Abstract
The clinical feature of invasive pulmonary aspergillosis (IPA) in immunocompromised patients is well studied in the past decades. While the manifestations of IPA in immunocompetent patients remain unclear. The purpose of this study was to determine the clinical and radiological manifestations of invasive pulmonary aspergillosis (IPA) in patients without immunosuppression, as well as the reasons for the misdiagnosis of IPA. We retrieved and retrospectively reviewed the records of 102 patients from whom surgical lung specimens of chronic inflammatory granulomas were harvested. 26 patients were eventually diagnosed with pulmonary aspergillosis on Grocott methenamine silver staining. We investigated these patients in detail. We found that the rate of misdiagnosis before the lung surgery was as high as 73%. The most common symptom was hemoptysis, and the main feature in radiology was nodule or mass lesion. Air crescent sign or Halo sign were not common in our study. The atypical radiological manifestations and non-specific clinical findings make the diagnosis of IPA difficult and lead to a high misdiagnosis rate.
Keywords: Invasive pulmonary aspergillosis, immunocompetent, radiological features, clinical characteristics
Introduction
Invasive pulmonary aspergillosis (IPA) is an opportunistic infection that occurs in severely immunocompromised patients, especially, patients who have undergone hematopoietic stem cell transplantation (HSCT) or have hematological malignancy [1,2]. According to the guidelines in China [3], the Standardizing the diagnosis and treatment of invasive pulmonary fungal diseases and those of the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) [4], the diagnosis of fungal infection is classified as proven, probable, and possible invasive fungal disease in immunocompromised patients. The final diagnosis of IPA depends on the combination of various elements, including host factors, radiological signs, clinical symptoms, mycological results, and histopathological findings. The above guidelines emphasize the importance of host factors in the diagnosis of fungal infection. However, many individuals without any risk factors are infected with Aspergillus [5-8], and it is difficult to establish a diagnosis of aspergillosis in patients without host factors.
In the current study, we assessed the clinical and radiological findings in patients who were eventually diagnosed with aspergillosis in order to determine the reason for the initial misdiagnosis, and to provide clinicians with more information about the diagnosis of aspergillosis.
Material and methods
Subjects
Surgical lung specimens of chronic granulomatous inflammation harvested between August 2005 and July 2013 were identified from the archives of Anhui Provincial Hospital (Hefei, China). The search yielded 102 surgical lung specimens of chronic inflammatory granulomas. These paraffin-embedded tissues were sliced and stained again in order to determine the underlying etiology. The staining methods used were as follows: hematoxylin-eosin (HE) staining to show the tissue structure, Grocott methenamine silver (GMS) staining to confirm Aspergillus infection, and acid-fast staining to recognize tuberculosis infection.
The medical records of the patients from whom the specimens were harvested were retrospectively reviewed for age, sex, presenting symptoms, treatment, and radiological manifestations. The laboratory data abstracted included pulmonary-function tests and white blood cell count. In total, we identified 26 patients who had been diagnosed with aspergillosis on histopathological examination with GMS staining, according to the guidelines used in China and the EORTC/MSG guidelines [3,4]. This study was approved by the ethics committee of Anhui Provincial Hospital.
Standard and radiological definitions
All the patients in our study were diagnosed with proven pulmonary aspergillosis according to the EORTC/MSG guidelines [4]. Currently accepted classical host factors for aspergillosis [3,4] include the following: (1) a history of neutropenia (neutrophil count < 500 cells/mm3) for more than 10 days before the onset of fungal disease, (2) allogeneic stem cell transplantation, (3) corticosteroid use for > 3 weeks with a mean minimum dose of 0.3 mg/kg/day prednisone or its equivalent (except for, allergic bronchopulmonary aspergillosis treatment), (4) use of drugs that suppress T-cells, during the past 90 days, for example, cyclosporine, tumor necrosis factor-α blockers, specific monoclonal antibodies, and nucleoside analogues, and (5) inherited severe immunodeficiency disease, such as severe combined immunodeficiency and chronic granulomatous disease. Chronic obstructive pulmonary disease (COPD) was diagnosed according to the Global Initiative for Chronic Obstructive Lung Disease standard [9].
