Abstract
Objective: To identify the medicinal species in the Zi Hua Di Ding (ZHDD) prescription commonly used by the Dong ethnic people, and investigate the potential mechanism involved with the anti-fungal properties on Trichophyton rubrum. Methods: DNA barcode technique was used to identify the species in the ZHDD prescription. In vitro study was performed to investigate the antifungal properties of the identified Viola philippica on the T. rubrum. Microscopy was used to observe the growth of the T. rubrum. Results: The ZHDD prescription is a mixture of V. philippica and V. inconspicua, and its antifungal properties was superior to the single component. V. philippica could affect the surface and flexibility of hypha, and contribute to the generation of branches. In addition, it could damage the biofilm. Conclusions: Using the DNA barcode technique, we identify the ZHDD prescription is a mixture of V. philippica and V. inconspicua, and its antifungal properties was superior to the single component. V. philippica could affect the growth and biofilm formation of T. rubrum.
Keywords: Dong ethnic drug, DNA barcode, Trichophyton rubrum, antifungal property, Viola philippica
Introduction
Viola philippica is a small perennial herb with violet flowers, which is mainly distributed in China, Japan and Korea [1]. Its dry plant including the root, known as Herba Violae, has been acknowledged as an important constituent of the traditional Chinese medicine ‘Zi Hua Di Ding’, a drug with many documented local uses [2]. For example, Zi Hua Di Ding (ZHDD) prescription, a mixture of powder obtained from several plants of Violaceae, has been used by the residents of Dong ethnic minority living in southeast of Guizhou Province for treating tinea pedis by smearing the powder onto the affected position. According to a survey of complementary and alternative medicine use in the Dong ethnic minority, a majority of population using ZHDD prescription report a complete response of beriberi within 2-4 weeks with nearly no relapse. However, rare studies have been conducted for the phytochemistry of this medicinal herb.
Diversity of natural environmental conditions is important for the quality variation and ecotype classification of herbal medicine [3]. Plants for medicinal usage cover a wide range of taxa and closely related species. Although V. philippica is the only specimen approved for preparation of commercial drug according to the Pharmacopoeia of People’s Republic of China (2010 version), various commonly-used plant species are still used to prepare for an ethnic prescription in different regions. The identification of cultivars of Violaceae was difficult as they showed extremely similar appearance especially those with no fruit and flowers. When collecting the V. philippica, mixture of plants is inevitable. Fortunately, the ‘wrong collection’ resulted in better therapeutic effects compared with single component. To our best knowledge, no study has been conducted to identify the component of ethnic drug, and investigate the potential efficiency.
In this study, DNA barcode was used to identify the species of medicinal plant mentioned in the ZHDD prescription commonly used by the physicians of the Dong people. Also, we investigated the potential mechanism of ZHDD prescription on the antifungal properties on the T. rubrum to illustrate the potential reason for the efficiency of tinea pedis.
Materials and methods
Materials
Medicinal plants were collected from a local maintain with an altitude of > 600 m in Liping country, southeast region of Guizhou Province. The plants were washed using fresh water and dried. The plants were identified by Dr. Zhirong Sun. Three species were collected, and the information of the species was listed in Table 1.
Table 1.
Characteristics of the plant species
| ID | Characteristics of plants |
|---|---|
| Sample 1 | Violet flower, with no spigeal stem, short root stock, white root, stipule in pale color, capsular fruit in spherical profile, seeds in oosphere profile, in jasmine color |
| Sample 2 | Violet flower, with no spigeal stem, short root stock, white root, stipule in brown color, capsular fruit in spherical profile, seeds in oosphere profile, in flavor green color |
| Sample 3 | No spigeal stem, white root, no flower, fruit or seeds identified |
| A | A mixture of sample 1-3 |
DNA barcode identification
Rapid authentication of these plants was performed using the DNA barcode technique according to the previous report [4]. Briefly, DNA extraction from leaf tissues (100 mg) was performed using the commercial kit (TianGen Biotech Co., Ltd., Beijing, China) according to the manufacturer’s instructions. Specific primers for ITS2 were designed, and the sequences were 5’-ATGCGATACTTGGTGTGAAT-3’, and 5’-GACGCTTCTCCAGACTACAAT-3’. The PCR amplification was performed in a 25 µl volume containing 2 µl DNA template, µM of each primer (synthesized by Sangon Co., Ltd., Shanghai, China), and U DNA polymerase. The reaction conditions were pre-denaturation at 94°C for 5 min, followed by 40 cycles of denaturation at 94°C for 30 s, annealing at 56°C for 30 s, and extension at 72°C for 45 s. Purified PCR products were sequenced using a 3730XL sequencer (Applied Biosystems, Grand Island, NY, USA). All sequences of the samples containing the ITS2 were retrieved according to the previous report and GenBank annotations. Species identification was performed using BLASTN program.
