Abstract
Objective: Evaluate the behavior and function of human umbilical vein endothelial cells (HUVECs) on decellularized extracellular matrix (ECM) deposited by bone marrow mesenchymal stem cells (BMSCs). Methods: Prepared through chemical approach, decellularized ECM was characterized by use of immunofluorescence staining. The morphology, attachment, proliferation and migration of HUVECs cultured on six-well tissue culture plastic (TCP) and decellularized ECM were investigated. Results: Decellularized ECM was successfully prepared without three-dimensional architecture disruption. This biological scaffold is similar to nature vascular ECM, preserved various matrix proteins such as type I collagen, type III collagen and fibronection. HUVECs on decellularized ECM showed well attachment and regular arrangement. Decellularized ECM could also significantly enhance the migration and proliferation potential of HUVECs in contrast to TCP. Conclusion: Deposited by BMSCs, ECM can affect the behavior of endothelial cell and could be used as a promising material in tissue engineering.
Keywords: Decellularized extracellular matrix, tissue engineering, vascularization
Introduction
Researchers have focused on fabricating different types of tissue engineering scaffolds to substitute the damaged tissues for years [1]. Recently, synthetic materials have been developed dramatically and received wide attention [2-6]. They can be made into different shapes such as tube [7], sponge [8], film [9] or sheet [10] for specific clinic application purpose. Although some properties of these scaffolds have been improved, shortcomings such as single composition and lacking of important growth cytokines still impede their clinical application. Compared with synthetic materials, the advantages of decellularized ECM are their numerous biomolecules, complex composition, and biocompatibility. In addition, the intricate mixture of microstructure after removing antigenic cellular components could provide the similar microenvironment to the seeded cells and then regulates dynamic cellular behavior. All of these characteristic make cell deposited ECM scaffold promising in broader applications in regenerative medicine.
Nowadays the decellularized ECM derived from tissues, organs, cells have been extensively studied for manipulation stem cell function [11,12]. Our group has previously established the decellularization technology and proved that the BMSCs-derived ECM could promote BMSCs proliferation and facilitated their differentiation into hepatocyte-like cells [13]. Herein, we demonstrate that BMSCs-derived ECM has great potential in tissue engineering to regenerate various types of tissues and organs in the future.
However, it is well known that vascularization is a fundamental biological process in tissue engineering, adequate blood perfusion in graft could not only deliver necessary nutrients but also reduce graft infection via removing of bacteria [14]. So in bioengineered tissue fabrication, the key to the successful utilization of the cell deposited ECM scaffold is to ensure its compatibility with endothelial cells (ECs), which participate in vasculogenesis. Many studies have suggested that ECM is crucial in vascular morphogenesis by modulating EC adhesion, proliferation and migration. Therefore, we anticipate that BMSCs-derived ECM may provide ECs an angiogenic microenvironment. To our knowledge, study that deal with the impact of BMSCs deposited ECM on ECs was not reported, while it is of great importance to the further clinical application of the biomaterial.
Herein, our objective was to explore whether this acellular ECM could modulate ECs behavior and functions. In the current study, the human umbilical vein endothelial cell (HUVEC), a typical EC, was grown on two different substrates, six-well tissue-culture plate (TCP) and decellularized ECM. Their morphology, attachment, proliferation, and migration on six-well TCP and decellularized ECM were investigated. Results indicated that BMSCs-derived ECM strongly support HUVECs migration and proliferation serving as an ideal candidate biomaterial for better clinical application. This study may provide a deeper understanding of ECs behaviors on BMSCs deposited ECM microenvironments.
Materials and methods
Preparation of decellularized ECM deposited by BMSCs
Bone marrow mesenchymal stem cells were obtained from Sciencecell (Carlsbad, CA). Method for preparation of decellularized ECM deposited by BMSCs was in accordance with our previous report [13]. Briefly, TCP was firstly pretreated with a 0.2% gelatin solution (Sigma, St. Louis, MO) at 37°C for 1 h and then crosslinked by 1% glutaraldehyde (Sigma) and 1 M ethanolamine (Sigma) 30 min at room temperature. BMSC were seeded on pretreated TCP until 90% confluent. At the same time, 100 μM of L-ascorbic acid phosphate (Sigma) was added to the culture medium and cultured for another 8 days. Subsequently, a solution with 0.5% Triton X-100 (Sigma) and 20 mM NH4OH (Sigma) were added to remove the cells. The obtained decellularized ECM scaffold was stored in PBS containing penicillin (100 units/ml) and streptomycin (100 mg/ml) at 4°C until further use.
