Abstract
Background and aims: Biliary tract caner (BTC) is one of rare malignant disease with poor prognosis. Gemcitabine has been widely used as chemotherapeutic agent for advanced BTC treatment. Several molecules involved in gemcitabine metabolism, including human equilibrative nucleoside transporter (hENT1) and ribonucleotide reductase subunit M1 (RRM1), have been investigated as predictive biomarkers of chemotherapy efficacy. The aim of present study is to determine whether hENT1 and RRM1 could be used as the biomarkers to assess the efficacy of chemotherapy and predict survival in patients with advanced BTC. Methods: The analysis was performed on samples from 44 patients with unresectable or recurrent BTC who were treated with gemcitabine as first-line therapy. We determined levels of hENT1 and RRM1 with immunohistochemistry (IHC). Also, its prognostic and predictive role on tumor response and several clinical factors for survival were evaluated with Kaplan-Meier or Cox analysis. Results: The patients who were clinical benefit (partial response [PR] or stable disease [SD]) had high level of hENT1 (P = 0.046) and low level of RRM1 (P = 0.033). Moreover, hENT1 expression was a significant factor for progression free survival (PFS) (P = 0.005) and overall survival (OS) (P = 0.048) in Cox univariate analysis. Also, hENT1 was an independent prognostic factor of OS based on Cox multivariate analysis (P = 0.005). Conclusions: The expression of hENT1 and RRM1 was associated with gemcitabine efficacy. hENT1 was one of reliable predictive marker of survival in patients with advanced BTC patients.
Keywords: Biliary tract cancer, gemcitabine, human equilibrative nucleoside transporter 1, ribonucleotide reductase subunit M1
Introduction
Biliary tract carcinomas (BTC) are a group of tumors arising from the epithelial cells of intra- and extra-hepatic biliary ducts and the gallbladder, characterised by poor prognosis [1]. Gallbladder carcinoma and extrahepatic bile ducts carcinoma (cholangiocarcinoma) are the most common biliary tract cancer. Cholangiocarcinoma is classified into intrahepatic and extrahepatic disease according to its anatomical location within the biliary tree [2]. Surgical resection remains the only potentially curative therapeutic strategy, however, more than half of patients present with advanced stage and lost chance for surgery. The 5-year overall survival is about 20-32% for patients with intrahepatic cholangiocarcinoma [3]. Adjuvant chemotherapy has been already to improve chance of cure, yet there is no established standard chemotherapy until to now. The combination chemotherapy with gemcitabine or a platinum-based agent is regarded as a standard treatment, even if those treatment remains dismal [4]. To date, we still lack an effective therapy to improve survival of patients. Thus, clinical markers that can predict response to the specific therapy and the prognosis should be explored to guide individual therapy.
The human equilibrative nucleoside transporter 1 (hENT1) is a ubiquitous protein and is the major transporter by which gemcitabine enters cultured human cells and hematopoietic progenitor cells [5,6]. When gemcitabine is transported into cell by hENT1, is phosphorylated by deoxycytidine kinase (dCK) to its active diphosphate and triphosphate in a rate-limiting step. As a result, the DNA chain termination, is a major mechanism underlying the cytotoxicity of gemcitabine [7]. Ribonucleotide reductase subunit M1 (RRM1) is component of the ribonucleotide reductase (RNR) complex, locus on human chromosome 11p15.5 shows frequent loss of heterozygosity in cancer patients [8]. RRM1 depletion affects genetic instability during tumor initiation [9]. Most importantly, RRM1 expression levels have been extensively as markers of DNA repair capacity in tumor cells and is generally associated with sensitivity to platinum [10]. Of which has been validated as a predictive biomarker for response to gemcitabine (G) using in situ protein levels in a phase III trial for patients with non-small cell lung cancer [11].
The hENT1 and RRM1 had been proposed as one of predictive biomarkers for chemotherapy sensitivity in patients with pancreatic cancer and lung cancer [6,11,12]. A research about BTC have determined prognostic predictive values of hENT1 and RRM1 in chemotherapy based on gemcitabine alone [13]. It indicated that hENT1 is one of most reliable predictive marker of survival in patients with advanced BTC. At present study, we assessed the expressions of hENT1 and RRM1 in tumor samples from 44 patients with advanced BTC, who received first-line gemcitabine alone, and combined with 5-fluorouracil or cis-platinum. The aim of this study was to investigate the association between the expression of those proteins and chemotherapy effectiveness.
