Abstract
Cluster of differentiation 44 (CD44), a principal cell surface receptor for hyaluronic acid, has been implicated in tumorigenesis and metastasis. However, the relationship between CD44 expression and the patients with gastric cancer remains controversial. A meta-analysis was performed to quantitatively review the correlation of CD44 expression with the clinicopathological data of the patients with gastric cancer. We conducted a final analysis of the patients from 18 studies. Combined odds ratios (OR) suggested that CD44 expression was related with stage, tumor size, and LN metastasis of gastric cancer, and CD44v6 was related with LN metastasis, lymphatic invasion, and venous invasion. Our results suggested that CD44 and CD44v6 expression could be used to predict the metastasis of gastric cancer.
Keywords: CD44, gastric cancer, metastasis, tumorigenesis, invasion
Introduction
Gastric cancer is one of the leading causes of cancer-related mortality worldwide. A total of 989,600 new stomach cancer cases and 738,000 deaths are estimated to have occurred in 2008 [1]. In China, gastric cancer currently ranks third among the most common cancers, and will remain a significant cancer burden during the next decade [2].
Cluster of differentiation 44 (CD44) is a principal cell surface receptor for hyaluronic acid, a major component of extracellular matrices [3]. The CD44 gene is located on chromosome 11p13 and contains 20 exons, 10 of which are expressed in the standard form (CD44s) [4]. CD44 isoforms, containing variant exon 6 (CD44v6), are generated by alternative splicing of at least 12 exons [5]. CD44 has been reported to play important roles in adherence to the extracellular matrices, motility, matrix degradation, proliferation and cell survival [6,7]. CD44v6 also plays an important biological role in the invasion and metastasis of tumor [8]. Previous studies showed that increased expression of CD44 or CD44v6 was found in gastrointestinal tumors and was associated with tumor invasion, lymph node metastasis and patients’ survival [9-12].
The association between CD44 or CD44v6 and the clinicopathological parameters of gastric cancer patients has been studied for many years. A single study may fail to completely demonstrate this complicated relationship because of a small sample size. Therefore, we performed a meta-analysis in an attempt to resolve this issue.
Materials and methods
Search strategy
Science Direct, EMBASE, and PubMed were searched to identify potentially relevant published literature. The following criteria were used to search English language articles and abstracts: ‘CD44’ and ‘gastric carcinoma’ or ‘gastric cancer’ or ‘stomach neoplasms’. Existing systematic reviews and reference lists were also checked for any potentially relevant additional studies.
Selection criteria
The studies included in this meta-analysis could be either randomized controlled studies (RCTs) or observational studies (case-control or cohort) that evaluated the association between CD44 expression and gastric cancer. Articles were excluded from the analyses if there was insufficient published data for determining an estimate of RR and a CI, or if the full text couldn’t be found. If there were several publications from the same population, only the most recent reports were selected for analysis.
Data extraction
Data were independently extracted from each report by two authors (Wei Wang and Ning Zhang), using a data recording form developed for this purpose. Data tables were made to extract all relevant data from texts, tables and figures of each included studies, including author, year, country, patient number, and detection method. Any discrepancies between the two investigators were resolved by discussion and consultation with a third reviewer (Cheng-Hai Zhao).
Statistical analysis
The statistical process was performed according to the guidelines proposed by the Meta-Analysis of Observational Studies in Epidemiology group [13]. Cochrane Review Manager, version 5.2 (Cochrane Library, Oxford, UK) was used to calculate the available data from each investigation. In addition, if sufficient studies were included, we intended to construct a funnel plot of all studies to investigate the likelihood of publication bias.
Results
Search results
Detailed search steps were described in Figure 1. Two hundred and eighty-one articles were identified initially using the search strategy above. After titles and abstracts were previewed, 53 identified studies concerning CD44 and gastric cancer were further evaluated. Thirty-five of residual 53 papers were excluded due to nonhuman experiments, review, or letter to editor. Eventually, 18 eligible studies were included in the present meta-analysis and listed in Table 1.