Immunofluorescence staining
The ECM scaffold was washed twice with PBS and fixed with ice-cold methanol for 10 minutes. Then it was blocked for 1 hour with 1% BSA in PBS. Subsequently, the ECM scaffold was placed in primary antibodies for type III collagen, type I collagen and fibronection (Abcam, Cambridge, MA) overnight at 4°C. The ECM scaffold was washed with PBS for 10 min on shaker and incubated with an Alexa Fluor 488-labeled secondary antibody (Invitrogen, Grand Island, NY) in dark at 4°C for 1 hour. Finally, the ECM scaffold was washed to remove the residual fluorescent dye. The fluorescence images were taken with a fluorescence microscope (Olympus Corporation, Tokyo, Japan).
Cell culture
HUVECs were purchased from ATCC (Manasass, VA). The cells were cultured in Dulbecco’s Modification Eagle’s Medium (DMEM)-high glucose supplemented with 10% fetal bovine serum, penicillin (100 units/ml) and streptomycin (100 mg/ml). Fresh medium were replaced every 2 days. The cells were maintained at 37°C in a humidified atmosphere containing 5% CO2.
Wound assay
Wound assays were carried out to estimate the migration of HUVECs. HUVECs were seeded in two different conditions: six-well TCP and ECM at a density of 1 × 105 cells per well. When cells grew to confluence, one small area was disrupted by scratching a line through the layer by using 200 μL tips. The cells were then rinsed with PBS twice and 2 ml serum-free DMEM was then added to each well. Wound width was measured microscopically at 0h and 24 h.
The wound repair rate was calculated as follows (Equation 1):
The wound repaire rate% = (wound width at Oh - wound width at 24h)/wound width at Oh × 100%
Cell proliferation assay
HUVECs were grown in six-well TCPs at a density of 3 × 104 cells per well. Samples were harvested at day 4 and digested in a papain solution at 60°C for 4 h. The papain solution contained 125 mg/mL papain (Sigma) and 10 mM L-cysteine (Sigma) in PBE buffer (100 mM phosphate and 10mM EDTA, pH 6.5). Samples were vortexed for 10 seconds every hour. The quantity of DNA was measured with Quant-iT PicoGreen dsDNA assay kit (Invitrogen) according to manufacture’s instructions. The samples were read on SynergyMx Multimode Reader (Bio Tek, Winooski, VT)
Cell cycle analysis
HUVECs were plated in six-well plates at a density of 4 × 104 cells/well. The cells were collected at day 4 and washed in PBS by centrifugation. Subsequently, pellet were resuspended in 70% cold ethanol solution at 4°C overnight. The ethanol-suspended cells were centrifuged and then suspended with PBS. After processed with 20 μg/ml RNase A for 30 min at 37°C, samples were incubated in propidium iodide (Sigma) for 30 minutes in dark at room temperature. A total number of 1.2 × 104 cells were collected for each sample. The samples were analyzed on a FACScan (Beckton Dickinson, CA, USA) and data were analyzed with ModiFit software.
Statistical analysis
All experiments were carried out with three times and reported as means ± standard deviation. Statistical differences of measurement data were determined using Student’s t-Test Difference was considered significant at P < 0.05.
Results
Characterization of decellularized ECM
Preservation of the ECM proteins is an optimal goal in fabricating a material for tissue engineering. Immunofluorescence staining for decellularized ECM showed that it contained proteins type I collagen (Figure 1A), and type III collagen (Figure 1B) and fibronectin (Figure 1C).
Figure 1.
