Abstract
Background and Objective: The aim of this study was to evaluate the efficacy of DWI in differentiation of patients with residual cavity and type 1 hydatid cyst (HC) in the liver. Methods: 32 patients were included. 12 of these patients had type 1 HC and the remainders (n = 20) had postoperative residual cavities. In all patients, axial T2-weighted and DWI images were obtained. An apparent diffusion coefficient (ADC) map of the images was automatically generated and the ADC values were measured on this map for all patients. Mann-Whitney U test was used for comparison of continuous variables between two groups. Results: The mean diameters of type 1 hydatid cyst and residual cavity groups were 83.42 mm, 49.30 mm, respectively (P = 0.001). There were no significant differences in gender and age between the groups (both P > 0.05). The mean ADC values of type 1 hydatid cyst and residual cavity groups were 2.58 ± 0.13 × 10-3 s/mm2, 2.58 ± 0.16 × 10-3 s/mm2, respectively (P = 0.953). Conclusion: DWI might not be suitable to differentiate the postoperative residual cavity from the type 1 hydatid cyst in the liver due to similarity of ADC values between postoperative residual cavity and type 1 hydatid cyst.
Keywords: Hydatid cyst, liver, recurrence, diffusion-weighted magnetic resonance imaging
Introduction
Human hydatid disease is caused by the larvae of cestodes of the genus Echinococcus. This zoonosis is characterized by long term growth of metacestode (hydatid) cysts in humans and mammalian intermediate hosts. Hydatid cysts mainly locate in the liver or lungs in the endemic areas and two major species of hydatid cysts which infect humans are E. granulosus and E. multilocularis, causing cystic echinococcosis (CE) and alveolar echinococcosis (AE) [1]. If CE and AE are managed insufficiently, they cause chronic disease and high mortality [2].
Three therapeutic procedures can be utilized for the management of hydatid disease: chemotherapy, PAIR (puncture, aspiration, injection, re-aspiration), and surgery (partial or total cyst resection). Surgical management of liver hydatid cysts necessitates experience and caution owing to the risk of spread and recurrence [3,4]. Radical resections including partial hepatectomy or total pericystectomy are the gold standard in most patients [5]. Open partial cystectomy, performed for open cyst evacuation and sterilization of parasitic debris, is easy, safe, and rapid. Although it has a high incidence of local recurrence (4-25%), open partial cystectomy can be a plausible procedure in difficult patients [6-8].
Relapse is a main problem after liver hydatid cyst operation. Ultrasonography only is not enough to identify recurrences following surgical treatment. The antibody titers can remain positive long after operation, and only demonstration of the scolices in the remaining cavity provides final diagnosis. Residual cavity can be misdiagnosed as a recurrence. Ultrasonographic appearance of postoperative residual cavity can be similar to type 1 hydatid cyst [6,9]. On the other hand, type 2, 3 and 5 hydatid cysts have pathognomonic US and CT findings thanks to the presence of membrane separation, daughter cysts surrounded by a capsule and wall calcification around the cyst [10,11]. Postoperative residual cavity remains to be a main problem in endemic regions, because it may be confused with primer or post-operative cysts (biloma, lymphocele). Furthermore, there is no consensus on the diagnostic methods for detection and follow-up of recurrences [12]. Diagnosis of hydatid cyst involves a combination of imaging techniques and serological analysis [13].
Diffusion-weighted magnetic resonance imaging (DWI) is based on the restriction of the random microscopic motion of water molecules in different tissues [14]. To the best of our knowledge, there are studies using DWI in the classification of hepatic hydatid cysts, in the differential diagnosis of simple cyst and hepatic hydatid cysts as well as in the differential diagnosis of abscess and hepatic hydatid cysts [15-18]. But no research study has been performed yet to examine the efficacy of DWI in follow-up of patients treated with open partial cystectomy for liver hydatid cysts. By this way, the aim of this study was to evaluate the efficacy of DWI in differentiation of patients with residual cavity and type 1 hydatid cyst in the liver.