Material and methods
Subjects
We performed a retrospective evaluation the patients with histologically confirmed unresectable or postoperative advanced adenocarcinoma of biliary tract. In the end, a total of 44 patients treated with first-line gemcitabine monotherapy, or combined with 5-fluorouracil or cis-platinum, at Tianjin Medical University Cancer Institute and Hospital, were included between January 2006 and January 2013. BTC patients comprised extrahepatic bile duct cancer and gallbladder cancer. All surgical specimens were reviewed and classified according to the WHO classification by an experienced pathologist who was unknown of clinical or imaging findings. Patients’ inclusion and exclusion criteria are as followings: (1) all histological types of BTC patients were defined as adenocarcinoma; (2) were treated with gemcitabine as one of major firs-line agent and receipted more than two cycles chemotherapy treatment; (3) the patients died after surgery less than one month were excluded. Data from the medical records were collected, including factors potentially considered to be of prognostic value: age at initial treatment, sex, tumor location (intra or extrahepatic cholangiocarcinoma or gallbladder), number of chemotherapy cycles. Pathologic tumor-node-metastasis (TNM) stages were established using the International System for staging bile duct cancer adopted by the American Joint Committee on cancer and International Union against Cancer.
Efficacy assessments were conducted by computed tomography (CT) or magnetic resonance imaging (MRI) every six weeks, according to RECIST (version 1.0).
Immunohistochemistry
Fresh serial sections were cut at 2 μm from the original diagnostic paraffin-embedded tissue blocks, mounted on slides, dried overnight at 37°C, deparaffinized in xylene and rehydrated through a graded series of alcohol solutions. The slides were treated with 3% hydrogen peroxide in methanol for 15 minutes to block endogenous peroxide activity. To retrieve the antigenicities, slides were heated for 10 minutes at Antigen retrieval was performed in 140°C for 2 minutes by autoclave in Target Retrieval Solution (Zhongshan Golden Bridge Co., Beijing, China) and cooled at room temperature for at least 30 minutes. After washing with phosphate-buffered saline (PBS), the sections were incubated in blocking serum for 30 minutes at room temperature to reduce nonspecific binding, followed by incubation with anti-hENT1 rabbit polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) with a dilution 1:100, RRM1 rabbit monoclonal antibody (Abcam Co., Cambridge, UK) with a dilution 1:400. After that, the sections were incubated in a humidified chamber overnight at 4°C. Then, the sections were incubated with horseradish peroxidase (HRP) conjugated mouse anti-rabbit IgG antibody complex (Zhongshan Golden bridge Co., Beijing, China) for 30 minutes at 37°C with a dilution 1:500. A diaminobenzidine (DAB) liquid chromagen was placed on the samples for visualizing proteins. The sections were rinsed, counterstained with hematoxylin, dehydrated through graded alcohol and xylene, and cover slipped.
Immunohistochemical evaluation
As previously reported [14], each tissue sample was scored according to the intensity of nuclear or cytoplasmic staining (no staining, 0; weak staining, 1+; moderate staining 2+; and strong staining, 3+), and percentage of positive cells (no staining, 0; < 10%, 1; 11%-50%, 2; > 50%, 3). The ratings were confirmed by two independent pathologists. An immunoreactive score was calculated by multiplying the staining intensity by the percentage of cells showing staining. We defined that a score < 6 as low expression (negative), and ≥ 6 as high expression (positive).
Statistical analysis
The significance of the correlation between expressions and clinicopathological characteristics was assessed by the chi-square test (Fisher’s exact test). Survival probabilities were calculated using the Kaplan-Meier method. To test for independent relevance of the candidate prognostic factors, a univariate or multivariate Cox proportional hazards regression model was fit for hENT1 and RRM1. Progression-free survival (PFS) according to clinical judgement was measured from the date of first chemotherapy to the date of first treatment progression or death without any progressive disease. Overall survival was measured from the date of first treatment with chemotherapy to the date of death or last follow-up evaluation. Data on survivors were censored at the last follow-up. For all tests, two-sided P values < 0.05 were defined as statistically significant. All statistical analysis was performed using SPSS 15.0 statistical software (SPSS, Inc, Chicago, Illinois, U S A).
Results
Clinicopathological characteristics of enrolled BTC patients and association with expression levels of hENT1 and RRM1
The basic clinicopathological characteristic of 44 patients was summarized in Table 1. As is shown, there were 20 male and 24 female patients with a median age of 58 years old (range 37-72 years old). Patients received a median number of three cycles of chemotherapy (range 2-8). There were 29 BTC patients with TNM stage II-III, and 15 patients with TNM stage IV at initial diagnosis. Among of them, there were 20 patients with cancerous lesions located in cholangiocarcinoma (CC) and 24 patients with cancerous lesions located in gallbladder (GB). The major site of metastasis included liver, lymph nodes and pancreas. 16 patients suffer from liver metastasis. And 9 patients had liver combined with lymph node metastasis. 16 patients had other sites of metastasis, of which included lung, pancreas, pleura and so on. The last follow-up date was on April 1, 2014. The median progression-free survival time was 5 months (range 1-18 months). There were 35 patients died of tumor progression. The median overall survival was 8.5 months (range 2-26 months). All of 44 patients had been successfully prepared cancerous samples, and evaluated the expression of hENT1 and RRM1 by immunohistochemistry.