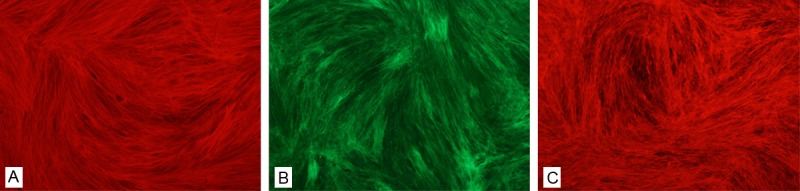
Characterization of decellularized ECM. Immunofluorescence staining for decellularized ECM showed that it contained proteins type I collagen (A), and type III collagen (B) and fibronection (C). (Original magnification 10×).
Cell morphology and attachment
The cell morphology was observed by light microscope at 24 h after seeding. HUVECs exhibited different morpholgy when grown on two different conditions. They exhibited a large and spindle shape, and random organized on six-well TCP (Figure 2B). Compared to the TCP samples, the cells on ECM were much smaller and elongated, with a regular arrangement following the ECM organization as shown in Figure 2A.
Figure 2.
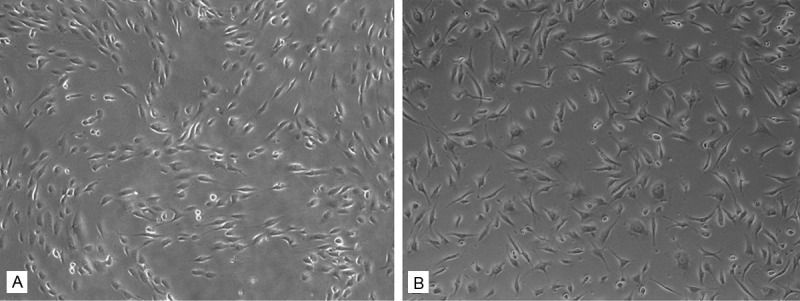
Cell morphology and attachment. HUVECs exhibited different morpholgy when grown on two different conditions. They exhibited a large and spindle shape, and random organized on six-well TCP (B). Compared to the TCP sample, the cells on ECM were much smaller and elongated, with a regular arrangement following the ECM organization (A). (Original magnification 5×).
Cell migration on decellularized ECM
The migration ability of ECs plays a vital role in vasculogenesis process. As shown in Figure 3, HUVECs grown on ECM nearly reached confluence at 24 h. These results showed that HUVECs migrated much faster on ECM compared with TCP group at 24 h, with the wound repair rate of 49.7% and 24.83%, respectively (*P < 0.05). (Figure 3B).
Figure 3.
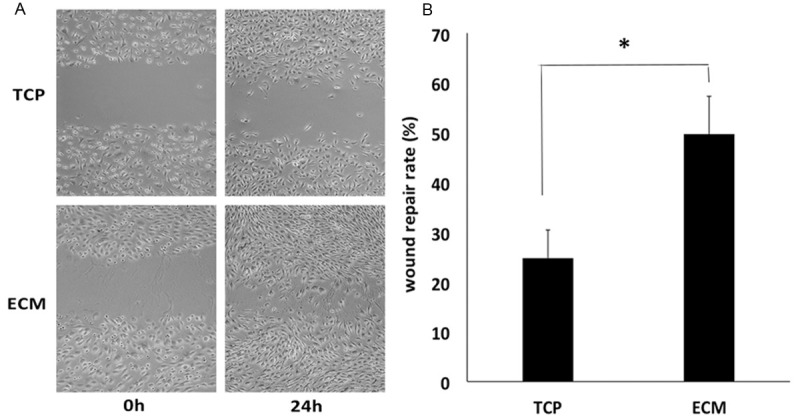
Cell migration ability. HUVECs grown on ECM nearly reached confluence at 24 h (A). (Original magnification 5×). These results showed that HUVECs migrated much faster on ECM compared with TCP group at 24 h, with the wound repair rate of 49.7% and 24.83%, respectively (*P < 0.05) (B).
Cell proliferation assay
The quantitative DNA assay was employed to evaluate the proliferative ability of HUVECs on ECM or TCP. The cell proliferation on ECM was significantly different from that on TCP. After a 4-day culture, the DNA content of HUVECs on ECM was 3.8 fold higher than that of cells on TCP (*P < 0.05) (Figure 4).
Figure 4.