Method
Study design and patients
This prospective study was conducted at the Radiology Department of our University School of Medicine. Prior to subject recruitment, the study protocol was reviewed and approved by the university ethics committee, in accordance with the ethical principles for human investigations, as outlined by the Second Declaration of Helsinki and written informed consents were obtained from all the patients. From June-2011 to November-2011 consecutively 32 patients were included. 12 of these patients had type 1 hydatid cysts and the remainders (n = 20) had postoperative residual cavities. Flow diagram of the study was given in Figure 1. Diagnosis of type 1 hydatid cysts was performed using the Gharbi ultrasound classification (Table 1) and serological tests. Patients with residual cavities had been operated due to Gharbi type 1 (n = 2), 2 (n = 7), 3 (n = 10) and 4 (n = 1) HC. Our operative approach was open partial cystectomy with external drainage. Postoperative follow-up program was routinely done using the enzyme linked immunosorbent assay (ELISA), indirect hemagglutination test for echinococcosis (IHA) and abdominal ultrasonography in the sixth and the twelfth postoperative month. In follow up, patients with residual cavities were detected by US. In these patients, the antibody titers start to decrease or become negative between 6th and 12th months. Therefore, these patients were accepted as patients with non-relapse residual cavity. Then, MRI was performed in all patients. In the operated population, MRI was taken at about 12th month of follow-up after the liver hydatid cyst surgery.
Figure 1.
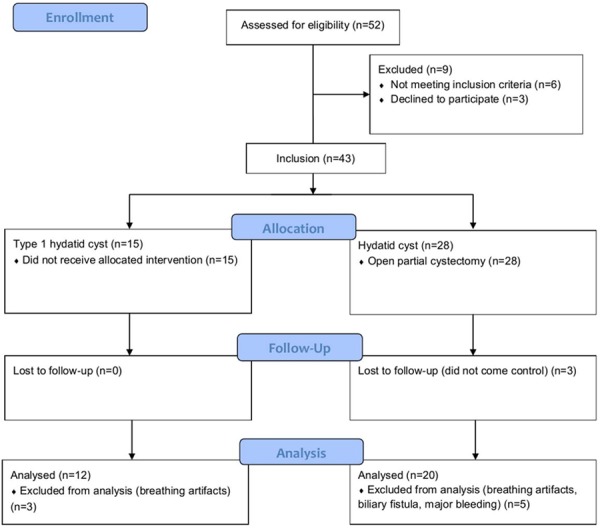
Flow diagram of the study.
Table 1.
Gharbi and WHO-Informal Working Group on Echinococcosis (WHO-IWGE) classification of hydatid cysts
| Gharbi | WHO-IWGE | Ultrasound characteristics |
|---|---|---|
| - | CL | Unilocular, cystic lesion with uniform anechoic content, cyst wall not visible |
| Type 1 | CE 1 | Unilocular, simple cyst with uniform anechoic content or fine echoes called hydatid sand (“snow flake sign”) |
| Type 3 | CE 2 | Multivesicular, multiseptated cyst, daughter cysts (“wheel-like”, “rosette-like” or “honeycomb-like” structures) |
| Type 2 | CE 3 | Anechoic content with detachment of laminated membrane (“water-lily sign”), cysts which may contain daughter cysts |
| Type 4 | CE 4 | Heterogenous hypoechoic or hyperechoic degenerative contents. |
| Type 5 | CE 5 | Cyst characterized by partial or complete calcified wall |
CL: Cystic Lesion; CE: Cystic Echinococcosis.
Exclusion criteria for the study were defined as follows; lesions smaller than 1 cm, other liver diseases, inadequate MRI studies, contraindications for MRI. One patient who had major bleeding into the residual cavity after surgery and two patients who had biliary fistula were excluded from the study. Furthermore, 3 cases of type 1 hydatid cysts located in the left lobe and 2 cases of the residual cavities located in the left lobe were excluded from the patient group in order to reduce the negative effects on ADC values of breathing artifacts.
MRI protocol and imaging analysis
Magnetic resonance imaging was performed using a 1.5 Tesla Magnetom Symphony A Tim System (Siemens, Erlangen, Germany). In all patients, axial T2-weighted and DWI images were taken directed at the upper abdomen. Images were taken with the patient in a supine position with a 16-channel body coil placed over the liver. Cardiac gating was not used in all patients. Respiratory triggering was simultaneously applied in 3 patients with residual cavities and 2 patients with type 1 hydatid cysts. Breath holding was preferred in the remaining patients. T2-weighted images were taken as TR 3440, TE 87, and NEX 1. By selecting TR 6000 ms, TE 88 ms, FOV 380 mm, matrix 128 × 256, NEX 4 on single shot, spin echo, echo planar (SS-SE-EP) DWI, images were obtained with values of b 0, 1000 s/mm2. An ADC map of the images was automatically generated and the ADC values were measured on this map for all patients. The circular region of interest (ROI) used for quantitative measurements of ADC values of the type 1 hydatid cysts and the residual cavities was located to cover almost the entire lesion (Figures 2, 3). The ROI was set so that the measurement area did not extend beyond the cyst. For each patient meeting the criteria defined above, three measurements were taken from the same level. The mean of the three ADC values obtained was used for evaluation. A comparison was made of type 1 hydatid cysts and residual cavities which resulted from the obtained values. Localization and mean diameter of the lesions were recorded. The readers were blinded to all clinical information. All DWI assessments were performed by the two experienced radiologists.