The definitions of chest imaging findings used in our study followed the guidelines of the Fleischner Society [10] and were as follows: (1) A soft-tissue opacity that completely covered the background of the lung was defined as a “nodule” if its diameter was < 3 cm and as a “mass” if its diameter was ≥ 3 cm. (2) A “ground-glass opacity” was defined as a hazy area of increased opacity in the lung, with preservation of bronchial and vascular margins. (3) The “halo” sign was defined as the computed tomography (CT) finding of a ground-glass opacity surrounding a nodule or mass. (4) An air crescent was a collection of air in a crescent shape that separated the wall of a cavity from an inner mass. (5) Consolidation appeared as a homogeneous increase in pulmonary parenchymal attenuation that obscured the margins of vessels and airway walls. (6) A cavity was defined as a gas-filled space, seen as a lucency or low-attenuation area, within an area of pulmonary consolidation, a mass, or a nodule. (7) The “tree-in-bud” pattern represented centrilobular branching structures that resembled a budding tree. (8) Bronchial dilatation was assessed with respect to the accompanying pulmonary artery (“signet ring” sign), and was associated with a lack of tapering of the bronchi and the presence of bronchi within 1 cm of the pleural surface.
The available radiographic data were reviewed by an expert radiologist and a chest expert. The histological findings were retrospectively reexamined by an expert pathologist and an expert microbiologist. We defined immune-competent as patients without any of the host factors listed above.
Statistical analysis
The data were summarized and analyzed. Continuous variables were summarized as either means and standard deviations or medians with interquartile ranges. For categorical variables, the percentages of patients in each category were calculated. All analyses were performed using SPSS for Windows (version 16.0).
Results
Demographic characteristics and underlying diseases
In all, 26 patients were diagnosed with proven pulmonary aspergillosis, according to the GMS staining findings. There were slightly more female patients (n = 15) than male patients (n = 11), with a female-to-male ratio of approximately 1:0.73. The mean age was 48 years (range, 27-77 years). No patient had classic host factors.
Of the 26 patients, 14 (53.8%) had underlying diseases, including tuberculosis (4 patients), bronchiectasis (3 patients), diabetes mellitus (3 patients), pulmonary sequestration (2 patients), breast cancer (1 patient), and chronic liver disease (1 patient; Table 1).
Table 1.
Demographic characteristics and underlying diseases
| Characteristic | Value |
|---|---|
| Mean age, yr (range) | 48 (27-77) |
| Female sex, no. (%) | 15/26 (57.7) |
| Underlying diseases, no. (%) | 14/26 (53.8) |
| Tuberculosis, no. (%) | 4/26 (15.4) |
| Diabetes mellitus, no. (%) | 3/26 (11.5) |
| Bronchiectasis, no. (%) | 3/26 (11.5) |
| Pulmonary sequestration, no. (%) | 2/26 (7.7) |
| Cancer, no. (%) | 1/26 (3.8) |
| Chronic liver disease, no. (%) | 1/26 (3.8) |
Clinical manifestations, laboratory tests and diagnosis
The clinical manifestations, laboratory tests, and diagnosis are summarized in Table 2. Hemoptysis was the most common complaint (16 patients, 61.5%), followed by cough (13 patients, 50.0%; dry cough, 6 patients, 23.1%). Thoracodynia and bloody phlegm were present in 4 patients (15.4%) and 3 patients (11.5%), respectively. Only 3 patients (11.5%) had chest stuffiness. On admission, none of the patients had fever.
Table 2.