Table 1.
Clinicopathological characteristics based on RRM1 and hENT-1 expression for advanced biliary tract cancer patients
| Characteristics | Total | RRM1 | hENT-1 | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Negative | Positive | P | Negative | Positive | P | ||
| Age | |||||||
| < 58 | 23 (52.27%) | 6 (13.64%) | 17 (38.63%) | 10 (22.73%) | 13 (29.55%) | ||
| ≥ 58 | 21 (47.73%) | 9 (20.45%) | 12 (27.27%) | 0.241 | 8 (18.18%) | 13 (29.55%) | 0.717 |
| Sex | |||||||
| Female | 20 (45.45%) | 9 (20.45%) | 11 (25.00%) | 13 (29.55%) | 7 (15.91%) | ||
| Male | 24 (54.55%) | 6 (13.64%) | 18 (40.91%) | 0.163 | 5 (11.36%) | 19 (43.18%) | 0.003 |
| TNM stage | |||||||
| II-III | 28 (63, 63%) | 11 (25.00%) | 17 (38.63%) | 11 (25.00%) | 17 (38.63%) | ||
| IV | 16 (36.37%) | 4 (9.10%) | 12 (27.27%) | 0.336 | 7 (15.91%) | 9 (20.45%) | 0.772 |
| Tumor types | |||||||
| CC | 20 (45.45%) | 9 (20.45%) | 11 (25.00%) | 0.163 | 10 (22.73%) | 10 (22.73%) | |
| GB | 24 (54.55%) | 6 (13.64%) | 18 (40.91%) | 8 (18.18%) | 16 (36.37%) | 0.263 | |
| Metastasis site | |||||||
| Liver | 25 (56.82%) | 7 (15.91%) | 18 (40.91%) | 0.328 | 7 (15.91%) | 18 (40.91%) | 0.046 |
| Others | 19 (43.18%) | 8 (18.18%) | 11 (25.00%) | 11 (25.00%) | 8 (18.18%) | ||
CC, Cholangiocarcinoma; GB, Gallbladder.
There were 22 BTC patients with RRM1 positive expression (scores ≥ 6) and 26 BTC patients with hENT1 positive expression. Examples of positive and negative tumor staining are shown in Figure 1A-D and Figure 2A-D. RRM1 immunostaining was localized predominantly in the membrane and cytoplasmic. While hENT1 was expressed in nucleus and also in cytoplasmic (Figure 2). We applied chi-square test to identify the significances between RRM1, hENT1 expression and clinicopathological characteristic. As shown in Table 1, the expressions of hENT1 were associated with age and metastasis site. In the rest of parameters failed to show significances in comparing with the expression of RRM1 or hENT1.
Figure 1.
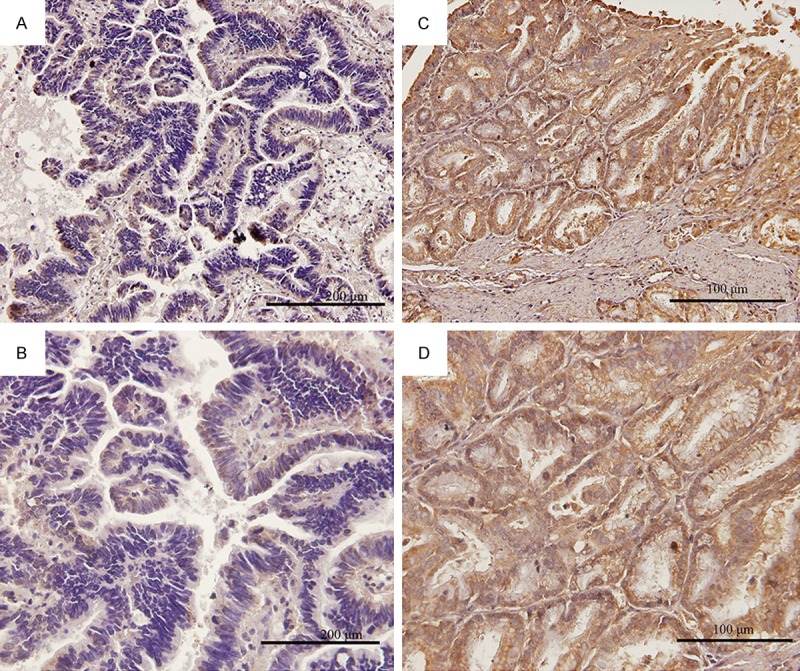
Immunohistochemical analysis of human equilibrative nucleoside transporter 1 (hENT1) expressions in biliary tract cancer tissues. Representative immunohistochemical results, negative staining (A, magnification ×200) and (B, magnification ×400), positive staining (C, magnification ×200) and (D, magnification ×400).
