Abstract
Sex determining region Y-box 2 (SOX2) has been identified as a putative cancer stem cells (CSCs) marker in Head and Neck Cancers (HNC). However, the clinicopathological and prognostic significance of SOX2 in HNC patients remains controversial. We reviewed the literature by performing a meta-analysis based on the data from 7 studies (9 cohorts) to evaluate the association between SOX2 and clinicopathological/prognostic parameters in patients with HNC. Pooled hazard ratio (HR) or odds ratio (OR) with its 95% confidence interval (CI) was used as the effect size estimate. Our analysis results suggested that high SOX2 expression predicted unfavorable OS (HR: 1.54, 95% CI: 1.09-2.18) and DFS (HR: 1.54, 95% CI: 1.13-2.10) of patients with HNC. In addition, increased SOX2 was also significantly associated with high tumor grade (OR: 1.86, 95% CI: 1.06-3.28), advanced TNM stage (OR: 4.22, 95% CI: 2.62-6.80), lymph node metastasis (OR: 2.25, 95% CI: 1.50-3.35) and distant metastasis (OR: 1.99, 95% CI: 1.26-3.15). Our study suggested that SOX2 expression can be served as a candidate unfavorable prognostic biomarker for HNC patients, indicating that it might be a potential therapeutic target.
Keywords: SOX2, head and neck cancers, prognosis, meta-analysis
Introduction
Head and neck cancer is one of the most prevalent type of malignancy worldwide, with roughly half million new cases each year, and its incidence is still increasing in several geographic areas and its trend is now affecting younger individuals [1]. The most common histological type of head and neck cancer, including oral cavity, nasopharynx, hypopharynx, larynx and nasal cavity, are squamous cell carcinoma (SCC) [2]. The mortality due to HNC is mainly caused by local recurrence and local metastasis to cervical lymph node, and occasionally by distant organ metastasis [3]. Despite advancements in the field of oncology and great methods of detecting the disease at earlier stages in the last 30 years, we still observe high morbidity and resistance to conventional therapy [4]. It is becoming increasingly evident that an improvement in the survival of HNC requires improved understanding of the high risk of HNC patients who are prone to tumor metastasis and poor prognosis.
Recent studies on the pathobiology of HNC have led to the discovery of a small population of cancer cells that is highly tumorigenic, capable of self-renewal, and behave as tumor progenitor cells. Such behavior is consistent with the features of cancer stem cells (CSCs) [5,6]. It is believed that existence of CSCs may be the reason for the lack of treatment effectiveness and high relapse and metastasis rate of HNC patients [4]. Targeted elimination of these CSCs has been considered a new conceptual framework for HNC treatment [7,8].
SOX2, a member of the sex determining region Y-box family, is a key transcription factor involved in maintaining the pluripotency of CSCs in self-renewal and differentiation, and plays a critical role in determining the fate of stem cells [9,10]. Recent studies indicated that SOX2 was aberrantly expressed in several human tumors including lung cancer, esophageal carcinoma, pancreatic carcinoma, breast cancer, ovarian carcinoma, hepatocellular carcinoma and head and neck cancers [11-15]. However, SOX2 expression pattern and the correlation with clinicopathological features and clinical outcome were highly variable among cancers. Some studies revealed that expression of SOX2 conferred a better prognosis [16-18], but others found an association with worse clinical outcome as well as adverse clinical parameters, including recurrence, lymph node and distant metastasis [2,19-21]. Based on these controversies, a meta-analysis was conducted in order to gain deeper insight into the clinicopathological and prognostic significance of SOX2 in HNC.
Materials and methods
Search strategy
PubMed, Embase and Web of Science were used to search for the original articles analyzing the prognostic value of SOX2 in human cancer, by means of keywords variably combined: (“SOX2” OR “SOX-2” OR “Sex determining region Y-box 2” OR “SRY-Related HMG-Box Gene 2”) AND (“cancer” OR “carcinoma” OR “neoplasm” OR “tumor” OR “malignancy”) AND (“prognosis” OR “prognostic” OR “outcome” OR “survival”). Last search was updated on 1 July 2014, and no lower date limit was used. Reports in English were eligible for inclusion. The reference list was also checked for relevant articles. Investigators were contacted and asked to supply additional data when essential data were unavailable from original literatures.
Eligibility criteria
All candidate articles were reviewed by two independent reviewers (ZYD and JYS), and discrepancies were resolved by discussion. Inclusion criteria were as follows: (i) The diagnosis of Head and Neck Cancers was made based on pathological examination; (ii) disease-free survival (DFS), overall survival (OS) and other clinicopathological indicators were the main outcomes of interest; (iii) SOX2 expression status was detected by immunohistochemistry (IHC); (iv) the values of hazard ratios (HRs) and 95% CI between SOX2 expression and survival status could be obtained from the literature directly or recalculated based on the survival curve in the articles; and (v) for duplicate articles, only the most complete and/or recently published one was included. Exclusion criteria were as follows: (i) abstracts, letters, editorials, expert opinions, reviews and case reports; (ii) studies with insufficient data for estimating HR and 95% CI; (iii) literature which failed to present the cut-off value defining “elevated SOX2”; (iv) literature written in language other than English; (v) non-human research.