Figure 1.
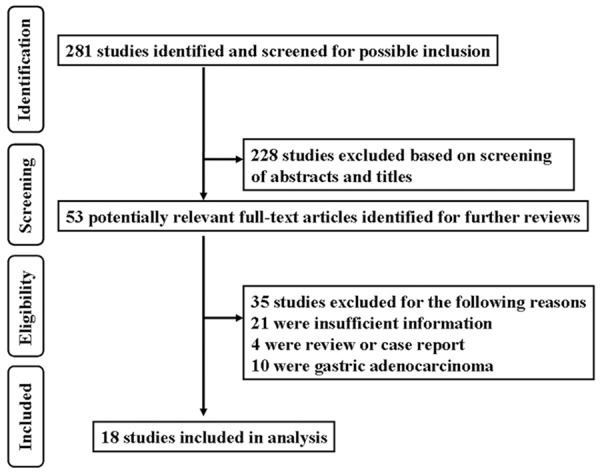
Flow diagram of identifying potential studies in our meta-analysis.
Table 1.
Characteristics of studies included in the meta-analysis
| First author | Year | Country | Cases | Ages (mean) | Type | Method |
|---|---|---|---|---|---|---|
| da Cunha [12] | 2010 | Portugal | 43 | not shown | CD44v6 | IHC |
| Okayama [14] | 2013 | Japan | 135 | 63.4 y | CD44v6 | IHC |
| Horikawa [15] | 2013 | Japan | 147 | 66 ± 11 y | CD44 | RT-PCR |
| Chen [16] | 2013 | China | 152 | 55 y | CD44 | IHC |
| Mayer [17] | 1993 | Germany | 60 | not shown | CD44 | IHC |
| Müller [18] | 1997 | Germany | 529 | 64.9 y | CD44v6 | IHC |
| Wakamatsu [19] | 2012 | Japan | 190 | not shown | CD44 | IHC |
| Kurozumi [20] | 1998 | Japan | 572 | 61 ± 11 y | CD44 | IHC |
| Yamaguchi [21] | 2002 | Japan | 201 | not shown | CD44v6 | WB |
| Chen [22] | 2013 | China | 43 | 58.5 ± 13.4 y | CD44v6 | RT-PCR |
| Liang [23] | 2012 | China | 59 | 61.8 ± 10.5 y | CD44v6 | IHC |
| Kim [24] | 1997 | Korea | 26 | not shown | CD44v6 | RT-PCR |
| Chen [25] | 2005 | China | 31 | not shown | CD44v6 | IHC |
| Yoo [26] | 1999 | Korea | 261 | 56 y | CD44 | JHC |
| Xin [27] | 2001 | China | 155 | not shown | CD44v6 | IHC |
| Chong [28] | 1997 | Japan | 104 | 62.8 y | CD44v6 | IHC |
| Cao [29] | 2014 | China | 203 | 60.5 y | CD44 | IHC |
| Qiu [30] | 2014 | China | 309 | 60.4 ± 10.4 y | CD44 | IHC |
RT-PCR, reverse transcription PCR; IHC, immunohistochemistry; WB, Western blot.