Figure 2.
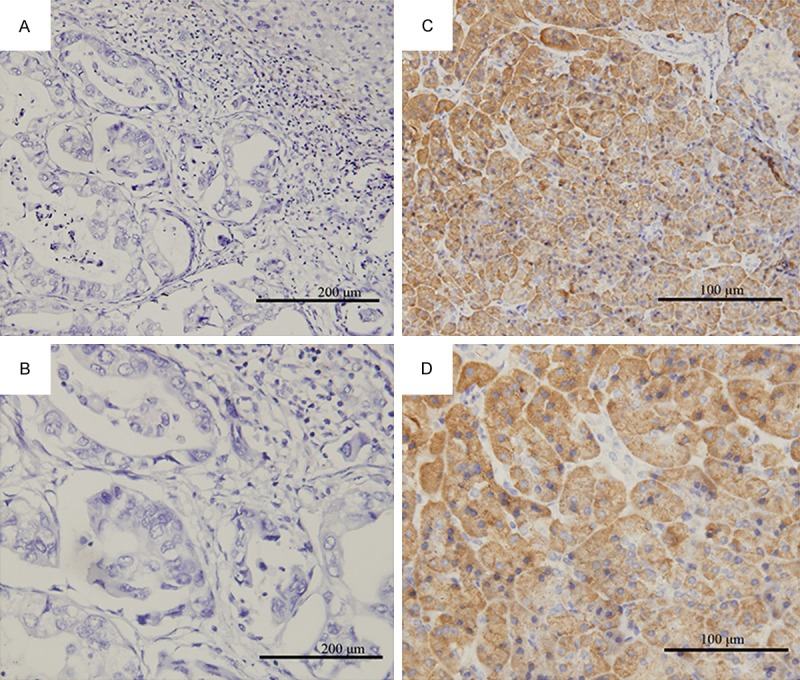
Immunohistochemical analysis of ribonucleotide reductase subunit M1 (RRM1) expressions in biliary tract cancer tissues. Representative immunohistochemical results, negative staining (A, magnification ×200) and (B, magnification ×400), positive staining (C, magnification ×200) and (D, magnification ×400).
Chemotherapy effectiveness and expression of RRM1 and hENT1
The relationships of chemotherapy effectiveness and expression of RRM1 and hETN1 are summarized in Table 2. At present study, 9 patients with BTC were treated with gemcitabine, other 35 patients were used the combination chemotherapy regiments, of which were combined with 5-fluorouracil (FU, 5 patients) or cis-platinum (P, 30 patients). In total, 25 patients were partial response (PR) or stable disease (SD). Interestingly, expression of RRM1 was significantly lower in patient with PR or SD than those with progression of disease (PD). On the other hand, expression of hENT1 was significantly higher in the patients with PR or SD. In view of this, both RRM1 and hENT1 might have an important role in affecting chemotherapy efficacy of advanced BTC with significantly difference.
Table 2.
RRM1 and hENT-1 expression association with chemotherapy response
| Clinical outcomes after chemotherapy | |||
|---|---|---|---|
|
|
|||
| Variables | SD+PR | PD | P |
| G | 5 (11.36%) | 4 (9.10%) | |
| G+FU/G+P | 4 (9.10%)/16 (36.37%) | 1 (2.27%)/14 (31.82%) | 0.932 |
| RRM1 | |||
| Negative | 16 (36.37%) | 6 (13.64%) | |
| Positive | 9 (20.45%) | 13 (29.55%) | 0.033 |
| hENT-1 | |||
| Negative | 7 (15.91%) | 11 (25.00%) | |
| Positive | 18 (40.91%) | 8 (18.18%) | 0.046 |
G, gemcitabine; FU, 5-fluorouracil; P, cis-platinum; PR, partial response; SD, stable disease; PD, progression disease.