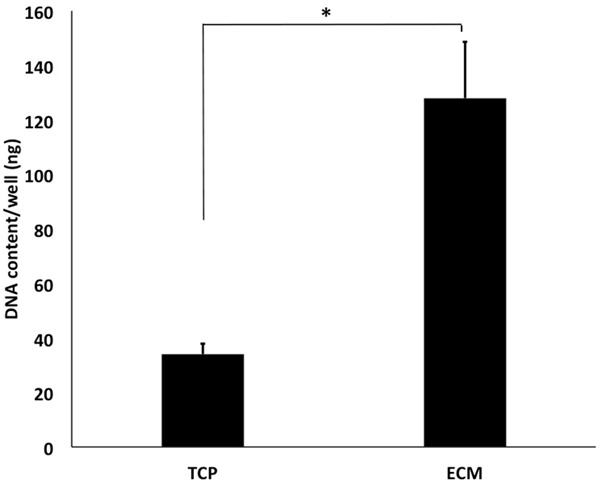
Cell proliferation assay. The quantitative DNA assay was employed to evaluate the proliferative ability of HUVECs on ECM or TCP. The cell proliferation on ECM was significantly different from that on TCP. After a 4-day culture, the DNA content of HUVECs on ECM was 3.8 fold higher than that of cells on TCP (*P < 0.05).
Cell cycle analysis
Cell cycle analysis by flow cytometry was carried out to investigate the mechanism of the promoting effect of ECM on HUVEC growth. After a 4-day culture, the phase distribution of HUVECs was analyzed (Figure 5). The percentage of cells in the phase on ECM was higher than that of control cells. HUVECs grown on ECM had 26.47% in the S phase, while the percentage of cells in S on TCP was 21% (*P < 0.05). These results indicated that ECM was capable of regulating the cell cycle entering into the S phase.
Figure 5.
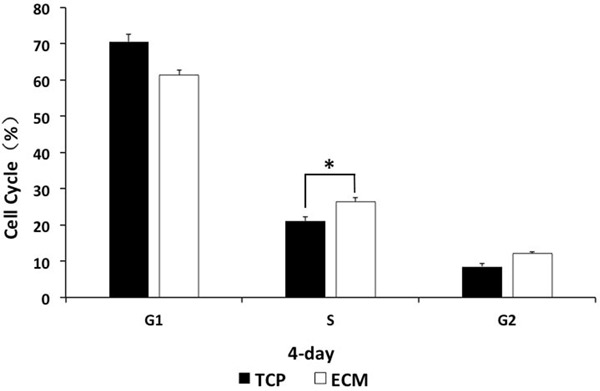
Cell cycle analysis. After a 4-day culture, the phase distribution of HUVECs was analyzed. HUVECs grown on ECM had 26.47% in the S phase, while the percentage of cells in S on TCP was 21% (*P < 0.05). These results indicated that ECM was capable of regulating the cell cycle entering the S phase.
Discussion
We in the present study explored the behavior and function of HUVECs on decellularized ECM, which we believe may affect the long-term clinical application of BMSCs derived ECM. Our study indicated that this decellularized ECM could serve as an appropriate scaffold material in tissue engineering for supporting ECs and possibly prompt their vascularization.
Scaffold material and proper cell seeding cell are two key elements ensuring the tissue biofabrication. Decellularized ECM has been used for regenerating tissues or organs by recellularizing with tissue-specific cells for many years. It is well known that long-term survival of the grafts is based on sufficient blood supply to provide necessary nutrients. So adequate blood perfusion plays a vital role in integrating grafts with host tissues. Research groups have developed various approaches to enhance vascularization, such as implantation of ECs, scaffolds functionalization, adding exogenous growth factors and so on [15-17]. Because it normally takes time for host vascular growing into scaffolds, one of the choices is to prevascularize the engineered tissue in vitro, especially for large engineered tissues [18]. When the graft is transplanted into recipients, it could anastomose with the recipient blood vessel rapidly. Therefore, seeding ECs on an appropriate decellularized ECM scaffold that highly mimics vascular ECM in native tissues is a promising choice of graft prevascularization and shows wide application prospects.
