, compared to sham group; ##P < 0.01, #P < 0.05, $P < 0.05, compared to vehicle group.
Effects of safranal and propentofylline on mRNA levels of OX-42 and GFAP expression in dorsal horn after SNT
mRNA levels of GFAP and OX-42 in ipsilateral dorsal horn of SNI animals increased significantly at day 1, 7 and 21 d post surgery. Propentofylline attenuated the increase of GFAP at 7 and 21 d post surgery and attenuated the increase of OX-42 at 1, 7 and 21 d post surgery. Safranal attenuated the increase of GFAP and OX-42 at 7 and 21 d post surgery (Figure 4).
Figure 4.
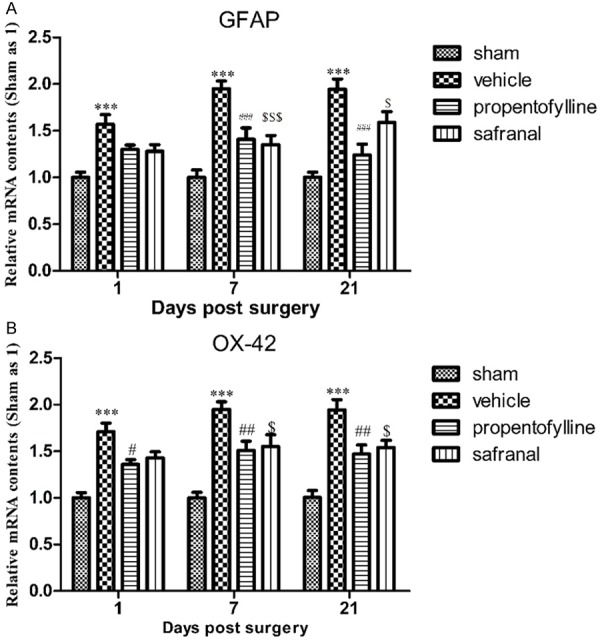
mRNA levels of GFAP (A) and OX-42 (B) in ipsilateral dorsal horn of lumbar enlargement of rats post SNT surgery. mRNA levels of GFAP and OX-42 increased significantly at 1, 7 and 21 d post surgery. Propentofylline significantly inhibited the up-regulation of GFAP at 7 and 21 d post surgery and inhibited the up-regulation of OX-42 at 1, 7 and 21 d post surgery. Safranal inhibited the up-regulation of GFAP and OX-42 at 7 and 21 d post surgery. ***P < 0.001, compared to sham group; ###P < 0.001, ##P < 0.01, $$$P < 0.001, $P < 0.05, compared to vehicle group.
Effects of safranal and propentofylline on mRNA levels of TNF-α in ipsilateral lumbar enlargement after SNT
mRNA levels of TNF-α and IL-1β in ipsilateral dorsal horn increased significantly at 1 and 7 d post surgery. Propentofylline inhibited the up-regulation of TNF-α at 7 d post surgery and inhibited the up-regulation of IL-1β at 1 and 7 d post surgery. Safranal inhibited the up-regulation of TNF-α and IL-1β at 7 d post surgery (Figure 5).
Figure 5.
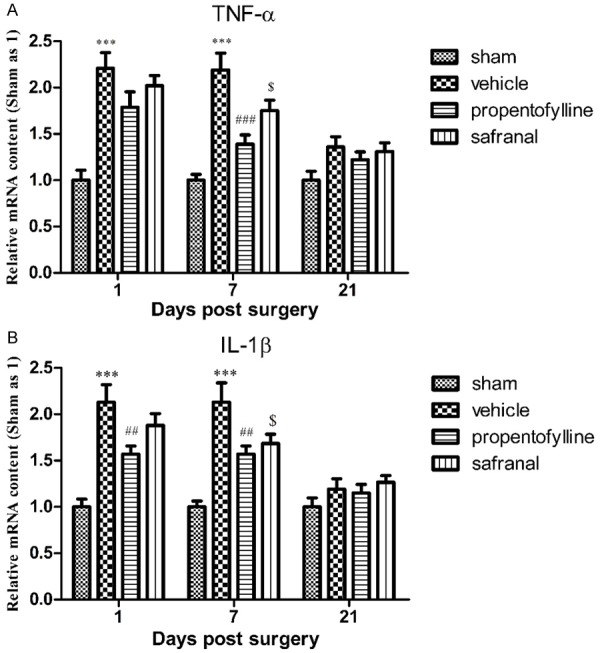
mRNA levels of TNF-α (A) and IL-1β (B) in ipsilateral dorsal horn of lumbar enlargement of rats post SNT surgery. mRNA levels of TNF-α and IL-1β increased significantly at 1 and 7 d post surgery. Propentofylline significantly inhibited the up-regulation of TNF-α at 7 d post surgery and inhibited the up-regulation of IL-1β at 1 and 7 d post surgery. Safranal inhibited the up-regulation of TNF-α and IL-1β at 7 d post surgery. ***P < 0.001, compared to sham group; ###P < 0.001, ##P < 0.01, $P < 0.05, compared to vehicle group.