Association of cancer stem cell marker CD44 with the clinicopathological parameters of the patients with gastric cancer
There was no clear correlation between CD44 expression and sex (pooled OR = 1.14, 95% CI: 0.85-1.55, P = 0.38) (Figure 2A), and differentiation of gastric cancer (pooled OR = 1.30, 95% CI: 0.97-1.75, P = 0.08) (Figure 2B). However, CD44 expression was associated with stage (pooled OR = 2.05, 95% CI: 1.12-3.75, P = 0.02) (Figure 2C), tumor size (pooled OR = 1.42, 95% CI: 1.08-1.87, P = 0.01) (Figure 2D), and LN metastasis (pooled OR = 1.50, 95% CI: 1.14-1.98, P = 0.004) (Figure 2E). Furthermore, we found that CD44v6 was related with LN metastasis (pooled OR = 2.26, 95% CI: 1.40-3.64, P = 0.0008) (Figure 3E), lymphatic invasion (pooled OR = 1.45, 95% CI: 1.05-2.01, P = 0.02) (Figure 3F), and venous invasion (pooled OR = 1.62, 95% CI: 1.20-2.18, P = 0.001) (Figure 3G), but not with sex (pooled OR = 1.04, 95% CI: 0.73-1.46, P = 0.84) (Figure 3A), differentiation of gastric cancer (pooled OR = 2.23, 95% CI: 0.85-5.83, P = 0.10) (Figure 3B), stage (pooled OR = 0.68, 95% CI: 0.36-1.28, P = 0.23) (Figure 3C), and tumor type (pooled OR = 0.95, 95% CI: 0.68-1.34, P = 0.79) (Figure 3D). No obvious publication bias was observed in these studies (Figure 4).
Figure 2.
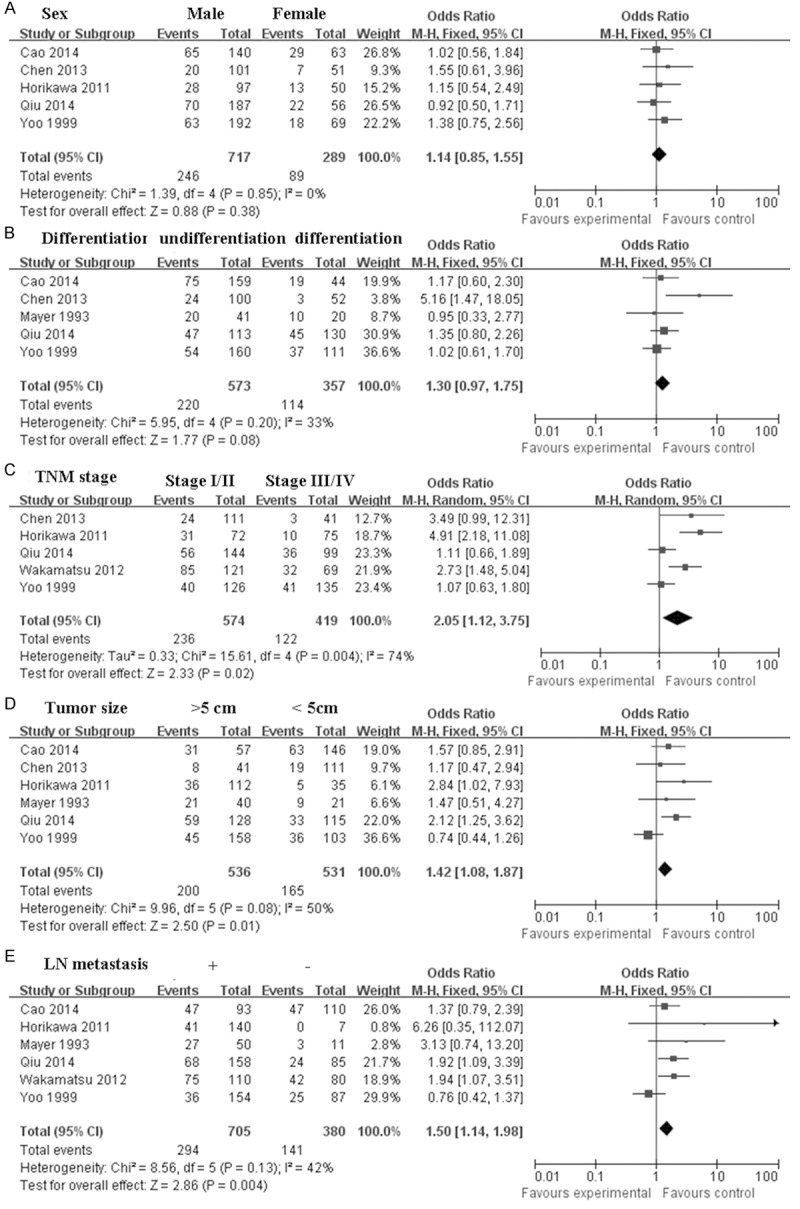
Meta-analysis of CD44 and the clinical characteristics of patients with gastric cancer. A. Sex; B. Differentiation; C. Stage; D. Tumor size; E. LN metastasis.