Drug sensitive test
To prepare the ZHDD extract, sample 1-3 were extract using ethanol (50%) and water. After condensation, the dried samples were obtained, and on this basis, the extract with a concentration of 0.1 g/ml was obtained.
For the antifungal test, the T. rubrum (l × 105 cFu/ml) was cultivated in the 24-well cell culture plate containing ZHDD solution or solution of sample 1-3 with a concentration of 50 mg/ml, 25 mg/ml, 12.5 mg/ml, 6.25 mg/ml, 3.125 mg/ml and 1.563 mg/ml, respectively. Fungi treated using terbinafine dissolved with sterilized NaCl solution (0.85%) with a concentration of 5, 2.5, 1.25, 0.625, 0.3125, 0.156, 0.078, 0.039 μg/ml set as positive control.
Minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC) were used to determine the antifungal properties according to the previous description. Quality control was performed according to the CLSI standard [5].
Results
Identification of plant species in ZHDD prescription
Two plant strains were chosen for each sample (sample 1-3). The ITS2 sequences of sample 1 and 3 showed the same DNA sequence as that of V. philippica obtained from GenBank database (Figure 1), demonstrating the sample 1 and 3 were V. philippica. For the ITS2 sequence of sample 2, high sequence similarity of (94% and 97%) was noticed with the V. inconspicua (Figure 2). Therefore, we concluded the species of sample 2 was V. inconspicua. Moreover, a higher similarity was noticed between sample 2 and sample 1 or 3, demonstrating these cultivars belong to the Violaceae.
Figure 1.
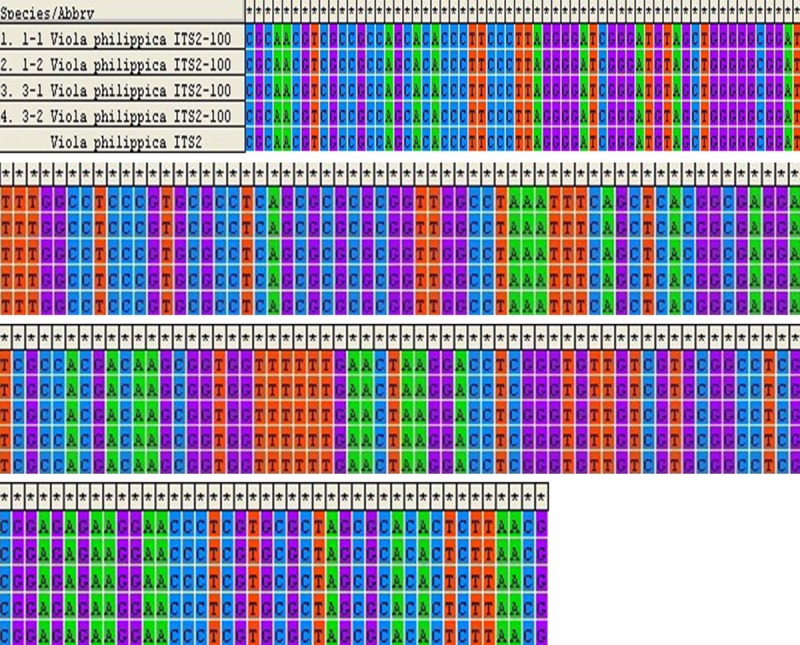
Sequence alignment of ITS2 of sample 1 and 3 compared with the ITS2 of Viola philippica.
Figure 2.