Discussion
Saffron is a traditional medicine used for pain relief [3], and modern pharmacological studies have demonstrated the antiallodynia effect of its extracts including safranal, the main volatile constituent, in different animal models [6-12]. However, no study has been reported to reveal the antiallodynia mechanism of safranal in neuropathic pain. And in this study, with the SNT model of rats, we confirmed the antiallodynia effect of safranal and investigated its influence on glial activation and inflammatory cytokine production in spinal cord.
Roles of glial activation and inflammatory cytokines in central nervous system have been well established in neuropathic pain induced by peripheral nerve injury [16]. Expression elevation of glial fibrillary acidic protein (GFAP) and OX-42 (in human known as integrin alpha M (ITGAM) or MAC-1, CR3/CD11b) has been widely accepted as markers of astrocytic and microglial activation, respectively [24]. Previous study reported long-lasting elevation of GFAP and OX-42 expression in spinal cord post spinal nerve transection in rats [22,24]. Propentofylline, a methylxanthine derivative, has been found a glial regulating agent [25,26] and showed anti-allodynia effect in SNT rats [24]. In this study, we observed the antiallodynia and glial activation suppression effect of both propentofylline and safranal at 7 and 21 d post surgery. This suggested that the antiallodynia effect of safranal under neuropathic pain conditions is associated with its inhibiting effect on glial activation in central nervous system.
Inflammatory cytokines such as TNF-α and IL-1β are normally expressed at a low level in spinal cord. After peripheral nerve injury, the infiltrated immune cells and the activated glial cells provide source for the increase of these inflammatory cytokines, which may in turn contribute to the development of neuropathic pain [16]. Many studies have demonstrated the expression changes of proinflammatory cytokines, including TNF-α and IL-1β, in spinal cord after peripheral nerve injury. Xu et al. observed a peak elevation of TNF-α expression in spinal cord at 7 d and returned to baseline at 21 d post spinal nerve transection [27]. Lee et al. examined the mRNA levels of cytokines in the spinal cord post chronic constriction injury of sciatic nerve and observed an elevation of TNF-α within 3 days and elevation of IL-1β within 7 days post injury [28]. In this study, we observed an inhibiting effect of propentofylline on TNF-α and IL-1β up-regulation in spinal level which is in agreement with previous studies [29,30]. Similarly, we also observed the inhibiting effect of safranal on the TNF-α and IL-1β up-regulation. The anti-inflammatory effect of safranal has been reported in previous studies on inflammatory pain animal and sensitized animal [10,13]. Our result suggests the anti-inflammatory constituent for the antiallodynia activity of safranal.
In summary, we confirmed the antiallodynia effect of safranal, main volatile fraction of saffron, in a neuropathic pain model induced by spinal nerve transection. Meanwhile, our results firstly elucidate that the antiallodynia effect of safranal under neuropathic pain conditions should be, in some degree, attributed to its suppression on glial activation and proinflammatory cytokines production in central nervous system.
Acknowledgements
This work was supported by the Scientific Research Foundation of the Chinese PLA Medical Programs, No. ms031.
Disclosure of conflict of interest
None.