Clinical manifestations and diagnosis
| Characteristic | Number (%) |
|---|---|
| Clinical manifestations | |
| Hemoptysis | 16/26 (61.5) |
| Cough | 13/26 (50.0) |
| Expectoration | 7/26 (26.9) |
| Dry cough | 6/26 (23.1) |
| Bloody phlegm | 3/26 (11.5) |
| Chest stuffiness | 3/26 (11.5) |
| Thoracodynia | 4/26 (15.4) |
| Melosalgia | 1/26 (3.8) |
| Fever | 2/26 (7.7) |
| No symptoms | 1/26 (3.8) |
| Neutropenia | 0 |
| Diagnosis | |
| Misdiagnosis | 19/26 (73.1) |
| Hemoptysis of unknown origin | 1/19 (5.3) |
| Lung cancer | 10/19 (52.6) |
| Tuberculosis | 4/19 (21.1) |
| Inflammatory pseudotumor | 3/19 (15.8) |
| Bronchiectasis | 1/19 (5.3) |
No patient had a history of corticosteroid therapy. Antibiotic treatment was prescribed to 5 patients before admission, 2 of whom had been on long-term antibiotic treatment since they developed bronchiectasis. Three patients underwent bronchoscopy. In one of these patients, bronchoscopy of the left, upper lobe, anterior segment bronchus (LB3) revealed granulomatous obstructions. Subsequent histological examination revealed chronic inflammation. Laboratory tests showed that none of the patients had neutropenia, even though some patients had a low white blood cell count.
Of the 26 patients, only 7 (26.9%) were diagnosed with aspergillosis infection on admission. Thus, the rate of misdiagnosis was 73.1%. The most common misdiagnoses were lung cancer, tuberculosis, and inflammatory pseudotumor. A 47 year-old patient, who had had melosalgia since > 1 month, was diagnosed with lumbar disc herniation. In this patient, multiple nodules were found in both lungs on a radiographic examination performed prior to a scheduled arthroscopic microdiscectomy. The melosalgia was relieved after lung surgery.
Imaging examinations
All 26 patients underwent chest radiography on admission. The radiological characteristics were diverse. There was a nodule or a mass in 10 patients (most lesions measured < 3 cm in diameter). All nodules (Figure 1A, 1B) were of uneven density and contained a central, necrotic area of low density. Four patients had lobulation and spiculation, which were difficult to distinguish from tumors (Figures 1C, 1D, 2A and 2B). Five patients were found to have patches on CT. Cavity formation was reported in 6 patients, all of whom had thin-walled cavities. The air crescent sign was seen in 2 patients (Figure 3C, 3D), while the halo sign was found in 4 patients (Figure 1A, 1B). Consolidation (Figure 2C, 2D) with air bronchograms was reported in 2 patients. In addition, 4 patients showed bronchiectasis.
Figure 1.