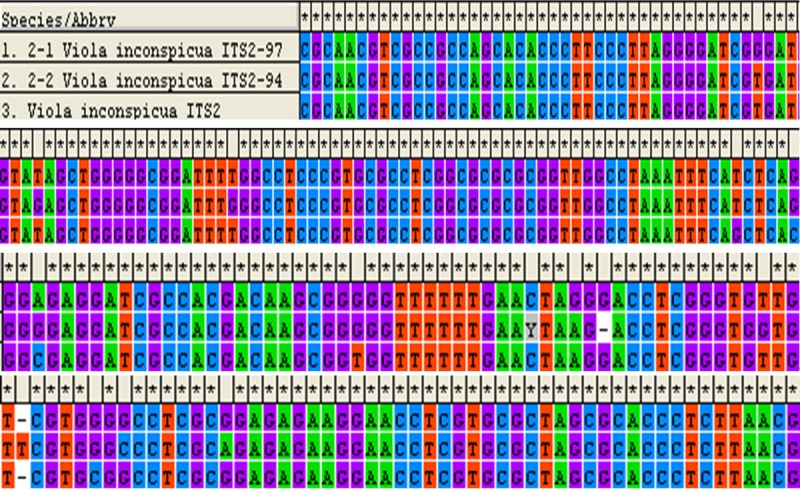
Sequence alignment of ITS2 of sample 2 compared with the ITS2 of V. inconspicua.
Determination of drug sensitive test
Table 2 summarized the MIC and MFC of the samples. Sample A showed higher antifungal properties with a MIC and MFC of 1.563 mg/ml and 6.25 mg/ml, respectively. For sample 1-3, similar antifungal properties were noticed.
Table 2.
Minimum inhibitory concentration and minimum fungicidal concentration on Trichophyton rubrum
| ID | MIC | MFC |
|---|---|---|
| Sample 1 | 12.5 mg/ml | 12.5 mg/ml |
| Sample 2 | 12.5 mg/ml | 25 mg/ml |
| Sample 3 | 12.5 mg/ml | 25 mg/ml |
| Sample A | 1.563 mg/ml | 6.25 mg/ml |
| Terbinafine | 1.25 μg/ml | 1.25 μg/ml |
MIC, minimum inhibitory concentration; MFC, minimum fungicidal concentration.
Effects of V. philippica on the growth of T. rubrum
In this study, we determined the effects of V. philippica on the growth of T. rubrum. The identified V. philippica was extract using ethanol and water solution, and was diluted into 50, 25, 12.5, 6.25, 3.125, and 1.563 mg/ml using Sabaurauds agar medium. The organisms obtained from the well incubated with 6.25 mg/ml V. philippica extract were smeared on the Sabaurands agar medium and were incubated at 30°C for 14 days.
As revealed in Figure 3, the hypha of the T. rubrum was long with no branches. The septation of the hypha was even and significant. In addition, dispersed spores were noticed. For the T. rubrum treated by V. philippica, the T. rubrum were twisted with multiple branches. The septation was eliminated or not obvious. Meanwhile, no spores were noticed. Based on these facts, we concluded that ZHDD may eliminate the septation and spores, and contribute to the irregular formation of branches for the hypha. All these may affect the growth and proliferation of T. rubrum, which finally inhibit the growth of fungi.
Figure 3.
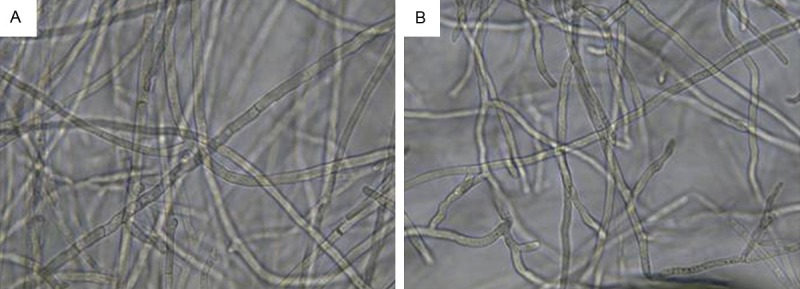
Growth of Trichophyton rubrum with (A) or without interference of V. philippica (B).
In this study, scanning electron microscopy was used to monitor the structure of T. rubrum (Figures 4 and 5). A large amount of deep-color substances were observed after treating using ZHDD. Meanwhile, a large number of small segmental hypha was noticed. Further, the membrane attached to the surface of the hypha was significantly damaged, and no secretion was noticed on the membrane. Based on this information, ZHDD could induce damage of the membrane surface, based on which to affect the growth and proliferation of T. rubrum.