Data extraction
Data extraction was performed independently by two authors (GCL and BQH) from eligible studies. Controversial problems were resolved by discussion and consensus. Two investigators reviewed all of researches that met inclusion and exclusion criteria. In order to ensure the quality of the meta-analysis, we followed the guidelines provided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [22]. Information retrieved from the researches included author, publication year, country of population, sample size, histological type, tumor stage, outcome indexes, detection method , SOX2 location, cut-off value, follow-up time, HR and their 95% CI.
Quality assessment
Study quality was assessed independently by two investigators (ZYD and DHW), by means of reading and evaluating according to Newcastle-Ottawa quality assessment scale (NOS) [23]. NOS scores of ≥ 6 were assigned as high-quality studies. Any disagreement was addressed by joint discussion.
Statistical analysis
Hazard ratio (HR) and 95% confidence intervals (95% CI) were obtained directly from each literature or from estimation according to the methods by Parmer [24] and Tierney [25]. For the analysis of the relationship between SOX2 and clinicopathological parameters, odds ratios (OR) and 95% CI were combined as the effective value. Statistical heterogeneity between cohorts was evaluated by x2 test and inconsistency index (I2) and was considered significant when x2 P-value < 0.1 or I2 > 50%. In the absence of statistically significant heterogeneity, the Mantel-Haenszel method in the fixed-effect model was used for the Meta analysis. Otherwise, the DerSimonian-Laird method in the random-effect model was selected. Publication bias was evaluated graphically by Begg’s funnel plot analysis and then statistically using Egger’s test with significant publication bias defined as P < 0.05. All analyses were performed with Review Manager Version 5 (RevMan, Cochrane Collaboration, Oxford, England) and Stata version 12.0 (StataCorp LP, College Station, TX).
Results
Selection and characteristics of studies
A total of 746 articles were identified initially using the search strategy above. Studies excluded with animal experiments, non-NHC-related studies, non-English articles, non-original articles, only 22 publications met the inclusion criteria for the present analysis (Figure 1). Of the 22 candidate studies, 13 studies were not directly related to specific outcomes, 1 did not provide enough data for estimating the HR and 95% CI and 1 failed to present complete information about the follow-up time and cut-off value. Thus 7 studies (9 cohorts) [2,19,20,26-29] published between 2010 and 2014 were included in our meta-analysis investigating OS/DFS or pathological features. The total number of patients included was 909, with sample sizes ranging from 51 to 161 patients. The median follow-up period ranged from 18 to 86.4 months. As the studies by Márquez [2] included three cohorts focused on three types of HNC and reported their clinical outcome separately, we marked them as Márquez (HPC), Márquez (LC) and Márquez (SNC) respectively in the following analysis. The characteristics of the included studies were summarized in Table 1. Six studies were from China, one study (three cohorts) from Spain. HR and 95% CI were produced directly by the multivariate analysis in five of the enrolled cohorts (Table S1). For the remaining studies, HRs and 95% CIs were calculated from Kaplan-Meier curves. NOS score was above 6 in all cohorts (Table S2).
Figure 1.
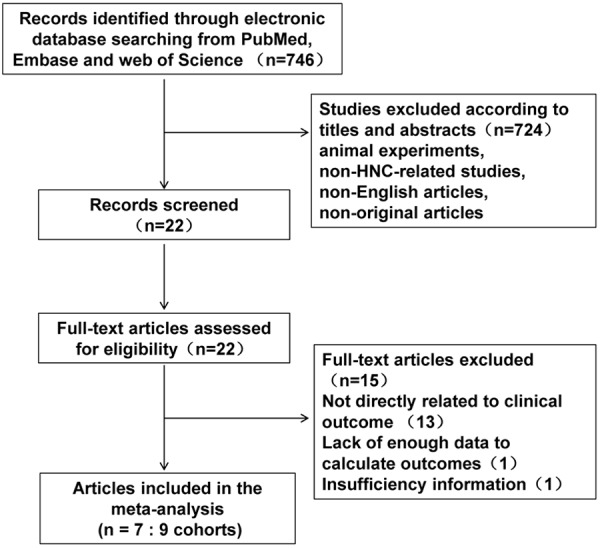
Flow diagram of studies selection procedure.
Table 1.