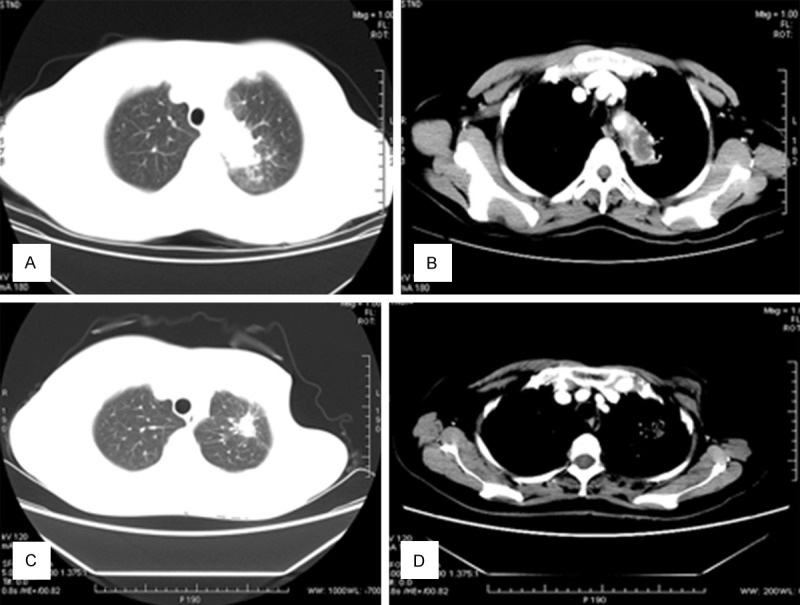
A, B. A 43-year-old woman with hemoptysis with no obvious cause. Chest CT shows a nodule with lobulation, and the halo sign. Intravenous contrast-enhanced CT reveals that the nodule is heterogeneously enhanced and contains an area of low density (necrosis), measuring 2×2.5 cm. The postoperative pathological examination revealed an Aspergillus infection. C, D. A 56-year old woman was diagnosed with breast cancer and underwent surgery 11 years ago. Thereafter, she had underwent several cycles of chemotherapy. Three years ago, she developed intermittent hemoptysis. CT shows a lesion with high density and an irregular boundary and spiculation in the left upper lung, surrounded by patchy. In the mediastinal window, the lesion is partly subtracted.
Figure 2.
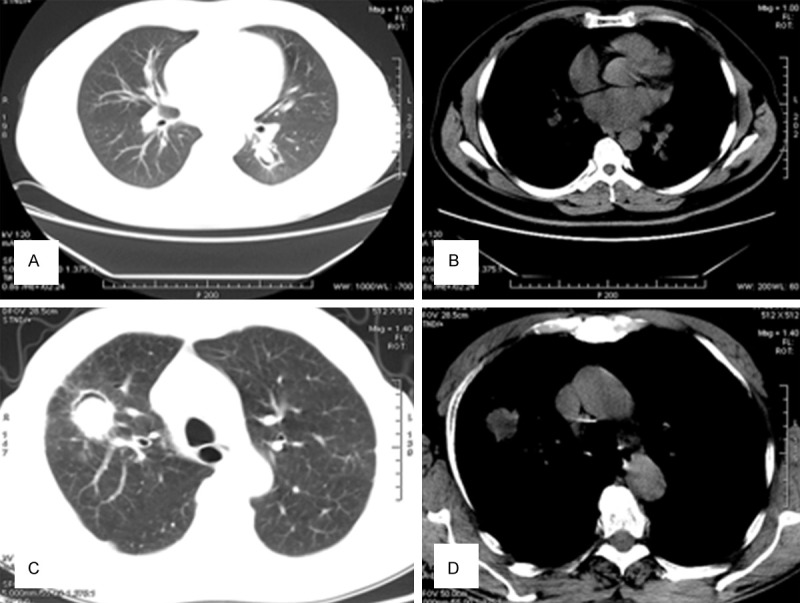
A, B. A 35-year old woman with a more than 2-year history of hemoptysis. A mass is observed in the right lung. The lesion has a clear boundary and lobulation, and measures 3×2.5 cm. C, D. In the right lung, consolidation is observed with an ill-defined border and uneven density, within which cavity formation can also be observed.
Figure 3.