Figure 3.
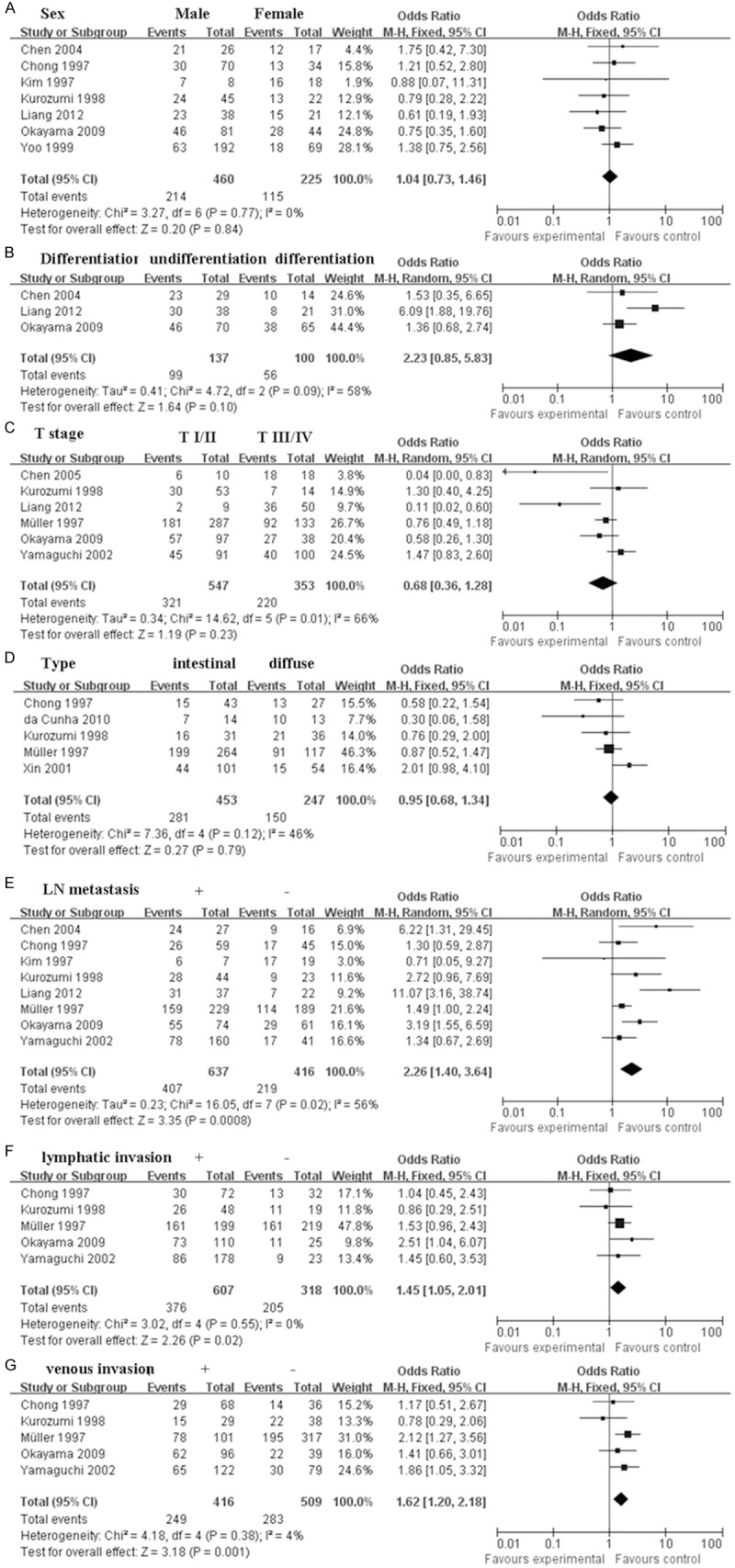
Meta-analysis of CD44v6 and the clinical characteristics of patients with gastric cancer. A. Sex; B. Differentiation; C. Stage; D. Type; E. LN metastasis; F. Lymphatic invasion; G. Venous invasion.