Figure 2.
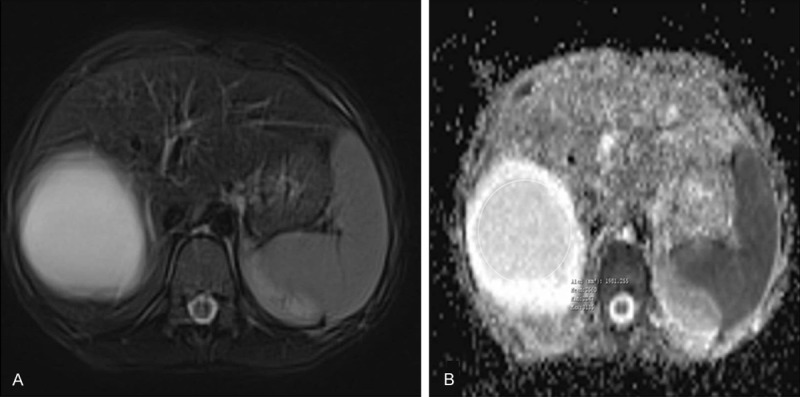
Magnetic resonance images of 9-year-old girl patient with type 1 hydatid cysts. A. Axial T2-weighted fast spin-echo MRI. B. ADC map show type 1 hydatid cysts in segment 7-8 of the liver. ADC value of postoperative residual cavity is 2.56 × 10-3 s/mm2.
Figure 3.
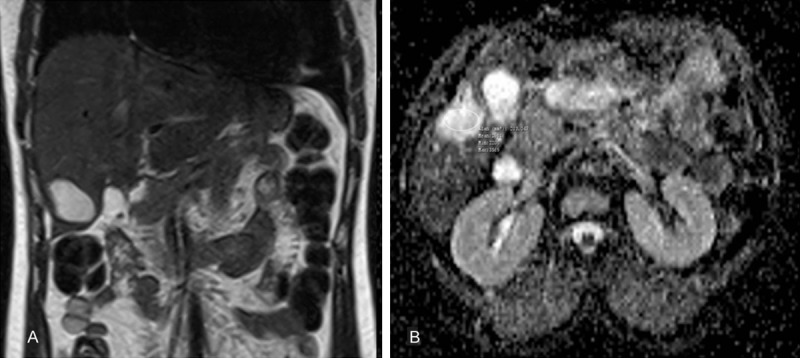
Magnetic resonance images of 37-year-old female patient with postoperative residual cavity treated with open partial cystectomy and external drainage for liver hydatid cysts about 12 months ago. A. Axial T2-weighted fast spin-echo MRI. B. ADC map show postoperative residual cavity in segment 6 of the liver. ADC value of postoperative residual cavity is 2.63 × 10-3 s/mm2.
Statistical analysis
All statistical analyses were performed using SPSS for Windows version 15.0 (SPSS, Chicago, IL, USA). Kolmogorov-Smirnov tests were used to test the normality of data distribution. The data were expressed as median, minimum and maximum values. The chi-square test was used to compare the categorical variables between groups. Mann-Whitney U test was used for comparison of continuous variables between two groups. A two-sided p value < 0.05 was considered statistically significant.
Results
Type 1 hydatid cysts were located in segment 4-5-8 (n = 1), 5 (n = 1), 5-6 (n = 1), 6 (n = 1), 6-7 (n = 2), 7-8 (n = 3), and 8 (n = 3) of the liver. Residual cavities were located in segment 5 (n = 3), 5-6 (n = 1), 5-8 (n = 1), 6 (n = 4), 6-7 (n = 2), 7 (n = 3), 7-8 (n = 3), and 8 (n = 3) of the liver. The mean diameters of type 1 hydatid cyst and residual cavity groups were 83.42 mm, 49.30 mm, respectively (P = 0.001) (Table 2).
Table 2.
Comparison of the demographic characteristics, mean diameters and mean ADC values of type 1 HC and residual cavity groups
| Type 1 HC (n = 12) | Residual cavity (n = 20) | P | |
|---|---|---|---|
| Gender, female/male | 10/2 | 15/5 | 0.587 |
| Age, years | 29.17 ± 12.60 | 34.75 ± 20.44 | 0.533 |
| Mean diameters (mm) | 83.42 ± 30.20 | 49.30 ± 19.42 | 0.001* |
| Mean ADC values (× 10-3 s/mm2) | 2.58 ± 0.13 | 2.58 ± 0.16 | 0.953 |
Data were given as mean ± standard deviation.