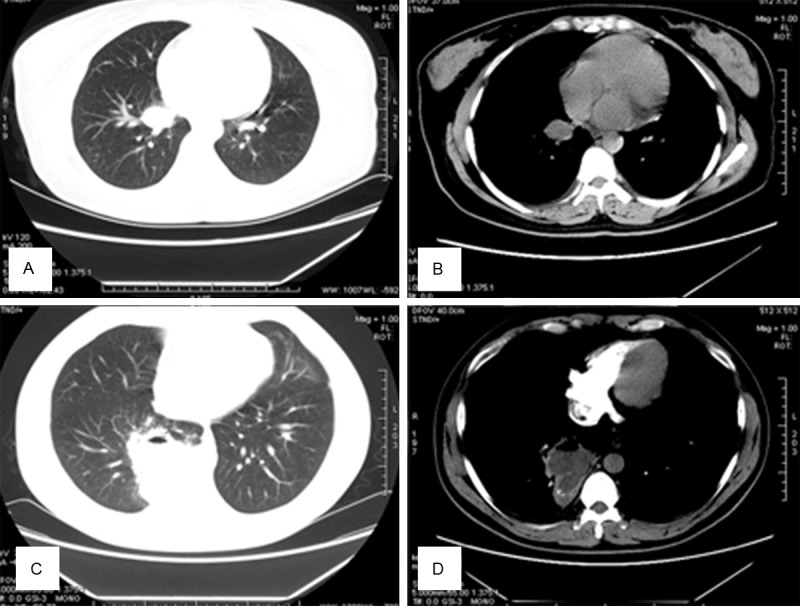
A, B. A 37-year-old man with bloody sputum since > 3 months. Chest CT reveals an ovoid opacity located within a lung cavity. C, D. A 59-year old man with cough and bloody sputum. Chest CT reveals a round, high-density, soft-tissue lesion (diameter, 4.2 cm) in the right, upper lung. A curved air shadow, i.e., the air crescent sign, is seen within the lesion. In the mediastinal window, subtraction is not obvious.
Multiple features were observed in the same individual, such as nodules and patches. None of the patients had mediastinal lymphadenopathy. Eight patients underwent intravenous contrast-enhanced CT, of whom 4 showed no significant enhancement. The remaining 4 patients had mild-to-obvious enhancement of the lesion. Of the 26 patients, 2 (7.7%) had bilateral lung lesions; half of the patients (n = 13) had lesions in the right lung. We also observed one case of aspergilloma, which typically appears as an ovoid or round opacity located within a lung cavity (Figure 3A, 3B). The radiological features are listed in Table 3.
Table 3.
Imaging examinations
| Characteristic | Number (%) |
|---|---|
| Nodule or mass lesion | 10/26 (38.5) |
| Nodules | 7/26 (26.9) |
| Masses | 3/26 (11.5) |
| Lobulation and spiculation | 4/10 (40.0) |
| Halo sign | 4/26 (15.4) |
| Cavity | 6/26 (23.1) |
| Consolidation | 3/26 (11.5) |
| Air bronchogram | 2/26 (7.7) |
| Air crescent sign | 2/26 (7.7) |
| Patch | 5/26 (19.2) |
| Bronchiectasis | 4/26 (15.4) |
Pathology
Histologically, IPA is characterized by necrosis of the lung parenchyma followed by acute or chronic inflammatory cell infiltration and especially, the presence of hyphae invading lung tissue. Occasionally, vascular invasion or dissemination to other organs can also be observed. Morphologically, Aspergillus typically grow as septate hyphae with characteristic branching at 45° angles, which can easily be observed on HE staining, but is best observed with GMS staining (Figure 4).
Figure 4.