Characteristics of included studies
| First author | Year | Country | Malignant disease | Histological type | Stage (I II/III IV) | Sample size (male) | Follow-up median (months) | Outcome indexes | Design of Data Collection | Detection method | Staining pattern | Cut-off value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Luo et al. | 2013 | China | Nasopharyngeal carcinoma | SCC | 33/89 | 122 (92) | 60.1 (8-92) | OS | Retrospective | IHC | nucleus | Score ≥ 6 (0-9) |
| Wang et al. | 2012 | China | Nasopharyngeal carcinoma | SCC | 40/68 | 108 (76) | 86.4 | OS | Retrospective | IHC | nucleus | Score ≥ 9 (0-12) |
| Ge et al. | 2010 | China | Hypopharyngeal carcinoma | SCC | 7/78 | 85 (84) | 52 (7-69.5) | OS/DFS | Retrospective | IHC | nucleus | Score ≥ 4 (0-7) |
| Dai et al. | 2014 | China | Salivary gland adenoid cystic | ACC | NA | 131 (75) | 38.6 (3-62) | OS/DFS | Retrospective | IHC | nucleus | Score ≥ 6 (0-9) |
| Du et al. | 2013 | China | Oral tongue carcinoma | SCC | NA | 82 (55) | 67 (4-81) | OS/DFS | Retrospective | IHC | nucleus | Score ≥ 2 (0-7) |
| Tang et al. | 2011 | China | Laryngeal carcinoma | SCC | 65/96 | 161 (152) | 46.3 (8-60) | OS | Retrospective | IHC | nucleus | Score ≥ 6 (0-7) |
| Márquez et al. | 2013 | Spain | Hypopharyngeal carcinoma | SCC | 7/95 | 102 (99) | 14 (0-95) | OS/DFS | Retrospective | IHC | nucleus | > 5% |
| 2013 | Spain | Laryngeal carcinoma | SCC | 13/54 | 67 (67) | 37 (1-97) | OS/DFS | Retrospective | IHC | nucleus | > 5% | |
| 2013 | Spain | Sinonasal carcinoma | SCC | 4/47 | 51 (37) | 18 (1-211) | OS/DFS | Retrospective | IHC | nucleus | > 5% |
Abbreviations: OS, overall survival; DFS, disease-free survival; IHC, Immunohistochemistry; SCC, squamous cell carcinoma; ACC, adenoid cystic carcinomas.
SOX2 expression and OS in HNC patients
There were 9 cohorts presenting the data of SOX2 and OS in HNC patients. The pooled estimates demonstrated a significant relationship between elevated SOX2 and shorter OS (HR = 1.59, 95% CI = 1.09-2.18, P = 0.01), with significant heterogeneity between studies (I2 = 44%, P = 0.07) (Figure 2A).
Figure 2.
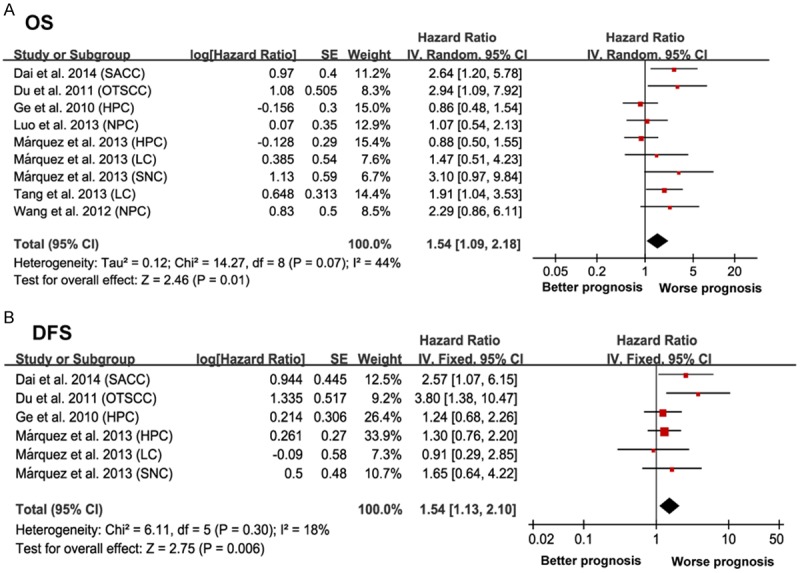
Forrest plots evaluating association between increased SOX2 expression and clinical outcomes in HNC. A. Forrest plot to assess the overall effect of SOX2 on OS in HNC patients. B. Forrest plot to assess the overall effect of SOX2 on DFS in HNC patients. Results are presented as individual and pooled hazard ratio (HR), and 95% confidence interval (CI).
To explore the heterogeneity, further subgroup analysis by different histological type suggested that both subgroups did not alter the prognostic role of SOX2 in OS (SCC: HR = 1.33, 95% CI = 1.03-1.74, P = 0.03, I2 = 40%, P = 0.11 and ACC: HR = 2.64, 95% CI = 1.20-5.78, P = 0.02). When different cancer types were considered, SOX2 was only a negative prognostic marker in patients diagnosed with laryngeal carcinoma (LC) (HR = 1.79, 95% CI = 1.05-3.14, P = 0.03, I2 = 0, P = 0.67). When grouped according to the regional distribution, Asian (China) cohorts with increased SOX2 expression suggested the significant results (HR = 1.64, 95% CI = 1.08-2.50, P = 0.02, I2 = 47%, P = 0.006). We then focused on the median follow-up time in each cohort, those with a median follow-up time over 36 month studies suggested the significant relationship (HR = 1.54, 95% CI = 1.16-2.05, P = 0.003, I2 = 37%, P = 0.15). In subtotal analyses of the sample size, the pooled outcome of those with a research object over 100 patients subgroup showed increased SOX2 expression was significantly associated with an unfavorable OS (HR = 1.45, 95% CI = 1.07-1.97, P = 0.02, I2 = 47%, P = 0.11) (Table 2).
Table 2.