The formation of capillary vessels is a complex procedure involving migration, proliferation and coalescence of ECs [19]. During vasculogenesis, ECM acts as a key role in regulating the function of ECs. An optimal scaffold should maximally preserve ECM components and minimally disrupt the native three-dimensional (3D) architecture of ECM. In our study, the presence of proteins in the ECM was examined by using immunofluorescence staining, various matrix protein such as type I collagen, type III collagen and fibronectin were shown to be well preserved in the BMSCs deposited ECM. Vascular ECM, which is highly enriched in proteins, proteoglycans, growth factors and cytokines, plays a vital role in controlling endothelial cells behavior during angiogenesis [20,21]. It is known that type I collagen and type III collagen are the most abundant collagen in blood vessels [22]. Fibronection, an adhesive glycoprotein, also presents in vascular ECM [20]. In addition, they are known to be responsible for the tensile strength and stiffness of the blood vessel wall [20]. Our study demonstrated that this decellularized scaffold highly retained the ECM components that are contained in nature vascular ECM. Moreover, the 3D morphology of decellularized ECM provided a permissive environment for the growth of HUVECs.
For tissue engineering applications, EC adhesion to the ECM is a prerequisite for the formation of blood vessels [21]. The attachment of HUVECs to decellularized ECM and six-well TCP was shown in Figure 2. It was clear that HUVECs adhered to ECM and spread well after seeding on the ECM. This is partly because the porous structure made by decellurization technical could support cell attachment. It has been reported that ECM components such as collagen and fibronection could promote HUVECs attachment and retention on scaffold [23,24]. The stable adhesion not only contributes to the survival and proliferation of cells, but also prevents thrombus and intimal hyperplasia [25].
Sufficient cell number is of importance for vascularization. Our date showed that the cell number of HUVECs on decellularized ECM exhibited large difference from six-well TCP on the day-4 culture. The DNA content of HUVECs on decellularized ECM was 3.8 fold higher than their TCP counterparts. Cell cycle analysis results also showed that the ECM scaffold promoted the G1-S transition of the HUVECs cell cycle. It suggested that BMSCs derived ECM could mimic vascular ECM microenvironment and support HUVECs growth. Studies have reported that BMSCs could secrete cytokines such as vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (FGF) through paracrine mechanisms [26]. It is possible that the growth factors contained in the ECM altered the expression of cell cycle regulatory molecular such as cycling E and cycling D1, thereby indirectly promoted EC proliferation.
In our study, we observed that decellularized ECM has a significant effect on promoting HUVECs migration. During the process of vascularization, migration, the first step of sprouting of new capillary vessels from the existing vasculature, has an essential role in vascularization [27]. According to a previous report, collagen and fibronection were considered to have the ability to facilitate cell migration [28-30]. Comparing with individual component of ECM, this cell-deposited ECM contains considerable proteins that mimic the microenvironment in vivo. Although many kinds of ECM components have been reported to promote endothelial cells migration, the main protein that drives cell migration is unknown.
Taken together, in this study the behavior of HUVECs on BMSC derived ECM was investigated. We found that this decellularized ECM, containing various matrix proteins similar to nature ECM, enhanced migration and proliferation potential of HUVECs. To our knowledge, it was the first approach that explored the behavior of HUVECs on BMSCs derived ECM. However, the mechanism that ECM modulates the activity of HUVECs is unknown. More work should be done to explore the underlying relationship between HUVECs and decellularized ECM at molecular level. In addition, paracrine factors secreted by BMSCs within ECM need to be investigated in the future.
Acknowledgements
This publication was supported by grant of the National Natural Science Foundation of China (81271115), National Natural Science Foundation of China (51103182), Science and Technology Planning Project of Guangdong Province (No. 2011A060901013).
Disclosure of conflict of interest
None.