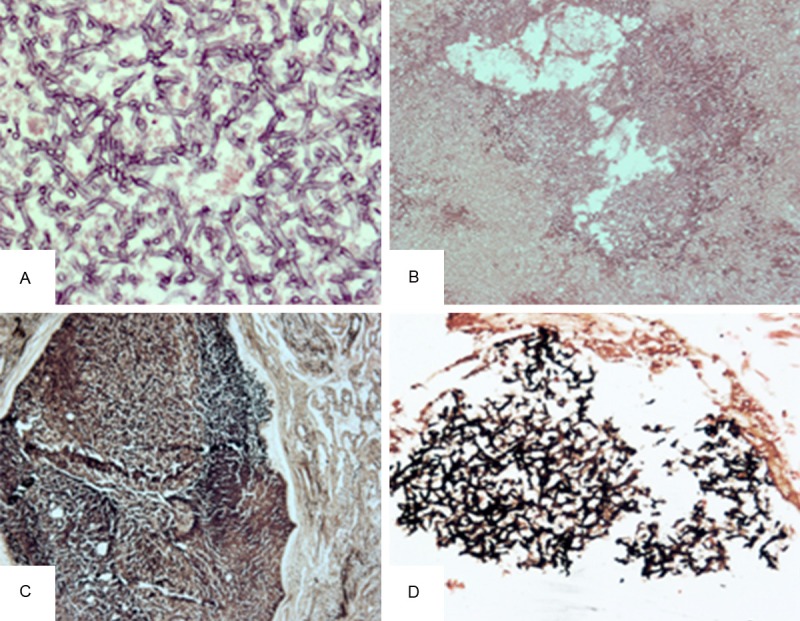
A, B. Aspergillus hyphae on HE staining. There are numerous Aspergillus hyphae with 45° branching (A: original magnification, ×400; B: original magnification, ×200). C. The lesion is surrounded by fibrous tissue (original magnification, ×100). D. GMS staining shows fungal elements (original magnification, ×200).
Discussion
An early diagnosis of IPA based on histopathological or mycological evidence is difficult to establish [11,12], especially, in the absence of host factors. The demonstration of invasive hyphae on histological examination or a positive culture from a normally sterile environment is diagnostic of proven invasive fungal disease. The diagnosis of IPA requires a combination of host factors, and clinical and mycological criteria. It is very difficult to obtain evidence to confirm pulmonary aspergillosis in the absence of host factors. Thus, the incidence of aspergillosis and the clinical and radiological manifestations of IPA in individuals without classic host factors remain unclear.
Our investigation indicates that hemoptysis is an important symptom; this symptom has also been reported in immunocompromised patients [13,14]. In addition, cough and thoracodynia were common symptoms. Some patients had no symptoms at all. According to our investigation, the clinical findings in IPA patients without host factors are non-specific and contribute little to the diagnosis. None of our patients had classic host factors, but 14 of 26 patients (53.8%) had underlying diseases. Several studies [15-19] have indicated that patients with underlying diseases, such as cystic fibrosis and COPD, are at risk for pulmonary aspergillosis.
In our investigation, 4 main radiological findings were identified: nodules and masses (10 patients), cavity (6 patients), patches (5 patients), and bronchiectasis (4 patients). Our results are similar to those of a survey conducted by Qin et al. [20] in patients with liver transplantation; they found that the main radiological manifestations were nodules and patchy consolidation. Franquet et al. [21] have reported that mass-like lesions are the predominant finding in immunocompromised individuals. A large epidemiological survey of fungal infections in China determined that the main radiological manifestations of pulmonary aspergillosis were cavity, consolidation, and pleural effusion [22]. Masses and nodules are occasionally difficult to distinguish from malignancies, especially, when the lesion exhibits lobulation and spiculation or a diffuse and bilateral distribution in the lungs. In our study, 10 patients were misdiagnosed with lung cancer. A recent report has indicated that nodules in pulmonary aspergillosis may mimic cancer, with a high 18F-fluorodeoxyglucose (FDG) uptake on positron-emission tomography (PET-CT) examinations [23].
The air crescent sign commonly occurs in IPA, especially, in neutropenic patients, during reco-very from the disease [24]. One study reported that the air crescent sign was seen in 48% of patients with IPA [25]. In our study, however, only 2 (7.7%) patients exhibited the air crescent sign. The halo sign is also a classic finding in pulmonary aspergillosis. Reginale et al. [26] evaluated the significance of the halo sign in IPA, and found that most (61%) of their patients (patients with allogeneic HSCT, hematological malignancy, or recent neutropenia) had the halo sign, but consolidation, nodules, cavities, and the air crescent sign were less common. In our investigation, 4 (15.4%) patients had the halo sign.