Figure 4.
Funnel plot for publication bias test.
Discussion
The role of CD44 expression in gastric cancer has been explored for nearly thirty years. CD44 was identified as a surface glycoprotein and a lymphocyte homing receptor found on lymphoid and epithelial cells in 1982 [31]. Its main function on lymphocytes is mediating interaction with the endothelium [32]. CD44v6, one of the major variants of CD44, could alter the conjugation of CD44s and hyaluronic acid (HA), or enhance the metastasis of tumor by conjugation with HA [23]. Despite there being many studies, the validity of CD44 and CD44v6 as a therapeutic or diagnostic target in gastric cancer has not been fully investigated and some findings are still controversial. In this meta-analysis, we found that CD44 could influence stage, tumor size, and LN metastasis. And CD44v6 was related with LN metastasis, lymphatic invasion, and venous invasion. Günthert et al. [33] demonstrated a significant relationship between CD44v6 expression and lymph node metastasis, lymphatic invasion when they transfected plasmids expressing CD44 or CD44v6 into nonmetastatic rat pancreatic carcinoma cells.
Our study provided a more believable result due to a larger size sample, and provides explanations for the inconsistencies observed in previous studies. However, some possible limitations of our meta-analysis should be acknowledged and taken into consideration. First, original information was not available in all of the selected studies. Second, the results may be influenced by the lack of observations regarding gene-environment interactions. Third, a meta-analysis is not able to solve problems with confounding factors that could be inherent in the included studies.
In summary, despite the limitations listed above, this present study shows a significant correlation between CD44 expression and stage, tumor size, and LN metastasis of gastric cancer. CD44v6 was related with LN metastasis, lymphatic invasion, and venous invasion. The value of the current meta-analysis compensates for the individual lack of precision of most studies, a problem alleviated by pooling. Further studies are required to evaluate their potential use in predicting patients’ outcome.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81370517) and the General Project Scientific Research from the Department of Education of Liaoning Province (L2012288).
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Yang L. Incidence and mortality of gastric cancer in China. World J Gastroenterol. 2006;12:17–20. doi: 10.3748/wjg.v12.i1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–13. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- 4.Screaton GR, Cáceres JF, Mayeda A, Bell MV, Plebanski M, Jackson DG, Bell JI, Krainer AR. Identification and characterization of three members of the human SR family of premRNA splicing factors. EMBO J. 1995;14:4336–49. doi: 10.1002/j.1460-2075.1995.tb00108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Screaton GR, Bell MV, Jackson DG, Cornelis FB, Gerth U, Bell JI. Genomic structure of DNA encoding the lymphocyte homing receptor CD44 reveals at least 12 alternatively spliced exons. Proc Natl Acad Sci U S A. 1992;89:12160–4. doi: 10.1073/pnas.89.24.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagano O, Saya H. Mechanism and biological significance of CD44 cleavage. Cancer Sci. 2004;95:930–5. doi: 10.1111/j.1349-7006.2004.tb03179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signaling regulators. Nat Rev Mol Cell Biol. 2003;4:33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 8.Köbel M, Weichert W, Crüwell K, Schmitt WD, Lautenschläger C, Hauptmann S. Epithelial hyaluronic acid and CD44v6 are mutually involved in invasion of colorectal adenocarcinomas and linked to patient prognosis. Virchows Arch. 2004;445:456–464. doi: 10.1007/s00428-004-1095-0. [DOI] [PubMed] [Google Scholar]