Survival analysis according to RRM1 and hENT1 expression
Next, Kaplan-Meier was used to analyse PFS and OS. Those patients with higher hENT1 had a longer PFS in comparing with those low levels (P = 0.002, P = 0.135, respectively) (Figure 3A). Specifically, the median PFS in high levels of hENT1 staining patients was 5 months, as compared to 4 months in those with low hENT1 expression. Besides, the median OS in high levels of hENT1 staining patients were 13 months, while the patients with low hENT1 levels were 7 months (Figure 3B). However, it failed to reach statistical difference. Those patients with low expression RRM1 showed a longer PFS (Figure 4A) and OS (Figure 4B) time, but this increase did not reach the statistical significance. Then, univariate analysis showed that the expression of hENT1 was associated significantly with PFS (P = 0.005) and OS (P = 0.048) (Table 3). More importantly, multivariate analysis indicated that the expression of hENT1 was associated significantly with PFS (P = 0.005), which indicated that hENT1 was an independently factor of PFS for the patients with advanced BTC. However, in the regardless of parameters, age, sex, TNM stages, chemotherapy, and tumor type were not associated with OS (Table 3).
Figure 3.
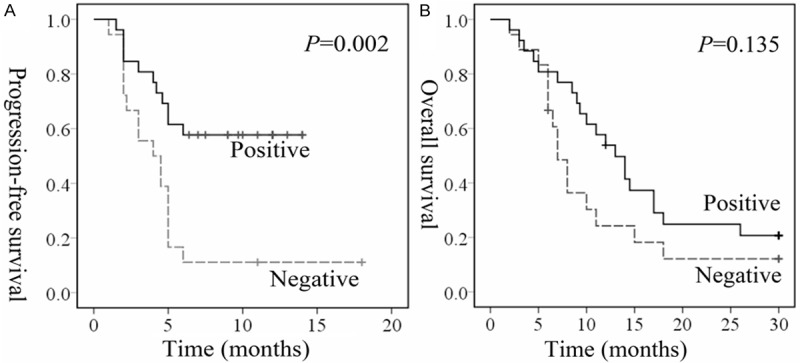
Time to progression in biliary tract cancer patients receiving chemotherapy according to hENT1 expression levels by tumor cells. Kaplan-Meier estimates of progression free survival (PFS) (A) and overall survival (OS) (B).
Figure 4.
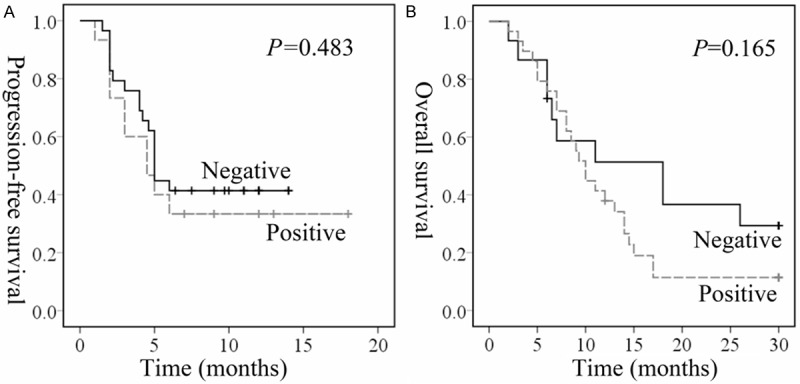
Time to progression in biliary tract cancer patients receiving chemotherapy according to RRM1 expression levels by tumor cells. Kaplan-Meier estimates of progression free survival (PFS) (A) and overall survival (OS) (B).
Table 3.
Cox univariate and multivariate analysis in progression-free survival and overall survival
| Variables | Progression-free survival | Overall survival | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Univariate | Multivariate | Univariate | Multivariate | |||
|
|
|
|||||
| P | HR | P | P | HR | P | |
| Age (< 58/≥ 58) | 0.742 | 0.881 | NA | 0.710 | 1.177 | NA |
| Sex (female/male) | 0.122 | 0.549 | NA | 0.059 | 0.426 | NA |
| TNM stage (II/III-IV) | 0.890 | 1.033 | 0.850 | 0.611 | 1.148 | 0.466 |
| Tumor types (CC/GB) | 0.463 | 1.328 | 0.599 | 0.240 | 1.673 | 0.868 |
| Chemotherapy method | 0.547 | 0.946 | 0.927 | 0.120 | 0.669 | 0.114 |
| RRM1 (N/P) | 0.507 | 0.767 | 0.718 | 0.945 | 0.968 | 0.173 |
| hENT-1 (N/P) | 0.005 | 0.330 | 0.005 | 0.048 | 0.413 | 0.143 |
CC, Cholangiocarcinoma; GB, Gallbladder; N, negative; P, Positive; HR, Hazard ratio.