Subgroup analysis of the studies reporting the prognostic value of SOX2 expression
| Outcome | Subgroups | Cohorts | HR (95% CI) | P | Model | Heterogeneity | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| I2 | P | ||||||
| OS | All | 9 | 1.54 (1.09-2.18) | 0.01 | Random | 44% | 0.07 |
| Cancer type | |||||||
| LC | 2 | 1.79 (1.05-3.14) | 0.03 | Fixed | 0 | 0.67 | |
| HPC | 2 | 0.87 (0.58-1.31) | 0.5 | Fixed | 0 | 0.95 | |
| NPC | 2 | 1.38 (0.79-2.24) | 0.26 | Fixed | 36% | 0.21 | |
| Histological type | |||||||
| SCC | 8 | 1.33 (1.03-1.74) | 0.03 | Fixed | 40% | 0.11 | |
| ACC | 1 | 2.64 (1.20-5.78) | 0.02 | Fixed | N/A | N/A | |
| Region | |||||||
| Asia | 6 | 1.64 (1.08-2.50) | 0.02 | Random | 47% | 0.09 | |
| Europe | 3 | 1.18 (0.75-1.87) | 0.48 | Fixed | 48% | 0.15 | |
| Median follow-up time | |||||||
| ≥ 36 month | 7 | 1.54 (1.16-2.05) | 0.003 | Fixed | 37% | 0.15 | |
| < 36 month | 2 | 1.49 (0.44-5.01) | 0.52 | Random | 73% | 0.06 | |
| Sample size (n) | |||||||
| ≥ 100 | 5 | 1.45 (1.07-1.97) | 0.02 | Fixed | 47% | 0.11 | |
| < 100 | 4 | 1.67 (0.84-3.31) | 0.14 | Random | 55% | 0.08 | |
Abbreviations: HR, hazard radio; CI, confidence internal; OS, overall survival; SCC, squamous cell carcinoma; ACC, adenoid cystic carcinomas; LC, laryngeal carcinoma; HPC, hypopharyngeal carcinoma; NPC, nasopharyngeal carcinoma.
SOX2 expression and DFS in HNC patients
A total of six cohorts focused on SOX2 expression and DFS in HNC patients. The pooled estimates demonstrated a significant relationship between elevated SOX2 and poor DFS (HR = 1.54, 95% CI =1.13-2.10, P = 0.006) without significant heterogeneity (I2 = 18%, P = 0.30) (Figure 2B).
SOX2 expression and HNC clinicopathological features
To gain further insight into the value of SOX2 as a biomarker, we investigated the association of positive SOX2 expression with various clinicopathological indicators (Figure 3 and Table S3). A fixed-effect model revealed associations between SOX2 expression and advanced TNM stage (III-IV) (OR = 4.22, 95% CI = 2.62-6.80, P < 0.00001, Figure 3B), lymph node metastasis (OR = 2.25, 95% CI = 1.50-3.35, P < 0.0001, Figure 3D) and distant metastasis (OR = 2.09, 95% CI = 1.31-3.34, P = 0.002, Figure 3F), However, no significant association was observed between SOX2 expression and tumor local recurrence (OR = 0.62, 95% CI = 0.29-1.36, P = 0.24, Figure 3E). A random-effect model revealed an association between SOX2 expression and high tumor grade (T3-T4) (OR=1.86, 95% CI = 1.06-2.18, P = 0.03, Figure 3A) but there was no significant correlation between SOX2 expression and histological grade (poor) (OR = 0.97, 95% CI = 0.51-1.85, P = 0.92, Figure 3C). These findings indicate that SXO2 expression implies a poor prognosis in HNC patients with advanced TNM stage or high tumor grade, as well as serving as an indicator of lymph node and distal organ metastasis.
Figure 3.
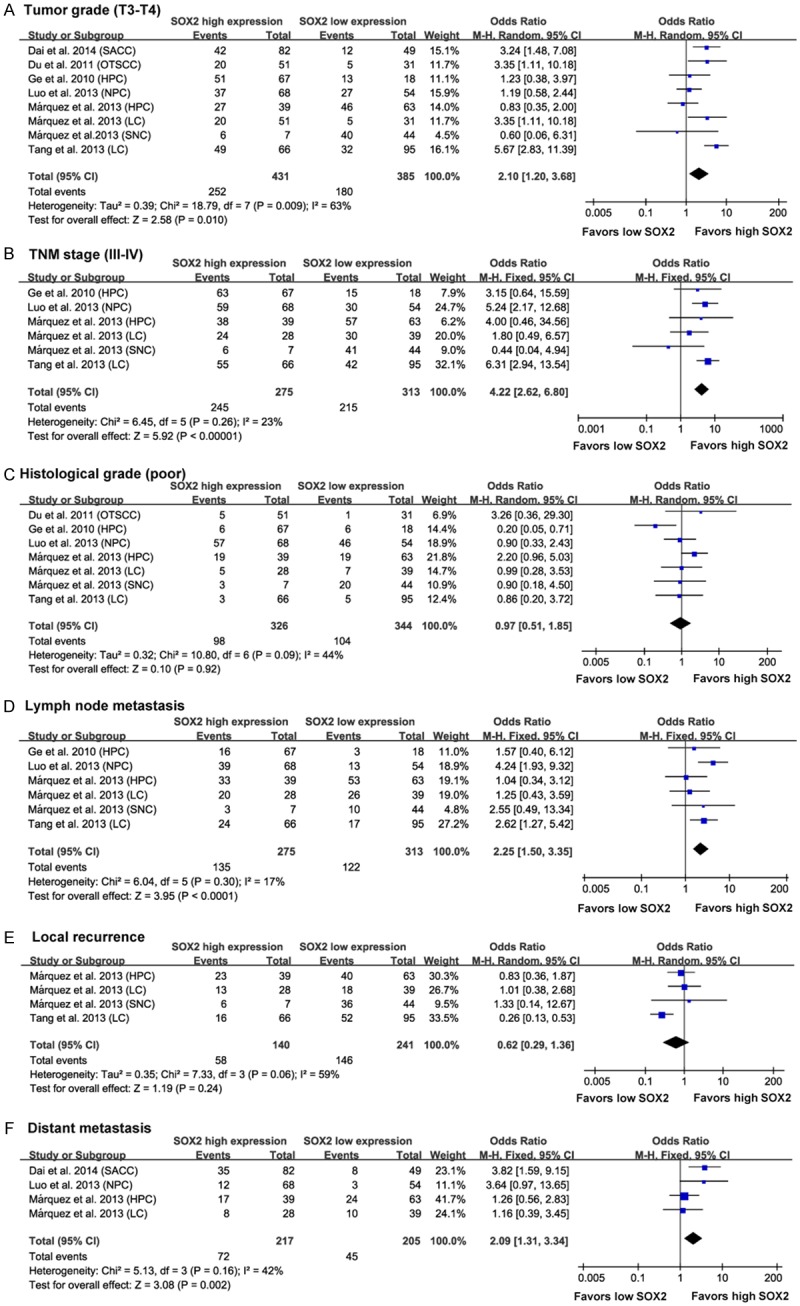