References
- 1.Zargari A. Medicinal plants. Tehran: University Press; 1990. pp. 672–5. [Google Scholar]
- 2.Abdullaev F, Espinosa-Aguirre J. Biomedical properties of saffron and its potential use in cancer therapy and chemoprevention trials. Cancer Detect Prev. 2004;28:426–32. doi: 10.1016/j.cdp.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Rios JL, Recio MC, Ginger RM, Manz S. An update review of saffron and its active constituents. Phytother Res. 1996;10:189–93. [Google Scholar]
- 4.Tamaddonfard E, Hamzeh-Gooshchi N. Effect of crocin on the morphine-induced antinociception in the formalin test in rats. Phytother Res. 2010;24:410–3. doi: 10.1002/ptr.2965. [DOI] [PubMed] [Google Scholar]
- 5.Ma S, Zhou S, Shu B, Zhou J. Pharmacological studies Crocus glycosides I. Effect on antiinflammatory and immune function. Zhongcaoyao. 1998;29:536–9. [Google Scholar]
- 6.Hosseinzadeh H, Younesi HM. Antiallodynia and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol. 2002;2:7–12. doi: 10.1186/1471-2210-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amin B, Hosseinzadeh H. Evaluation of aqueous and ethanolic extracts of saffron, Crocus sativus L. , and its constituents, safranal and crocin in allodynia and hyperalgesia induced by chronic constriction injury model of neuropathic pain in rats. Fitoterapia. 2012;83:888–95. doi: 10.1016/j.fitote.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 8.Karami M, Bathaie SZ, Tiraihi T, Habibi-Rezaei M, Arabkheradmand J, Faghihzadeh S. Crocin improved locomotor function and mechanical behavior in the rat model of contused spinal cord injury through decreasing calcitonin gene related peptide (CGRP) Phytomedicine. 2013;21:62–7. doi: 10.1016/j.phymed.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 9.Tamaddonfard E, Hamzeh-Gooshchi N. Effects of intraperitoneal and intracerebroventricular injection of crocin on acute corneal pain in rats. Phytother Res. 2010;24:1463–7. doi: 10.1002/ptr.3169. [DOI] [PubMed] [Google Scholar]
- 10.Tamaddonfard E, Farshid AA, Eghdami K, Samadi F, Erfanparast A. Comparison of the effects of crocin, safranal and diclofenac on local inflammation and inflammatory pain responses induced by carrageenan in rats. Pharmacol Rep. 2013;65:1272–80. doi: 10.1016/s1734-1140(13)71485-3. [DOI] [PubMed] [Google Scholar]
- 11.Assimopolou AN, Sinakos Z, Papageorgiou VP. Radical scavenging activity of Crocus sativus L. extract and its bioactive constituents. Phytother Res. 2005;19:997–1000. doi: 10.1002/ptr.1749. [DOI] [PubMed] [Google Scholar]
- 12.Boskabady MH, Byrami G, Feizpour A. The effect of safranal, a constituent of Crocus sativus (saffron), on tracheal responsiveness, serum levels of cytokines, total NO and nitrite in sensitized guinea pigs. Pharmacol Rep. 2014;66:56–61. doi: 10.1016/j.pharep.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Tamaddonfard E, Farshid AA, Maroufi S, Kazemi-Shojaei S, Erfanparast A, Asri-Rezaei S. Effects of safranal, a constituent of saffron, and vitamin E on nerve functions and histopathology following crush injury of sciatic nerve in rats. Phytomedicine. 2014;21:717–23. doi: 10.1016/j.phymed.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 14.Sweitzer SM, Colburn RW, Rutkowski M, DeLeo JA. Acute peripheral inflammation induces moderate glial activation and spinal IL-1b expression that correlates with pain behavior in the rat. Brain Res. 1999;829:209–21. doi: 10.1016/s0006-8993(99)01326-8. [DOI] [PubMed] [Google Scholar]
- 15.Farahmand SK, Samini F, Samini M, Samarghandian S. Safranal ameliorates antioxidant enzymes and suppresses lipid peroxidation and nitric oxide formation in aged male rat liver. Biogerontology. 2013;14:63–71. doi: 10.1007/s10522-012-9409-0. [DOI] [PubMed] [Google Scholar]