References
- 1.Freed LE, Guilak F, Guo XE, Gray ML, Tranquillo R, Holmes JW, Radisic M, Sefton MV, Kaplan D, Vunjak-Novakovic G. Advanced tools for tissue engineering: scaffolds, bioreactors, and signaling. Tissue Eng. 2006;12:3285–3305. doi: 10.1089/ten.2006.12.3285. [DOI] [PubMed] [Google Scholar]
- 2.Mo XM, Xu CY, Kotaki M, Ramakrishna S. Electrospun P (LLA-CL) nanofiber: a biomimetic extracellular matrix for smooth muscle cell and endothelial cell proliferation. Biomaterials. 2004;25:1883–1890. doi: 10.1016/j.biomaterials.2003.08.042. [DOI] [PubMed] [Google Scholar]
- 3.Zong X, Kim K, Fang D, Ran S, Hsiao BS, Chu B. Structure and process relationship of electrospun bioabsorbable nanofiber membranes. Polymer. 2002;43:4403–4412. [Google Scholar]
- 4.Choi JS, Kim BS, Kim JY, Kim JD, Choi YC, Yang HJ, Park K, Lee HY, Cho YW. Decellularized extracellular matrix derived from human adipose tissue as a potential scaffold for allograft tissue engineering. J Biomed Mater Res A. 2011;97:292–299. doi: 10.1002/jbm.a.33056. [DOI] [PubMed] [Google Scholar]
- 5.Uygun BE, Soto-Gutierrez A, Yagi H, Izamis ML, Guzzardi MA, Shulman C, Milwid J, Kobayashi N, Tilles A, Berthiaume F, Hertl M, Nahmias Y, Yarmush ML, Uygun K. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16:814–820. doi: 10.1038/nm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badylak SF. Xenogeneic extracellular matrix as a scaffold for tissue reconstruction. Transpl Immunol. 2004;12:367–377. doi: 10.1016/j.trim.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 7.Mo X, Weber HJ, Ramakrishna S. PCL-PGLA composite tubular scaffold preparation and biocompatibility investigation. Int J Artif Organs. 2006;29:790–799. doi: 10.1177/039139880602900809. [DOI] [PubMed] [Google Scholar]
- 8.He X, Kawazoe N, Chen G. Preparation of cylinder-shaped porous sponges of poly (L-lactic acid), poly (DL-lactic-co-glycolic acid) and poly (ε-carprolactone) Biomed Res Int. 2014;2014:106082. doi: 10.1155/2014/106082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chlapanidas T, Tosca MC, Faragò S, Perteghella S, Galuzzi M, Lucconi G, Antonioli B, Ciancio F, Rapisarda V, Vigo D, Marazzi M, Faustini M, Torre ML. Formulation and characterization of silk fibroin films as a scaffold for adipose-derived stem cells in skin tissue engineering. Int J Immunopathol Pharmacol. 2012;26:43–49. doi: 10.1177/03946320130260S106. [DOI] [PubMed] [Google Scholar]
- 10.Lui PP, Wong OT, Lee YW. Application of tendon-derived stem cell sheet for the promotion of graft healing in anterior cruciate ligament reconstruction. Am J Sports Med. 2014;42:681–689. doi: 10.1177/0363546513517539. [DOI] [PubMed] [Google Scholar]
- 11.Pei M, He F, Kish VL. Expansion on extracellularmatrix deposited by human bone marrow stromal cells facilitates stem cell proliferation and tissue-specific lineage potential. Tissue Eng Part A. 2011;17:3067–3076. doi: 10.1089/ten.tea.2011.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin H, Yang G, Tan J, Tuan RS. Influence of decellularized matrix derived from human mesenchymal stem cells on their proliferation, migration and multi-lineage differentiation potential. Biomaterials. 2012;33:4480–4489. doi: 10.1016/j.biomaterials.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 13.He H, Liu X, Peng L, Gao Z, Ye Y, Su Y, Zhao Q, Wang K, Gong Y, He F. Promotion of Hepatic Differentiation of Bone Marrow Mesenchymal Stem Cells on Decellularized Cell-Deposited Extracellular Matrix. Biomed Res Int. 2013;2013:406871. doi: 10.1155/2013/406871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milburn ML, Holton LH, Chung TL, Li EN, Bochicchio GV, Goldberg NH, Silverman RP. Acellular dermal matrix compared with synthetic implant material for repair of ventral hernia in the setting of peri-operative Staphy-lococcus aureus implant contamination: a rabbit model. Surg Infect (Larchmt) 2008;9:433–442. doi: 10.1089/sur.2007.044. [DOI] [PubMed] [Google Scholar]