Figure 4.

Growth of T. rubrum with no interference of V. philippica. A. The hypha was long with spores on it. B. The hypha was smooth with membrane attached on it. C. The membrane was intact with secretion on the membrane. The hypha was smooth and even.
Figure 5.

Growth of Trichophyton rubrum treated using V. philippica. A. The hypha was destroyed with no spore existed. B. A large number of substances in deep color were noticed in the hypha. The surface was smooth and fragile. C. The membrane at the surface of the hypha was destroyed. The mycelium was not even.
Discussion
Tinea pedis is a common superficial fungal infection in the general population with T. rubrum as the major causal organisms [6,7]. ZHDD prescription has been used for treating tinea pedis by the Dong ethnic People. Plants of Viola showed similar morphology, especially those before blooming and without fruits, which brings a great challenge for the identification of the species. Therefore, the prescription is actually a mixture of the medicinal herbs. In this study, DNA barcode technique was used for the identification of specimen mentioned in the prescription. Our results revealed that the prescription was consisted of two species, including V. philippica and V. inconspicus.
T. rubrum is the most common causal organism of tinea pedis [8]. In this study, the MICs of V. philippica and V. inconspicus were same with a value of 12.5 mg/ml, indicating these two species showed antifungal properties to T. rubrum. In addition, the MFCs of V. philippica and V. inconspicus were 12.5 mg/ml and 25 mg/ml, respectively. The killing effects of these species were associated with the irreversible injury to the growth of T. rubrum. Taken together, we concluded that the ZHDD prescription could affect the growth and proliferation of T. rubrum, and exert killing effects. Compared with the V. inconspicus, V. philippica was more effective in killing the T. rubrum. Although these two species showed antifungal and even fungi-killing effects, the efficiency of combinational application was superior to any single component.
To further determine the potential mechanism of the antifungal and killing effects, we determined the effects of V. philippica on the growth of the T. rubrum in vitro using scanning electron microscope. The septation of the hypha was even and significant. In addition, dispersed spores were noticed. In addition, the surface of the hypha was not smooth, and the flexibility of the hypha was poor after interference of V. philippica. On this basis, several branches were generated in the hypha, and were fragile. Further, the anatomical structure and physiological function of the hypha were affected. Meanwhile, the membrane structure and the attached substances in the surface of hypha were significantly damaged. However, as the ZHDD presc-ription of two herbal plants, even though the effects of V. philippica have been identified, we cannot conclude that V. philippica is the only active component for treating tinea pedis.
Interestingly, in this study, formation of biofilm was noticed in T. rubrum as revealed by electron scanning. According to the previous studies, formation of biofilm contributed to the drug-resistance in Candida albicans [9,10]. In our study, the biofilm was severely damaged by V. philippica, based on which we concluded that V. philippica is effective in inhibiting or even killing the T. rubrum.
To date, extremely rare studies have been conducted to investigate the potential mechanism of the ethnic drugs on certain diseases. The essence of ethnic drugs is a mixture of several components [11], which can exert certain effects such as killing the fungi. However, this cannot live up to the standards of modern medicine theory, which focuses on how the drug works and the potential function of single component [12]. For ethnic drugs, single component may show no contribution to the efficiency as revealed by pharmaceutical and toxicological tests. However, effectiveness was noticed after combining application of the single component.
DNA barcode contributes to the accurate and rapid authentication of medicinal plants of the same species. In this study, using DNA barcode technique, we identified the raw materials of ZHDD prescription were consisted of V. philippica and V. inconspicua. On this basis, we determined the antifungal effects of V. philippica on the growth of T. rubrum in vitro under scanning electron microscopy. Our results indicated that V. philippica extract could inhibit the growth and proliferation of T. rubrum in vitro. Also, the biofilm formed in the T. rubrum was destroyed by V. philippica extract, demonstrating it plays a crucial role in the antifungal effects of the ZHDD prescription. In the future, we will focus on the effects of V. inconspicua on the growth of T. rubrum.