- 9.Li M, Zhang B, Zhang Z, Liu X, Qi X, Zhao J, Jiang Y, Zhai H, Ji Y, Luo D. Stem cell-like circulating tumor cells indicate poor prognosis in gastric cancer. Biomed Res Int. 2014;2014:981261. doi: 10.1155/2014/981261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li K, Dan Z, Nie YQ. Gastric cancer stem cells in gastric carcinogenesis, progression, prevention and treatment. World J Gastroenterol. 2014;20:5420–6. doi: 10.3748/wjg.v20.i18.5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li H, Guo L, Li JW, Liu N, Qi R, Liu J. Expression of hyaluronan receptors CD44 and RHAMM in stomach cancers: relevance with tumor progression. Int J Oncol. 2000;17:927–32. [PubMed] [Google Scholar]
- 12.da Cunha CB, Oliveira C, Wen X, Gomes B, Sousa S, Suriano G, Grellier M, Huntsman DG, Carneiro F, Granja PL, Seruca R. De novo expression of CD44 variants in sporadic and hereditary gastric cancer. Lab Invest. 2010;90:1604–14. doi: 10.1038/labinvest.2010.155. [DOI] [PubMed] [Google Scholar]
- 13.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 14.Okayama H, Kumamoto K, Saitou K, Hayase S, Kofunato Y, Sato Y, Miyamoto K, Nakamura I, Ohki S, Sekikawa K, Takenoshita S. CD44v6, MMP-7 and nuclear Cdx2 are significant biomarkers for prediction of lymph node metastasis in primary gastric cancer. Oncol Rep. 2009;22:745–55. doi: 10.3892/or_00000496. [DOI] [PubMed] [Google Scholar]
- 15.Horikawa M, Iinuma H, Inoue T, Ogawa E, Fukushima R. Clinical significance of intraperitoneal CD44 mRNA levels of magnetically separated CD45-negative EpCAM-positive cells for peritoneal recurrence and prognosis in stage II and III gastric cancer patients. Oncol Rep. 2011;25:1413–20. doi: 10.3892/or.2011.1191. [DOI] [PubMed] [Google Scholar]
- 16.Chen S, Hou JH, Feng XY, Zhang XS, Zhou ZW, Yun JP, Chen YB, Cai MY. Clinicopathologic significance of putative stem cell marker, CD44 and CD133, in human gastric carcinoma. J Surg Oncol. 2013;107:799–806. doi: 10.1002/jso.23337. [DOI] [PubMed] [Google Scholar]
- 17.Mayer B, Jauch KW, Günthert U, Figdor CG, Schildberg FW, Funke I, Johnson JP. De-novo expression of CD44 and survival in gastric cancer. Lancet. 1993;342:1019–22. doi: 10.1016/0140-6736(93)92879-x. [DOI] [PubMed] [Google Scholar]
- 18.Müller W, Schneiders A, Heider KH, Meier S, Hommel G, Gabbert HE. Expression and prognostic value of the CD44 splicing variants v5 and v6 in gastric cancer. J Pathol. 1997;183:222–7. doi: 10.1002/(SICI)1096-9896(199710)183:2<222::AID-PATH923>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 19.Wakamatsu Y, Sakamoto N, Oo HZ, Naito Y, Uraoka N, Anami K, Sentani K, Oue N, Yasui W. Expression of cancer stem cell markers ALDH1, CD44 and CD133 in primary tumor and lymph node metastasis of gastric cancer. Pathol Int. 2012;62:112–9. doi: 10.1111/j.1440-1827.2011.02760.x. [DOI] [PubMed] [Google Scholar]
- 20.Kurozumi K, Nishida T, Nakao K, Nakahara M, Tsujimoto M. Expression of CD44 variant 6 and lymphatic invasion: importance to lymph node metastasis in gastric cancer. World J Surg. 1998;22:853–7. doi: 10.1007/s002689900481. discussion 857-8. [DOI] [PubMed] [Google Scholar]