- 15.Lovett M, Lee K, Edwards A, Kaplan DL. Vascularization strategies for tissue engineering. Tissue Eng Part B Rev. 2009;15:353–370. doi: 10.1089/ten.teb.2009.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groeber F, Kahlig A, Loff S, Walles H, Hansmann J. A bioreactor system for interfacial culture and physiological perfusion of vascularized tissue equivalents. Biotechnol J. 2013;8:308–316. doi: 10.1002/biot.201200160. [DOI] [PubMed] [Google Scholar]
- 17.Yu H, VandeVord PJ, Mao L, Matthew HW, Wooley PH, Yang SY. Improved tissue-engineered bone regeneration by endothelial cell mediated vascularization. Biomaterials. 2009;30:508–517. doi: 10.1016/j.biomaterials.2008.09.047. [DOI] [PubMed] [Google Scholar]
- 18.Post MJ, Rahimi N, Caolo V. Update on vascularization in tissue engineering. Regen Med. 2013;8:759–770. doi: 10.2217/rme.13.74. [DOI] [PubMed] [Google Scholar]
- 19.Herbert SP, Stainier DY. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat Rev Mol Cell Biol. 2011;12:551–564. doi: 10.1038/nrm3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raines EW. The extracellular matrix can regulate vascular cell migration, proliferation, and survival: relationships to vascular disease. Int J Exp Pathol. 2000;81:173–182. doi: 10.1046/j.1365-2613.2000.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis GE, Senger DR. Endothelial extracellular matrix biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res. 2005;97:1093–1107. doi: 10.1161/01.RES.0000191547.64391.e3. [DOI] [PubMed] [Google Scholar]
- 22.Shekhonin BV, Domogatsky SP, Muzykantov VR, Idelson GL, Rukosuev VS. Distribution of type I, III, IV and V collagen in normal and atherosclerotic human arterial wall: immunomorphological characteristics. Coll Relat Res. 1985;5:355–368. doi: 10.1016/s0174-173x(85)80024-8. [DOI] [PubMed] [Google Scholar]
- 23.Feugier P, Black RA, Hunt JA, How TV. Attachment, morphology and adherence of human endothelial cells to vascular prosthesis materials under the action of shear stress. Biomaterials. 2005;26:1457–1466. doi: 10.1016/j.biomaterials.2004.04.050. [DOI] [PubMed] [Google Scholar]
- 24.Sgarioto M, Vigneron P, Patterson J, Malherbe F, Nagel MD, Egles C. Collagen type I together with fibronectin provide a better support for endothelialization. C R Biol. 2012;335:520–528. doi: 10.1016/j.crvi.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Luescher TF, Barton M. Biology of the endothelium. Clin Cardiol. 1997;20:3–10. [PubMed] [Google Scholar]
- 26.Potapova IA, Gaudette GR, Brink PR, Robinson RB, Rosen MR, Cohen IS, Doronin SV. Mesenchymal stem cells support migration, extracellular matrix invasion, proliferation, and survival of endothelial cells in vitro. Stem Cells. 2007;25:1761–1768. doi: 10.1634/stemcells.2007-0022. [DOI] [PubMed] [Google Scholar]
- 27.Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res. 2007;100:782–794. doi: 10.1161/01.RES.0000259593.07661.1e. [DOI] [PubMed] [Google Scholar]
- 28.Dejana E, Languino LR, Polentarutti N, Balconi G, Ryckewaert JJ, Larrieu MJ, Donati MB, Mantovani A, Marguerie G. Interaction between fibrinogen and cultured endothelial cells. Induction of migration and specific binding. J Clin Invest. 1985;75:11–18. doi: 10.1172/JCI111661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zou L, Cao S, Kang N, Huebert RC, Shah VH. Fibronectin induces endothelial cell migration through β1 integrin and Src-dependent phosphorylation of fibroblast growth factor receptor- 1 at tyrosines 653/654 and 766. J Biol Chem. 2012;287:7190–7202. doi: 10.1074/jbc.M111.304972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herbst TJ, McCarthy JB, Tsilibary EC, Furcht LT. Differential effects of laminin, intact type IV collagen, and specific domains of type IV collagen on endothelial cell adhesion and migration. J Cell Biol. 1988;106:1365–1373. doi: 10.1083/jcb.106.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]