Forest plots showing results of studies on the association between elevated SOX2 and clinicopathological parameters in HNC patients. Forrest plots display the correlation between SOX2 expression and high tumor grade (T3-T4) (A), advanced TNM stage (III-IV) (B), histological grade (poor) (C), lymph node metastasis (D), local recurrence (E) and distant metastasis (F) in HNC. Results are presented as individual and pooled odds ratio (OR), and 95% confidence interval (CI).
Sensitivity analysis
Sensitivity analysis was performed through the sequential omission of individual studies. The corresponding pooled estimates of the relation of SOX2 expression to clinicopathological and prognostic outcomes were not altered significantly for any study factor after sequentially excluding each study, demonstrating that our data are stable and reliable.
Publication bias
A Begg’s funnel plot was presented for the visual assessment of overt publication bias for the included cohorts in SOX2 (Figure 4). The funnel plot did not showed obvious asymmetry for OS (Pr > |Z| = 0.175, Figure 4A) and DFS (Pr > |Z| = 0.452, Figure 4B). The P value of Egger’s test also indicated that there was not any publication bias in OS (P = 0.101) and DFS (P = 0.267) among these included studies. In addition, publication bias was also not observed among studies with regard to clinicopathological indicators (Table S3).
Figure 4.
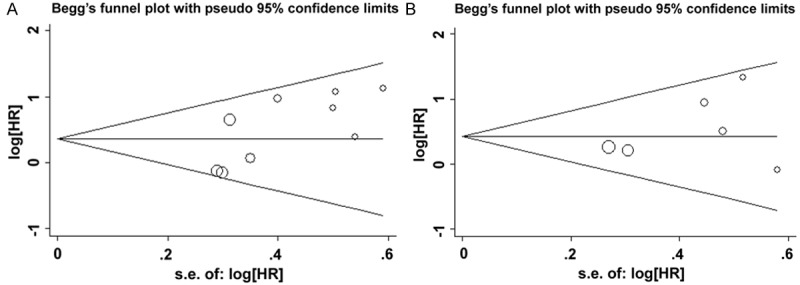
Begg’s funnel plots for the evaluation of potential publication bias in the impact of SOX2 expression on the clinical outcome of HNC. A. Begg’s funnel plots of publication bias test for the overall merged analysis of OS. Each point represents a separate study; B. Begg’s funnel plots of the publication bias test for the overall merged analysis of DFS.
Discussion
CSCs are defined as a small subpopulation of cancer cells that constitute a pool of self-sustaining cells with the exclusive ability to cause the heterogeneous lineages of cancer cells that comprise the tumor [30]. Correlation between the presence of CSCs in HNC and prognosis has been corroborated by numerous studies since 2007, when Prince described CSCs in HNC [6,31]. Recently, there are growing evidences suggest that stable expression of CSC markers in HNC could promote tumor cell growth, anti-apoptosis and metastasis, therefore play an important role in carcinogenesis and contributed to tumor aggressiveness and poor outcome [32].
SOX2 has been proven to be a key regulator for maintaining the pluripotency and self-renewal of CSCs in HNC. The role of the SOX2 in the carcinogenesis is attributed to their properties involved in the regulation of cell differentiation, proliferation, and survival in multiple essential processes [33]. Although overexpressed SOX2 has been wildly demonstrated in HNC, the role of SOX2 as a prognostic marker is still a matter of debate. In order to detect the precise relationship between SOX2 expression and the prognostic significance of HNC, we extracted the eligible data into groups, including DFS, OS and clinicopathological indicators.
