Abstract
The interaction between compatible pollen grains and the female stigma of Gladiolus gandavensis has been used as a model system for investigation of cell recognition in plants. The molecular architecture of the receptive stigma surface has been investigated, and determinants binding to both concanavalin A and β-glucosyl artificial carbohydrate antigen, as well as esterase activity, have been characterized, and conditions for their isolation have been established. The stigma surface, before and after modification, was found to bind 125I-labeled proteins nonspecifically. Pollen tube penetration of the papillar cuticle is prevented when the receptor sites for concanavalin A are occupied. The concanavalin-A-binding determinants of the stigma surface have been fractionated to reveal several glycoproteins in the molecular weight range 43,000-93,000 and a group of glycolipids of molecular weight approximately 22,000. These results are interpreted in terms of two major recognition events regulating pollination.
Keywords: pollen-stigma interactions, concanavalin A, cell surface receptors, binding sites, incompatibility
Full text
PDF
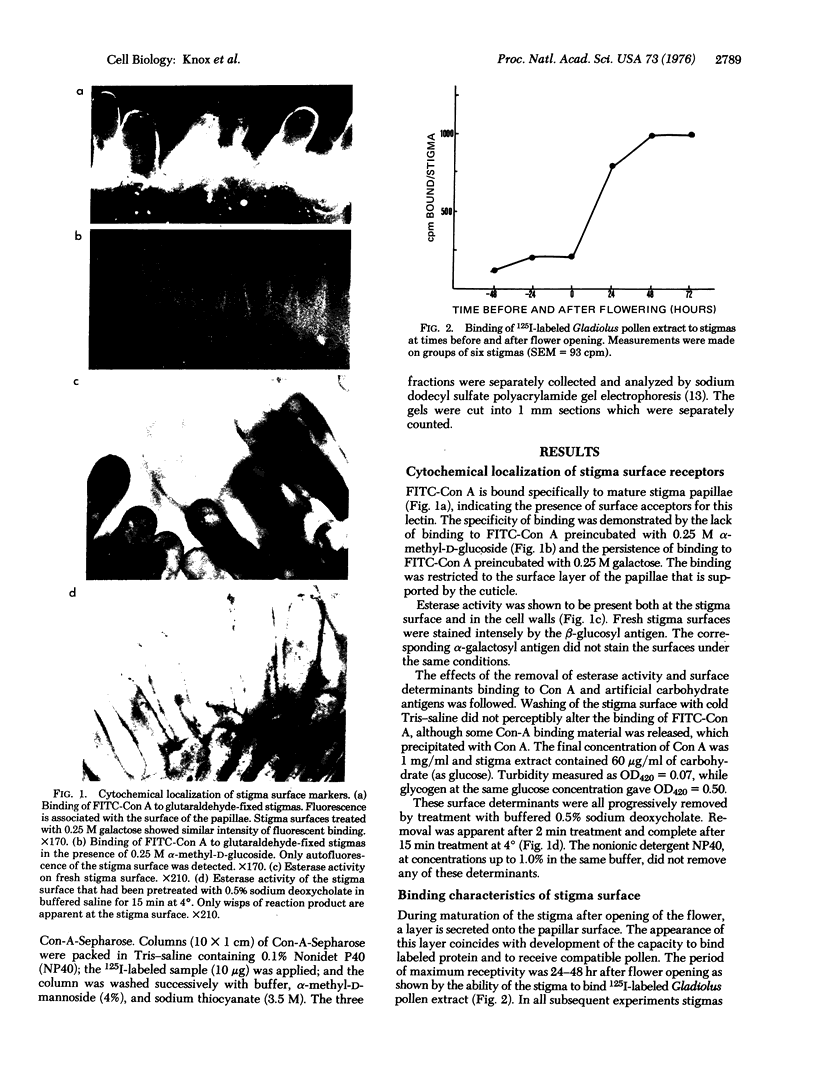
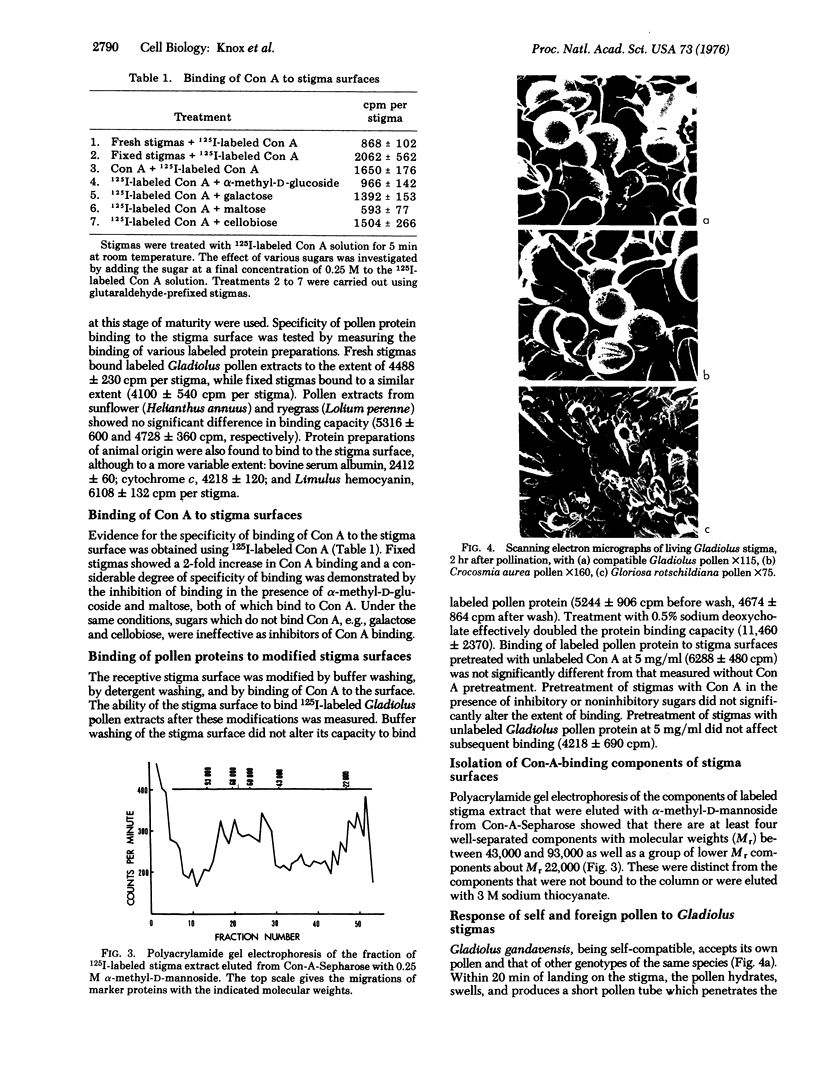