- 21.Yamaguchi A, Goi T, Yu J, Hirono Y, Ishida M, Iida A, Kimura T, Takeuchi K, Katayama K, Hirose K. Expression of CD44v6 in advanced gastric cancer and its relationship to hematogenous metastasis and long-term prognosis. J Surg Oncol. 2002;79:230–5. doi: 10.1002/jso.10082. [DOI] [PubMed] [Google Scholar]
- 22.Chen JQ, Zhan WH, He YL, Peng JS, Wang JP, Cai SR, Ma JP. Expression of heparanase gene, CD44v6, MMP-7 and nm23 protein and their relationship with the invasion and metastasis of gastric carcinomas. World J Gastroenterol. 2004;10:776–82. doi: 10.3748/wjg.v10.i6.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang YZ, Fang TY, Xu HG, Zhuo ZQ. Expression of CD44v6 and Livin in gastric cancer tissue. Chin Med J (Engl) 2012;125:3161–5. [PubMed] [Google Scholar]
- 24.Kim YS, Chi SG, Kim YW, Park YK, Yoon C. Molecular genetic characterization of alternatively spliced CD44 transcripts in human stomach carcinoma. J Korean Med Sci. 1997;12:505–13. doi: 10.3346/jkms.1997.12.6.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen XY, Wang ZC, Li H, Cheng XX, Sun Y, Wang XW, Wu ML, Liu J. Nuclear translocations of beta-catenin and TCF4 in gastric cancers correlate with lymph node metastasis but probably not with CD44 expression. Hum Pathol. 2005;36:1294–301. doi: 10.1016/j.humpath.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Yoo CH, Noh SH, Kim H, Lee HY, Min JS. Prognostic significance of CD44 and nm23 expression in patients with stage II and stage IIIA gastric carcinoma. J Surg Oncol. 1999;71:22–8. doi: 10.1002/(sici)1096-9098(199905)71:1<22::aid-jso5>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 27.Xin Y, Grace A, Gallagher MM, Curran BT, Leader MB, Kay EW. CD44V6 in gastric carcinoma: a marker of tumor progression. Appl Immunohistochem Mol Morphol. 2001;9:138–42. doi: 10.1097/00129039-200106000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Chong JM, Fukayama M, Hayashi Y, Funata N, Takizawa T, Koike M, Muraoka M, Kikuchi-Yanoshita R, Miyaki M, Mizuno S. Expression of CD44 variants in gastric carcinoma with or without Epstein-Barr virus. Int J Cancer. 1997;74:450–4. doi: 10.1002/(sici)1097-0215(19970822)74:4<450::aid-ijc16>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 29.Cao L, Hu X, Zhang J, Liang P, Zhang Y. CD44+ CD324- expression and prognosis in gastric cancer patients. J Surg Oncol. 2014;110:727–33. doi: 10.1002/jso.23690. [DOI] [PubMed] [Google Scholar]
- 30.Qiu Y, Hu Y, Zhang ZY, Ye L, Xu FH, Schneider ME, Ma XL, Du YX, Zuo XB, Zhou FS, Chen G, Xie XS, Zhang Y, Xia HZ, Wu JF, Du WD. Genetic association of osteopontin (OPN) and its receptor CD44 genes with susceptibility to Chinese gastric cancer patients. J Cancer Res Clin Oncol. 2014;140:2143–56. doi: 10.1007/s00432-014-1761-9. [DOI] [PubMed] [Google Scholar]
- 31.Gallatin WM, Weissman IL, Butcher EC. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature. 1983;304:30–4. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- 32.Idzerda RL, Carter WG, Nottenburg C, Wayner EA, Gallatin WM, St John T. Isolation and DNA sequence of a cDNA clone encoding a lymphocyte adhesion receptor for high endothelium. Proc Natl Acad Sci U S A. 1989;86:4659–63. doi: 10.1073/pnas.86.12.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Günthert U, Hofmann M, Rudy W, Reber S, Zöller M, Haussmann I, Matzku S, Wenzel A, Ponta H, Herrlich P. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell. 1991;65:13–24. doi: 10.1016/0092-8674(91)90403-l. [DOI] [PubMed] [Google Scholar]