The atypical radiological manifestations and non-specific clinical findings make the diagnosis of IPA difficult and lead to a high misdiagnosis rate. Patients with hemoptysis, cough, expectoration, or fever, who are found to have masses, nodules, cavities, patchy shadows, or consolidation with air bronchograms on radiological examination, especially, patients with underlying diseases, should undergo further examinations for pulmonary aspergillosis.
Disclosure of conflict of interest
None.
References
- 1.Marr KA, Carter RA, Boeckh M, Martin P, Corey L. Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood. 2002;100:4358–4366. doi: 10.1182/blood-2002-05-1496. [DOI] [PubMed] [Google Scholar]
- 2.De La Rosa GR, Champlin RE, Kontoyiannis DP. Risk factors for the development of invasive fungal infections in allogeneic blood and marrow transplant recipients. Transpl Infect Dis. 2002;4:3–9. doi: 10.1034/j.1399-3062.2002.00010.x. [DOI] [PubMed] [Google Scholar]
- 3.Deng WW. Standardizing the diagnosis and treatment of invasive pulmonary fungal diseases. Zhonghua Nei Ke Za Zhi. 2006;45:623. [PubMed] [Google Scholar]
- 4.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler CC, Kullberg BJ, Marr KA, Muñoz P, Odds FC, Perfect JR, Restrepo A, Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR, Zaoutis T, Bennett JE European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group; National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper JA, Weinbaum DL, Aldrich TK, Mandell GL. Invasive aspergillosis of the lung and pericardium in a nonimmunocompromised 33 ye-ar old man. Am J Med. 1981;71:903–907. doi: 10.1016/0002-9343(81)90396-x. [DOI] [PubMed] [Google Scholar]
- 6.Stevens DA, Melikian GL. Aspergillosis in the ‘nonimmunocompromised’ host. Immunol Invest. 2011;40:751–766. doi: 10.3109/08820139.2011.614307. [DOI] [PubMed] [Google Scholar]
- 7.Ergene U, Akcali Z, Ozbalci D, Nese N, Senol S. Disseminated Aspergillosis due to Aspergillus niger in Immunocompetent Patient: A Case Report. Case Rep Infect Dis. 2013;2013:385190. doi: 10.1155/2013/385190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu XY, Sun HM, Zhao BL, Shi Y. Diagnosis of airway-invasive pulmonary aspergillosis by tree-in-bud sign in an immunocompetent patient: case report and literature review. J Mycol Med. 2013;23:64–69. doi: 10.1016/j.mycmed.2012.12.050. [DOI] [PubMed] [Google Scholar]
- 9.Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, Stockley RA, Sin DD, Rodriguez-Roisin R. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 10.Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246:697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 11.Reichenberger F, Habicht JM, Gratwohl A, Tamm M. Diagnosis and treatment of invasive pulmonary aspergillosis in neutropenic patients. Eur Respir J. 2002;19:743–755. doi: 10.1183/09031936.02.00256102. [DOI] [PubMed] [Google Scholar]
- 12.Raad I, Hanna H, Huaringa A, Sumoza D, Hachem R, Albitar M. Diagnosis of invasive pulmonary aspergillosis using polymerase chain reaction-based detection of aspergillus in BAL. Chest. 2002;121:1171–1176. doi: 10.1378/chest.121.4.1171. [DOI] [PubMed] [Google Scholar]
- 13.Jewkes J, Kay PH, Paneth M, Citron KM. Pulmonary aspergilloma: analysis of prognosis in relation to haemoptysis and survey of treatment. Thorax. 1983;38:572–578. doi: 10.1136/thx.38.8.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thoracic B, Association T. Aspergilloma and residual tuberculous cavities--the results of a resurvey. Tubercle. 1970;51:227–245. [PubMed] [Google Scholar]