- 16.Sacerdote P, Franchi S, Moretti S, Castelli M, Procacci P, Magnaghi V. Cytokine Modulation is Necessary for Efficacious Treatment of Experimental Neuropathic Pain. J Neuroimmune Pharmacol. 2013;8:202–11. doi: 10.1007/s11481-012-9428-2. [DOI] [PubMed] [Google Scholar]
- 17.Tiwari V, Guan Y, Raja SN. Modulating the delicate glial-neuronal interactions in neuropathic pain: Promises and potential caveats. Neurosci Biobehav Rev. 2014;45C:19–27. doi: 10.1016/j.neubiorev.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liou JT, Lee CM, Lin YC, Chen CY, Liao CC, Lee HC, Day YJ. P-selectin is required for neutrophils and macrophage infiltration into injured site and contributes to generation of behavioral hypersensitivity following peripheral nerve injury in mice. Pain. 2013;154:2150–9. doi: 10.1016/j.pain.2013.06.042. [DOI] [PubMed] [Google Scholar]
- 19.Tsuda M, Masuda T, Tozaki-Saitoh H, Inoue K. Microglial regulation of neuropathic pain. J Pharmacol Sci. 2013;121:89–94. doi: 10.1254/jphs.12r14cp. [DOI] [PubMed] [Google Scholar]
- 20.Ríos JL, Recio MC, Giner RM, Má˜nez S. An update review of saffron and its active constituents. Phytother Res. 1996;10:189–93. [Google Scholar]
- 21.Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- 22.Tanga FY, Raghavendra V, DeLeo JA. Quantitative real-time RT-PCR assessment of spinal microglial and astrocytic activation markers in a rat model of neuropathic pain. Neurochem Int. 2004;45:397–407. doi: 10.1016/j.neuint.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Motoyoshi-Yamashiro A, Tamura M, Moriyama M, Takano K, Kawabe K, Nakajima H. Activation of cultured astrocytes by amphotericin B: stimulation of NO and cytokines production and changes in neurotrophic factors production. Neurochem Int. 2013;63:93–100. doi: 10.1016/j.neuint.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Sweitzer SM, Schubert P, DeLeo JA. Propentofylline, a glial modulating agent, exhibits antiallodynic properties in a rat model of neuropathic pain. J Pharmacol Exp Ther. 2001;297:1210–7. [PubMed] [Google Scholar]
- 25.Wu YP, MacRae A, Rudolphi K, Ling EA. Propentofylline attenuates microglial reaction in the rat spinal cord induced by middle cerebral artery occlusion. Neurosci Lett. 1999;260:17–20. doi: 10.1016/s0304-3940(98)00941-0. [DOI] [PubMed] [Google Scholar]
- 26.DeLeo J, Toth L, Schubert P, Rudolphi K, Kreutzberg GW. Ischemia-induced neuronal cell death, calcium accumulation, and glial response in the hippocampus of the mongolian gerbil and protection by propentofylline (HWA285) J Cereb Blood Flow Metab. 1987;7:745–51. doi: 10.1038/jcbfm.1987.129. [DOI] [PubMed] [Google Scholar]
- 27.Xu JT, Xin WJ, Zang Y, Wu CY, Liu XG. The role of tumor necrosis factor-alpha in the neuropathic pain induced by Lumbar 5 ventral root transection in rat. Pain. 2006;123:306–21. doi: 10.1016/j.pain.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 28.Lee HL, Lee KM, Son SJ, Hwang SH, Cho HJ. Temporal expression of cytokines and their receptors mRNAs in a neuropathic pain model. Neuroreport. 2004;22:2807–11. [PubMed] [Google Scholar]
- 29.Yao M, Chang XY, Chu YX, Yang JP, Wang LN, Cao HQ. Antiallodynic effects of propentofylline Elicited by interrupting spinal glial function in a rat model of bone cancer pain. J Neurosci Res. 2011;89:1877–86. doi: 10.1002/jnr.22711. [DOI] [PubMed] [Google Scholar]
- 30.Raghavendra V, Tanga F, Rutkowski MD, DeLeo JA. Anti-hyperalgesic and morphine-sparing actions of propentofylline following peripheral nerve injury in rats: mechanistic implications of spinal glia and proinflammatory cytokines. Pain. 2003;104:655–64. doi: 10.1016/S0304-3959(03)00138-6. [DOI] [PubMed] [Google Scholar]