In this meta-analysis, we first assessed the association of high SOX2 expression with OS and DFS in HNC patients. Our analysis suggested that elevated SOX2 was associated with poor OS and DFS in the indicated studies. Though with significant heterogeneity, most of the prognostic value was not undermined between SOX2 expression and OS by subgroup analysis based on different histological type, different tumor sites, region of the studied population, median follow-up time and sample size. Taken all these in to consideration, SOX2 was a promising prognostic marker helpful for the clinical decision-making process regarding HNC treatment and outcomes.
Regardless of progress in the HNC treatment recently, the survival rate of five years after diagnosing advanced HNC remains insufficient, approximately 50% [34]. One reason for high mortality associated with the advanced stage HNC is locoregional lymph node metastases due to the presence of a rich lymphatic network and the overall high number of lymph nodes in the neck region [35,36]. More important, the increasingly mortality of HNC should be also ascribable to local recurrence and distant metastasis, which emerged as the predominant cause of death in the face of the achievement of excellent local control for HNC [37]. In the present study, we also carried out pooled analyses of the association between SOX2 expression and clinicopathological features. The results indicated that high expression of SOX2 was closely correlated with high tumor grade, advanced TNM stage, lymph node and distant metastasis.
There has been growing evidences suggest that CSCs potential for epithelium- mesenchymal transition (EMT) during metastasis formation. CSCs might strongly resemble cells that have undergone an EMT, attributing these cells a role in local invasion [38,39]. As a key regulator of CSCs, SOX2 is proposed to play a role in the EMT in HNC, and confer invasive and metastases capacity on tumor cells. Luo [26] indicated overexpression of SOX2 in Nasopharyngeal Carcinoma (NPC) was significantly associated with high expression of N-cadherin, but adversely with low E-cadherin expression. Particularly, the distributions of SOX2 staining were more frequently located in the invasive front of tumors, and these cells often exhibited a fibroblast-like, spindle-shaped phenotype which was correlated strongly with EMT in tumor tissues. In the present analysis, high expression of SOX2 proteins in HNC was correlated significantly with a majority of tumor aggressive behaviors, such as local invasion, lymph node metastasis and distant metastasis. However, there was no significant association between SOX2 expression and tumor local recurrence, which could be ascribe to lack of efficient data focused on this correlation in the candidate studies and further investigation should be conducted to verify their relationship.
Several sources of heterogeneity should be considered in the present study. Pooled HRs from different articles with various cut-off values may partly account for the inter-study heterogeneity. Meanwhile, all of the studies included in our meta-analyses were retrospective and their experimental design may, to some extent, contribute to the heterogeneity. Besides, the heterogeneity could also be attributed to the differences in the histological types, tumor types and their treatments, the sample sizes, the regional distribution, the durations of follow-up and the inconsistency of clinicopathological parameters. We had conducted subgroup analysis and sensitive analysis to evaluate potential sources of bias and the observed inter-study heterogeneity. Furthermore, a meta-regression was also performed to find out the heterogeneity. Unfortunately, there were no variables analyzed in the meta-regression contributed to the heterogeneity.
Results from our study must be interpreted within the limitations of included studies. Firstly, although we strived to extract valid data from survival curves, in which HRs were not directly measured, these indirect data were less reliable than direct data from the original articles because these calculated HRs were the result of univariate analyses and might contain some deviations. Secondly, SOX2 expression in the indicated studies was measured mainly using IHC and these results were strongly dependent upon methodological factors, such as primary antibody and secondary antibody concentration. Meanwhile, there was also a large difference in the definition of cut-off values among the studies, and this can be a source of potential bias. Thirdly, high quality researches with complete reports, including clinicopathological and survival data, were limited, which may compromise our conclusions.
In conclusion, SOX2 expression exhibited the significant association with survival outcome and clinicopathological parameters in HNC. It can be used to figure out the high risk patients who may benefit less from the antitumor therapies and to adjust the management strategy accordingly. Whereas, given the limitation of the current analysis, the large prospective clinical studies based on homogeneous series of patients were needed to further confirm the prognostic value of SOX2.