Discussion
BTC are invasive carcinomas deriving from the epithelial lining of the gallbladder and bile ducts. Patients with advanced BTC have a poor prognosis with a median OS less than one year [15]. In this retrospective study, we compared the chemotherapy effectiveness between the patients with positive and negative of RRM1 or hENT1. We proved that patients with PR or SD after chemotherapy had low RRM1 and high hENT1 levels with significantly difference in comparing those PD patients. Although, Kaplan-Meier and Cox analysis did not indicate that RRM1 was associated with PFS or OS. The expression of hENT1 was a prognosis factor for PFS and OS according to univariate Cox analysis.
In many clinical trials conducted in BTC patients, a significant but modest efficacy of classical cytotoxic chemotherapy has been achieved. Literature data suggest that gemcitabine and gemcitabine-based platinum regimens offer a real but slight advantage over other regimens [16,17]. Only a minority of patients really benefits from chemotherapy in term of response rate and mostly in term of survival. In view of this reason, it is now necessary to find molecular or genetic factors predictive of response to chemotherapy, to better select proper patients with proper treatment.
hENT1 is the primary gatekeeper for intracellular uptake of gemcitabine, and RRM1 related to gemcitabine metabolism after intracellular entry. Expression of hENT1 and RRM1 might be as the indictors to assess efficacy of adjuvant gemcitabine-based chemotherapy for pancreatic carcinoma [18]. Previously studies had showed that hENT1 in tumor tissues was a favorable factor for BTC patients [5]. Few studies indicated the relationship of chemotherapy efficiency and hENT1 and RRM1 expression in patients with BTC. Only one study was executed by Murata et al. that included 28 patients with advanced BTC, determined the expression hENT1 and RRM1 as predictive factors for whom treated with gemcitabine alone. They found that hENT1 other than RRM1 was the most reliable predictive of survival [13]. This is consistency with our findings. We observed that the patients with high levels of hENT1 favorable disease response. According to previous research, which could be partially explanation by hENT1 promoting the transfer of chemotherapy drugs to tumor cells. Furthermore, one of characteristics of our cohort was that most of advanced BTC patients with metastasis. The patient with liver metastasis inclined to have high hENT1 levels. In other word, hENT1 might play an important role in regulating cancer cell migrate to liver.
Although, RRM1 did not show have a potential to be one of prognostic factor for BTC. After chemotherapy, patients with SD or PR had a low RRM1 expression levels. Of those findings might indicate that RRM1, not like hENT1, had a reverse role in regulating chemotherapy drugs transport in cancer cells. Except pancreatic cancer, several studies had explored the prognostic potentials in predict prognosis of non-small cell lung cancer [19-21], breast cancer [22], and myeloid leukemia [23]. As a result, RRM1 was not a prognostic biomarker for breast cancer. However, the expression of RRM1 protein or SNP variations could be severe as one of biomarkers for lung cancer and myeloid leukemia. Besides, gemcitabine diphosphate inhibits ribonucleotide reductase through binding to the active site of RRM1 leading to depletion of the deoxynucleotide pool and halt of DNA synthesis [24,25]. In view of this, the overexpression of RRM1 might prevent gemcitabine to halt the DNA synthesis and bring with poor response to advanced BTC patients.
However, the limitation of is that present study is retrospective evaluation with a small cohort. Prospective investigation including an adequate number of samples is needed to confirm the importance of chemotherapy sensitivity-related biomarkers. In addition, the relationships between chemotherapy effects in BTC and other gemcitabine sensitivity-related gene products such as RRM2, cytidine deaminase, and human concentrative nucleoside transporter 1 are remained to be elucidated for further investigations. In conclusion, we have shown that hENT1 and RRM1 were associated with gemcitabine response. The hENT1 levels were higher while RRM1 levels were lower in patients with PR or SD for gemcitabine chemotherapy. Moreover, hENT1 was an independent prognostic factor for advanced BTC patients.
Acknowledgements
Thanks for the support of National Natural Science Foundation of China (No. 81372394); National Science and Technology Major Project (No. 2013ZX09303001); Tianjin Municipal Health Bureau Science & Technology Foundation (2013KZ095); Tianjin Medical University Science Foundation (2013ky03).
Disclosure of conflict of interest
None.