In conclusion, the component of ZHDD prescription used for treating tinea pedis by the Dong ethnic People was identified using DNA barcode technique, which included V. philippica and V. inconspicua. Its antifungal properties were monitored using electron microscopy. ZHDD prescription plays an inhibiting role in the tinea pedis through inhibiting the growth and proliferation of T. rubrum, and damages the biofilm. Our study could provide helpful information for the research and development of ethnic drugs.
Acknowledgements
This project was supported by the grant from the Beijing University of Chinese Medicine for Ethnic Drug (No. 2011-MZYY-08) and the Independent Fund of Beijing University of Chinese Medicine (No. 2013-JYBZZ-XS-094).
Disclosure of conflict of interest
None.
References
- 1.Xie C, Veitch NC, Houghton PJ, Simmonds MS. Flavone C-glycosides from Viola yedoensis MAKINO. Chem Pharm Bull (Tokyo) 2003;51:1204–1207. doi: 10.1248/cpb.51.1204. [DOI] [PubMed] [Google Scholar]
- 2.Zhou HY, Hong JL, Shu P, Ni YJ, Qin MJ. A new dicoumarin and anticoagulant activity from Viola yedoensis Makino. Fitoterapia. 2009;80:283–285. doi: 10.1016/j.fitote.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Huang LF, Suo FM, Song JY, Wen MJ, Jia GL, Xie CX, Chen SL. Quality variation and ecotype division of Panax quinquefolium in China. Yao Xue Xue Bao. 2013;48:580–589. [PubMed] [Google Scholar]
- 4.Chen S, Yao H, Han J, Liu C, Song J, Shi L, Zhu Y, Ma X, Gao T, Pang X, Luo K, Li Y, Li X, Jia X, Lin Y, Leon C. Validation of the ITS2 region as a novel DNA barcode for identifying medicinal plant species. PLoS One. 2010;5:e8613. doi: 10.1371/journal.pone.0008613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Espinel-Ingroff A, Fothergill A, Ghannoum M, Manavathu E, Ostrosky-Zeichner L, Pfaller M, Rinaldi M, Schell W, Walsh T. Quality control and reference guidelines for CLSI broth microdilution susceptibility method (M 38-A document) for amphotericin B, itraconazole, posaconazole, and voriconazole. J Clin Microbiol. 2005;43:5243–5246. doi: 10.1128/JCM.43.10.5243-5246.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aly R, Fisher G, Katz I, Levine N, Lookingbill DP, Lowe N, Menter A, Morman M, Pariser DM, Roth HL, Savin RC, Shavin JS, Stewart D, Taylor JR, Tucker S, Wortzman M. Ciclopirox gel in the treatment of patients with interdigital tinea pedis. Int J Dermatol. 2003;42(Suppl 1):29–35. doi: 10.1046/j.1365-4362.42.s1.6.x. [DOI] [PubMed] [Google Scholar]
- 7.Evans EG. Tinea pedis: clinical experience and efficacy of short treatment. Dermatology. 1997;194(Suppl 1):3–6. doi: 10.1159/000246173. [DOI] [PubMed] [Google Scholar]
- 8.Leibovici V, Ramot Y, Siam R, Siam I, Hadayer N, Strauss-Liviatan N, Hochberg M. Prevalence of tinea pedis in psoriasis, compared to atopic dermatitis and normal controls-a prospective study. Mycoses. 2014;57:754–8. doi: 10.1111/myc.12227. [DOI] [PubMed] [Google Scholar]
- 9.Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, Ghannoum MA. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J Bacteriol. 2001;183:5385–5394. doi: 10.1128/JB.183.18.5385-5394.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez-Ribot JL. Large-scale biochemical profiling of the candida albicans biofilm matrix: new compositional, structural, and functional insights. MBio. 2014;5:e01781–14. doi: 10.1128/mBio.01781-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J, Li P, Wang Z. Brief discourse on development of psychology of modern traditional Chinese medicine. Zhonghua Yi Shi Za Zhi. 2014;44:155–157. [PubMed] [Google Scholar]
- 12.Lewinsohn R. Medical theories, science, and the practice of medicine. Soc Sci Med. 1998;46:1261–1270. doi: 10.1016/s0277-9536(97)10054-5. [DOI] [PubMed] [Google Scholar]