Acknowledgements
This work was supported by the National Nature Science Foundation of China (Grant No. 81172586).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Bertrand G, Maalouf M, Boivin A, Battiston-Montagne P, Beuve M, Levy A, Jalade P, Fournier C, Ardail D, Magne N, Alphonse G, Rodriguez-Lafrasse C. Targeting head and neck cancer stem cells to overcome resistance to photon and carbon ion radiation. Stem Cell Rev. 2014;10:114–126. doi: 10.1007/s12015-013-9467-y. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez-Marquez R, Llorente JL, Rodrigo JP, Garcia-Pedrero JM, Alvarez-Marcos C, Suarez C, Hermsen MA. SOX2 expression in hypopharyngeal, laryngeal, and sinonasal squamous cell carcinoma. Hum Pathol. 2014;45:851–857. doi: 10.1016/j.humpath.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Cherukuri DP, Nelson MA. Do elevated levels of eicosanoids play a role in head and neck cancer recurrence and metastasis? Implications for prevention and treatment. Cancer Biol Ther. 2004;3:853–854. doi: 10.4161/cbt.3.9.1124. [DOI] [PubMed] [Google Scholar]
- 4.Prince ME, Ailles LE. Cancer stem cells in head and neck squamous cell cancer. J. Clin. Oncol. 2008;26:2871–2875. doi: 10.1200/JCO.2007.15.1613. [DOI] [PubMed] [Google Scholar]
- 5.Szafarowski T, Szczepanski MJ. Cancer stem cells in head and neck squamous cell carcinoma. Otolaryngol Pol. 2014;68:105–111. doi: 10.1016/j.otpol.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007;104:973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhaijee F, Pepper DJ, Pitman KT, Bell D. Cancer stem cells in head and neck squamous cell carcinoma: a review of current knowledge and future applications. Head Neck. 2012;34:894–899. doi: 10.1002/hed.21801. [DOI] [PubMed] [Google Scholar]
- 8.Satpute PS, Hazarey V, Ahmed R, Yadav L. Cancer stem cells in head and neck squamous cell carcinoma: a review. Asian Pac J Cancer Prev. 2013;14:5579–5587. doi: 10.7314/apjcp.2013.14.10.5579. [DOI] [PubMed] [Google Scholar]
- 9.Boumahdi S, Driessens G, Lapouge G, Rorive S, Nassar D, Le Mercier M, Delatte B, Caauwe A, Lenglez S, Nkusi E, Brohee S, Salmon I, Dubois C, del Marmol V, Fuks F, Beck B, Blanpain C. SOX2 controls tumour initiation and cancer stem-cell functions in squamous-cell carcinoma. Nature. 2014;511:246–250. doi: 10.1038/nature13305. [DOI] [PubMed] [Google Scholar]
- 10.Hutz K, Mejias-Luque R, Farsakova K, Ogris M, Krebs S, Anton M, Vieth M, Schuller U, Schneider MR, Blum H, Wagner E, Jung A, Gerhard M. The stem cell factor SOX2 regulates the tumorigenic potential in human gastric cancer cells. Carcinogenesis. 2014;35:942–950. doi: 10.1093/carcin/bgt410. [DOI] [PubMed] [Google Scholar]
- 11.Cai YR, Zhang HQ, Qu Y, Mu J, Zhao D, Zhou LJ, Yan H, Ye JW, Liu Y. Expression of MET and SOX2 genes in non-small cell lung carcinoma with EGFR mutation. Oncol Rep. 2011;26:877–885. doi: 10.3892/or.2011.1349. [DOI] [PubMed] [Google Scholar]
- 12.Gen Y, Yasui K, Nishikawa T, Yoshikawa T. SOX2 promotes tumor growth of esophageal squamous cell carcinoma through the AKT/mammalian target of rapamycin complex 1 signaling pathway. Cancer Sci. 2013;104:810–816. doi: 10.1111/cas.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lengerke C, Fehm T, Kurth R, Neubauer H, Scheble V, Muller F, Schneider F, Petersen K, Wallwiener D, Kanz L, Fend F, Perner S, Bareiss PM, Staebler A. Expression of the embryonic stem cell marker SOX2 in early-stage breast carcinoma. BMC Cancer. 2011;11:42. doi: 10.1186/1471-2407-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun C, Sun L, Li Y, Kang X, Zhang S, Liu Y. Sox2 expression predicts poor survival of hepatocellular carcinoma patients and it promotes liver cancer cell invasion by activating Slug. Med Oncol. 2013;30:503. doi: 10.1007/s12032-013-0503-1. [DOI] [PubMed] [Google Scholar]
- 15.Yang Z, Pan X, Gao A, Zhu W. Expression of Sox2 in cervical squamous cell carcinoma. J BUON. 2014;19:203–206. [PubMed] [Google Scholar]
- 16.Honing J, Pavlov KV, Meijer C, Smit JK, Boersma-van Ek W, Karrenbeld A, Burgerhof JG, Kruyt FA, Plukker JT. Loss of CD44 and SOX2 expression is correlated with a poor prognosis in esophageal adenocarcinoma patients. Ann Surg Oncol. 2014;21(Suppl 4):657–64. doi: 10.1245/s10434-014-3763-x. [DOI] [PubMed] [Google Scholar]