References
- 1.Marino D, Leone F, Cavalloni G, Cagnazzo C, Aglietta M. Biliary tract carcinomas: from chemotherapy to targeted therapy. Crit Rev Oncol Hematol. 2013;85:136–148. doi: 10.1016/j.critrevonc.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Kaira K, Sunose Y, Ohshima Y, Ishioka NS, Arakawa K, Ogawa T, Sunaga N, Shimizu K, Tominaga H, Oriuchi N, Itoh H, Nagamori S, Kanai Y, Yamaguchi A, Segawa A, Ide M, Mori M, Oyama T, Takeyoshi I. Clinical significance of L-type amino acid transporter 1 expression as a prognostic marker and potential of new targeting therapy in biliary tract cancer. BMC cancer. 2013;13:482. doi: 10.1186/1471-2407-13-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirano S, Kondo S, Tanaka E, Shichinohe T, Tsuchikawa T, Kato K, Matsumoto J, Kawasaki R. Outcome of surgical treatment of hilar cholangiocarcinoma: a special reference to postoperative morbidity and mortality. J Hepatobiliary Pancreat Sci. 2010;17:455–462. doi: 10.1007/s00534-009-0208-1. [DOI] [PubMed] [Google Scholar]
- 4.Woo SM, Lee WJ, Kim JH, Kim DH, Han SS, Park SJ, Kim TH, Lee JH, Koh YH, Hong EK. Gemcitabine plus cisplatin versus capecitabine plus cisplatin as first-line chemotherapy for advanced biliary tract cancer: a retrospective cohort study. Chemotherapy. 2013;59:232–238. doi: 10.1159/000354539. [DOI] [PubMed] [Google Scholar]
- 5.Santini D, Schiavon G, Vincenzi B, Cass CE, Vasile E, Manazza AD, Catalano V, Baldi GG, Lai R, Rizzo S, Giacobino A, Chiusa L, Caraglia M, Russo A, Mackey J, Falcone A, Tonini G. Human equilibrative nucleoside transporter 1 (hENT1) levels predict response to gemcitabine in patients with biliary tract cancer (BTC) Curr Cancer Drug Targets. 2011;11:123–129. doi: 10.2174/156800911793743600. [DOI] [PubMed] [Google Scholar]
- 6.Mori R, Ishikawa T, Ichikawa Y, Taniguchi K, Matsuyama R, Ueda M, Fujii Y, Endo I, Togo S, Danenberg PV, Shimada H. Human equilibrative nucleoside transporter 1 is associated with the chemosensitivity of gemcitabine in human pancreatic adenocarcinoma and biliary tract carcinoma cells. Oncol Rep. 2007;17:1201–1205. [PubMed] [Google Scholar]
- 7.Li Y, Gu WJ, Liu HL. Induction of pancreatic cancer cell apoptosis and enhancement of gemcitabine sensitivity by RAP80 siRNA. Dig Dis Sci. 2012;57:2072–2078. doi: 10.1007/s10620-012-2132-5. [DOI] [PubMed] [Google Scholar]
- 8.Kim SH, Park ER, Joo HY, Shen YN, Hong SH, Kim CH, Singh R, Lee KH, Shin HJ. RRM1 maintains centrosomal integrity via CHK1 and CDK1 signaling during replication stress. Cancer Lett. 2014;346:249–56. doi: 10.1016/j.canlet.2013.12.031. [DOI] [PubMed] [Google Scholar]
- 9.Mannava S, Moparthy KC, Wheeler LJ, Natarajan V, Zucker SN, Fink EE, Im M, Flanagan S, Burhans WC, Zeitouni NC, Shewach DS, Mathews CK, Nikiforov MA. Depletion of deoxyribonucleotide pools is an endogenous source of DNA damage in cells undergoing oncogene-induced senescence. Am J Pathol. 2013;182:142–151. doi: 10.1016/j.ajpath.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Besse B, Olaussen KA, Soria JC. ERCC1 and RRM1: ready for prime time? J. Clin. Oncol. 2013;31:1050–1060. doi: 10.1200/JCO.2012.43.0900. [DOI] [PubMed] [Google Scholar]
- 11.Bepler G, Williams C, Schell MJ, Chen W, Zheng Z, Simon G, Gadgeel S, Zhao X, Schreiber F, Brahmer J, Chiappori A, Tanvetyanon T, Pinder-Schenck M, Gray J, Haura E, Antonia S, Fischer JR. Randomized international phase III trial of ERCC1 and RRM1 expression-based chemotherapy versus gemcitabine/carboplatin in advanced non-small-cell lung cancer. J. Clin. Oncol. 2013;31:2404–2412. doi: 10.1200/JCO.2012.46.9783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakano Y, Tanno S, Koizumi K, Nishikawa T, Nakamura K, Minoguchi M, Izawa T, Mizukami Y, Okumura T, Kohgo Y. Gemcitabine chemoresistance and molecular markers associated with gemcitabine transport and metabolism in human pancreatic cancer cells. Br J Cancer. 2007;96:457–463. doi: 10.1038/sj.bjc.6603559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murata A, Amano R, Yamada N, Kimura K, Yashiro M, Nakata B, Hirakawa K. Prognostic predictive values of gemcitabine sensitivity-related gene products for unresectable or recurrent biliary tract cancer treated with gemcitabine alone. World J Surg Oncol. 