- 15.Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Segal BH, Steinbach WJ, Stevens DA, van Burik JA, Wingard JR, Patterson TF Infectious Diseases Society of America. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:327–360. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]
- 16.Thompson GR 3rd, Patterson TF. Pulmonary aspergillosis. Semin Respir Crit Care Med. 2008;29:103–110. doi: 10.1055/s-2008-1063849. [DOI] [PubMed] [Google Scholar]
- 17.Sahlén AO, Suvarna SK, Wilkie ME. A case of invasive pulmonary aspergillosis in renal failure. Nephrol Dial Transplant. 2004;19:2687. doi: 10.1093/ndt/gfh418. [DOI] [PubMed] [Google Scholar]
- 18.Xu H, Li L, Huang WJ, Wang LX, Li WF, Yuan WF. Invasive pulmonary aspergillosis in patients with chronic obstructive pulmonary disease: a case control study from China. Clin Microbiol Infect. 2012;18:403–408. doi: 10.1111/j.1469-0691.2011.03503.x. [DOI] [PubMed] [Google Scholar]
- 19.Garnacho-Montero J, Amaya-Villar R, Ortiz-Leyba C, León C, Alvarez-Lerma F, Nolla-Salas J, Iruretagoyena JR, Barcenilla F. Isolation of Aspergillus spp. from the respiratory tract in critically ill patients: risk factors, clinical presentation and outcome. Crit Care. 2005;9:R191–199. doi: 10.1186/cc3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin J, Fang Y, Dong Y, Zhu K, Wu B, An Y, Shan H. Radiological and clinical findings of 25 patients with invasive pulmonary aspergillosis: retrospective analysis of 2150 liver transplantation cases. Br J Radiol. 2012;85:e429–435. doi: 10.1259/bjr/39784231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franquet T, Müller NL, Giménez A, Guembe P, de La Torre J, Bagué S. Spectrum of pulmonary aspergillosis: histologic, clinical, and radiologic findings. Radiographics. 2001;21:825–837. doi: 10.1148/radiographics.21.4.g01jl03825. [DOI] [PubMed] [Google Scholar]
- 22.Liu YN, She DY, Sun TY, Tong ZH, He B, Xiao Y, He LX, Qu JM, Liu XQ, Li ER, Chen P, Ma ZS, Shi Y, Feng YL, Jiang SJ, Xiong SD, Hu CP. A multicentre retrospective study of pulmonary mycosis clinically proven from 1998 to 2007. Zhonghua Jie He He Hu Xi Za Zhi. 2011;34:86–90. [PubMed] [Google Scholar]
- 23.Baliko Z, Sarosi V, Illes MB, Varga Z, Hegedus G, Molnar P, Szakall S. PET-CT imaging and reality. Pathol Oncol Res. 2011;17:393–395. doi: 10.1007/s12253-010-9299-x. [DOI] [PubMed] [Google Scholar]
- 24.Blum U, Windfuhr M, Buitrago-Tellez C, Sigmund G, Herbst EW, Langer M. Invasive pulmonary aspergillosis. MRI, CT, and plain radiographic findings and their contribution for early diagnosis. Chest. 1994;106:1156–1161. doi: 10.1378/chest.106.4.1156. [DOI] [PubMed] [Google Scholar]
- 25.Kim MJ, Lee KS, Kim J, Jung KJ, Lee HG, Kim TS. Crescent sign in invasive pulmonary aspergillosis: frequency and related CT and clinical factors. J Comput Assist Tomogr. 2001;25:305–310. doi: 10.1097/00004728-200103000-00027. [DOI] [PubMed] [Google Scholar]
- 26.Greene RE, Schlamm HT, Oestmann JW, Stark P, Durand C, Lortholary O, Wingard JR, Her-brecht R, Ribaud P, Patterson TF, Troke PF, Denning DW, Bennett JE, de Pauw BE, Rubin RH. Imaging findings in acute invasive pulmonary aspergillosis: clinical significance of the halo sign. Clin Infect Dis. 2007;44:373–379. doi: 10.1086/509917. [DOI] [PubMed] [Google Scholar]