- 17.Toschi L, Finocchiaro G, Nguyen TT, Skokan MC, Giordano L, Gianoncelli L, Perrino M, Siracusano L, Di Tommaso L, Infante M, Alloisio M, Roncalli M, Scorsetti M, Janne PA, Santoro A, Varella-Garcia M. Increased SOX2 gene copy number is associated with FGFR1 and PIK3CA gene gain in non-small cell lung cancer and predicts improved survival in early stage disease. PLoS One. 2014;9:e95303. doi: 10.1371/journal.pone.0095303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y, Huang Y, Huang Y, Chen J, Wang S, Zhou J. The prognostic value of SOX2 expression in non-small cell lung cancer: a meta-analysis. PLoS One. 2013;8:e71140. doi: 10.1371/journal.pone.0071140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dai W, Tan X, Sun C, Zhou Q. High expression of SOX2 is associated with poor prognosis in patients with salivary gland adenoid cystic carcinoma. Int J Mol Sci. 2014;15:8393–8406. doi: 10.3390/ijms15058393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang XB, Shen XH, Li L, Zhang YF, Chen GQ. SOX2 overexpression correlates with poor prognosis in laryngeal squamous cell carcinoma. Auris Nasus Larynx. 2013;40:481–486. doi: 10.1016/j.anl.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J, Chang DY, Mercado-Uribe I, Liu J. Sex-determining region Y-box 2 expression predicts poor prognosis in human ovarian carcinoma. Hum Pathol. 2012;43:1405–1412. doi: 10.1016/j.humpath.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 24.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–2834. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 25.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo W, Li S, Peng B, Ye Y, Deng X, Yao K. Embryonic stem cells markers SOX2, OCT4 and Nanog expression and their correlations with epithelial-mesenchymal transition in nasopharyngeal carcinoma. PLoS One. 2013;8:e56324. doi: 10.1371/journal.pone.0056324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ge N, Lin HX, Xiao XS, Guo L, Xu HM, Wang X, Jin T, Cai XY, Liang Y, Hu WH, Kang T. Prognostic significance of Oct4 and Sox2 expression in hypopharyngeal squamous cell carcinoma. J Transl Med. 2010;8:94. doi: 10.1186/1479-5876-8-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Liang Y, Chen Q, Xu HM, Ge N, Luo RZ, Shao JY, He Z, Zeng YX, Kang T, Yun JP, Xie F. Prognostic significance of SOX2 expression in nasopharyngeal carcinoma. Cancer Invest. 2012;30:79–85. doi: 10.3109/07357907.2011.630049. [DOI] [PubMed] [Google Scholar]
- 29.Du L, Yang Y, Xiao X, Wang C, Zhang X, Wang L, Zhang X, Li W, Zheng G, Wang S, Dong Z. Sox2 nuclear expression is closely associated with poor prognosis in patients with histologically node-negative oral tongue squamous cell carcinoma. Oral Oncol. 2011;47:709–713. doi: 10.1016/j.oraloncology.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 30.Clay MR, Tabor M, Owen JH, Carey TE, Bradford CR, Wolf GT, Wicha MS, Prince ME. Single-marker identification of head and neck squamous cell carcinoma cancer stem cells with aldehyde dehydrogenase. Head Neck. 2010;32:1195–1201. doi: 10.1002/hed.21315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han J, Fujisawa T, Husain SR, Puri RK. Identification and characterization of cancer stem cells in human head and neck squamous cell carcinoma. BMC Cancer. 2014;14:173. doi: 10.1186/1471-2407-14-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koukourakis MI, Giatromanolaki A, Tsakmaki V, Danielidis V, Sivridis E. Cancer stem cell phenotype relates to radio-chemotherapy outcome in locally advanced squamous cell head-neck cancer. Br J Cancer. 2012;106:846–853. doi: 10.1038/bjc.2012.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bourguignon LY, Wong G, Earle C, Chen L. Hyaluronan-CD44v3 interaction with Oct4-Sox2-Nanog promotes miR-302 expression leading to self-renewal, clonal formation, and cisplatin resistance in cancer stem cells from head and neck squamous cell carcinoma. J Biol Chem. 2012;287:32800–32824. doi: 10.1074/jbc.M111.308528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gallo O, Santoro R, Fiorini FR, Meccariello G, Lagana RM, Paiar F, Maio V. Prognostic role of internal jugular vein preservation in neck dissection for head and neck cancer. J Surg Oncol. 2013;108:579–583. doi: 10.1002/jso.23436. [DOI] [PubMed] [Google Scholar]
- 35.Vermorken JB, Specenier P. Optimal treatment for recurrent/metastatic head and neck cancer. Ann Oncol. 2010;21(Suppl 7):vii252–261. doi: 10.1093/annonc/mdq453. [DOI] [PubMed] [Google Scholar]
- 36.Liauw SL, Mancuso AA, Amdur RJ, Morris CG, Villaret DB, Werning JW, Mendenhall WM. Postradiotherapy neck dissection for lymph node-positive head and neck cancer: the use of computed tomography to manage the neck. J. Clin. Oncol. 2006;24:1421–1427. doi: 10.1200/JCO.2005.04.6052. [DOI] [PubMed] [Google Scholar]
- 37.Kramer RH, Shen X, Zhou H. Tumor cell invasion and survival in head and neck cancer. Cancer Metastasis Rev. 2005;24:35–45. doi: 10.1007/s10555-005-5046-2. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Z, Dong Z, Lauxen IS, Filho MS, Nor JE. Endothelial cell-secreted EGF induces epithelial to mesenchymal transition and endows head and neck cancer cells with stem-like phenotype. Cancer Res. 2014;74:2869–2881. doi: 10.1158/0008-5472.CAN-13-2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen C, Wei Y, Hummel M, Hoffmann TK, Gross M, Kaufmann AM, Albers AE. Evidence for epithelial-mesenchymal transition in cancer stem cells of head and neck squamous cell carcinoma. PLoS One. 2011;6:e16466. doi: 10.1371/journal.pone.0016466. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