2013;11:117. doi: 10.1186/1477-7819-11-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tai CJ, Lee CH, Chen HC, Wang HK, Jiang MC, Su TC, Shen KH, Lin SH, Yeh CM, Chen CJ, Yeh KT, Chang CC. High nuclear expression of phosphorylated extracellular signal-regulated kinase in tumor cells in colorectal glands is associated with poor outcome in colorectal cancer. Ann Diagn Pathol. 2013;17:165–171. doi: 10.1016/j.anndiagpath.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Hezel AF, Zhu AX. Systemic therapy for biliary tract cancers. Oncologist. 2008;13:415–423. doi: 10.1634/theoncologist.2007-0252. [DOI] [PubMed] [Google Scholar]
- 16.Dingle BH, Rumble RB, Brouwers MC. The role of gemcitabine in the treatment of cholangiocarcinoma and gallbladder cancer: a systematic review. Can J Gastroenterol. 2005;19:711–716. doi: 10.1155/2005/565479. [DOI] [PubMed] [Google Scholar]
- 17.Iqbal S, Rankin C, Lenz HJ, Gold PJ, Ahmad SA, El-Khoueiry AB, Messino MJ, Holcombe RF, Blanke CD. A phase II trial of gemcitabine and capecitabine in patients with unresectable or metastatic gallbladder cancer or cholangiocarcinoma: Southwest Oncology Group study S0202. Br J Cancer. 2011;68:1595–1602. doi: 10.1007/s00280-011-1657-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakagawa N, Murakami Y, Uemura K, Sudo T, Hashimoto Y, Kondo N, Sueda T. Combined analysis of intratumoral human equilibrative nucleoside transporter 1 (hENT1) and ribonucleotide reductase regulatory subunit M1 (RRM1) expression is a powerful predictor of survival in patients with pancreatic carcinoma treated with adjuvant gemcitabine-based chemotherapy after operative resection. Surgery. 2013;153:565–575. doi: 10.1016/j.surg.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 19.Wang L, Meng L, Wang XW, Ma GY, Chen JH. Expression of RRM1 and RRM2 as a novel prognostic marker in advanced non-small cell lung cancer receiving chemotherapy. Tumour biol. 2013;35:1899–1906. doi: 10.1007/s13277-013-1255-4. [DOI] [PubMed] [Google Scholar]
- 20.Mazzoni F, Cecere FL, Meoni G, Giuliani C, Boni L, Camerini A, Lucchesi S, Martella F, Amoroso D, Lucherini E, Torricelli F, Di Costanzo F. Phase II trial of customized first line chemotherapy according to ERCC1 and RRM1 SNPs in patients with advanced non-small-cell lung cancer. Lung Cancer. 2013;82:288–293. doi: 10.1016/j.lungcan.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 21.Dong X, Hao Y, Wei Y, Yin Q, Du J, Zhao X. Response to First-Line Chemotherapy in Patients with Non-Small Cell Lung Cancer According to RRM1 Expression. PLoS One. 2014;9:e92320. doi: 10.1371/journal.pone.0092320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jorgensen CL, Ejlertsen B, Bjerre KD, Balslev E, Nielsen DL, Nielsen KV. Gene aberrations of RRM1 and RRM2B and outcome of advanced breast cancer after treatment with docetaxel with or without gemcitabine. BMC Cancer. 2013;13:541. doi: 10.1186/1471-2407-13-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao X, Mitra AK, Pounds S, Crews KR, Gandhi V, Plunkett W, Dolan ME, Hartford C, Raimondi S, Campana D, Downing J, Rubnitz JE, Ribeiro RC, Lamba JK. RRM1 and RRM2 pharmacogenetics: association with phenotypes in HapMap cell lines and acute myeloid leukemia patients. Pharmacogenomics. 2013;14:1449–1466. doi: 10.2217/pgs.13.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Labroli MA, Dwyer MP, Shen R, Popovici-Muller J, Pu Q, Wyss D, McCoy M, Barrett D, Davis N, Seghezzi W, Shanahan F, Taricani L, Beaumont M, Malinao MC, Parry D, Guzi TJ. The identification of novel 5’-amino gemcitabine analogs as potent RRM1 inhibitors. Bioorg Med Chem. 2014;22:2303–10. doi: 10.1016/j.bmc.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 25.van der Donk WA, Yu G, Perez L, Sanchez RJ, Stubbe J, Samano V, Robins MJ. Detection of a new substrate-derived radical during inactivation of ribonucleotide reductase from Escherichia coli by gemcitabine 5’-diphosphate. Biochemistry. 1998;37:6419–6426. doi: 10.1021/bi9729357. [DOI] [PubMed] [Google Scholar]