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burnet F. M. "Self-recognition" in colonial marine forms and flowering plants in relation to the evolution of immunity. Nature. 1971 Jul 23;232(5308):230–235. doi: 10.1038/232230a0. [DOI] [PubMed] [Google Scholar]
- Clarke A. E., Knox R. B., Jermyn M. A. Localization of lectins in legume cotyledons. J Cell Sci. 1975 Oct;19(1):157–167. doi: 10.1242/jcs.19.1.157. [DOI] [PubMed] [Google Scholar]
- De Luca D., Decker J., Miller A., Sercarz E. Antigen binding to lymphoid cells from unimmunized mice: high frequency of beta-galactosidase binding cells at optimal conditions. Cell Immunol. 1974 Jan;10(1):1–18. doi: 10.1016/0008-8749(74)90146-4. [DOI] [PubMed] [Google Scholar]
- Knox R. B. Pollen-wall proteins: localization, enzymic and antigenic activity during development in Gladiolus (iridaceae). J Cell Sci. 1971 Jul;9(1):209–237. doi: 10.1242/jcs.9.1.209. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YARIV J., RAPPORT M. M., GRAF L. The interaction of glycosides and saccharides with antibody to the corresponding phenylazo glycosides. Biochem J. 1962 Nov;85:383–388. doi: 10.1042/bj0850383. [DOI] [PMC free article] [PubMed] [Google Scholar]