P < 0.05 (By Mann-Whitney U Test).
Demographic characteristics and mean ADC values of the patients were presented on Table 2. There were no significant differences in gender and age between the groups (both P > 0.05). The mean ADC values of type 1 hydatid cyst and residual cavity groups were 2.58 ± 0.13 × 10-3 s/mm2, 2.58 ± 0.16 × 10-3 s/mm2 respectively. Compared to patients with residual cavity, patients with type 1 hydatid cysts had almost similar ADC values (P = 0.953) (Figure 4).
Figure 4.
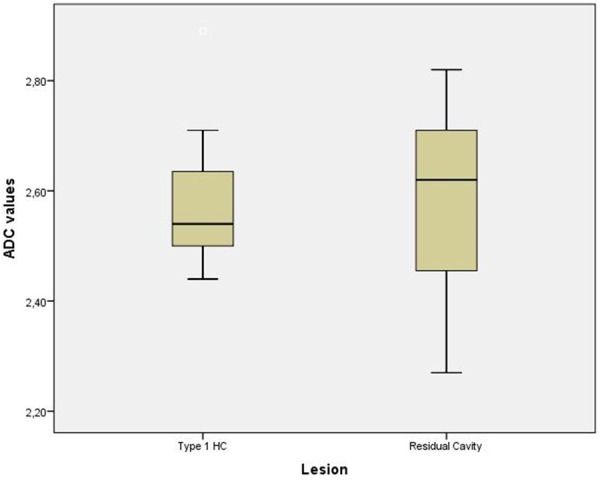
Box plots demonstrate ADC values for the patient groups. The boxes stretch from the 25th to the 75th percentiles. The horizontal line across each box is the median. The vertical lines with whiskers extending below and above the boxes indicate the minimal and maximal values respectively.
Discussion
To the best of our knowledge, this is the first report to evaluate and compare the ADC values of patients with type 1 hydatid cysts and residual cavities. The main findings of this study were that, (i) ADC values were not different between these patients, and (ii) DWI was not found to be a predictor in the differentiation of patients with residual cavity and type 1 hydatid cyst.
Echinococcosis is a zoonotic illness characterized by slowly growing cysts, mostly in liver and lungs. Hydatid diseases are found in sheep-farming areas, mainly in developing countries with poor hygiene [19]. Hydatid disease is endemic in particular areas of the world, including the Mediterranean countries, the Middle East, Eastern Europe, South America, Australia and New Zealand [20]. With immigration, the prevalence of the hydatid disease has raised in Europe and North America [21-24]. The management is primarily surgical; however medical therapy (mebendazole, albendazole) has a place in some cases [25].
In the current study open partial cystectomy with external drainage was performed in the 20 patients and a dose of 10-12 mg/kg of albendazole for 1 week prior to surgery and 3 months following surgery were given.
At present, diagnosis of hydatid cysts is easier than it was formerly thanks to the advance in new imaging techniques (US, CT, MRI), as well as in the development of reliable serological tests [26]. Hydatid immunoelectrophoresis, enzyme-linked immunosorbent assay (ELISA), and indirect hemagglutination (IHA) tests were performed for the diagnosis and post-operative follow up [27]. Serological tests have limitations for follow-up of patients after chemotherapy, PAIR, and surgery. The antibody titers increase after PAIR and surgery and then, the titers start to decrease at 3 months and become negative in a period of 12-14 months [28]. When the antibody titers start to increase according to the baseline values and/or residual cavities start to increase in size in the cross-sectional imaging, recurrence is considered. Postoperative residual cavity may be confused with primer or post-operative cysts in endemic regions. Furthermore, there is no consensus on the diagnostic methods for detection and follow-up of recurrences [12]. In our patients, the antibody titers started to decrease or became negative between 6th and 12th months and there was no the increase in the sizes of the residual cavities in follow-up ultrasound.
DWI is a non-invasive magnetic resonance imaging technique that is commonly chosen today. It identifies the tissues according to the diffusion properties of their water content, particularly with high b values. Initially, DWI was used in cases of acute brain infarction. Recently it has been used for the differentiation of epidermoid and arachnoid cysts, differential diagnosis for focal hepatic lesions and the discrimination of malignant and benign masses [29,30]. When compared with benign tumors, the cellular density of malignant tumors is higher. Hence the diffusion of water molecules is restricted in malignant tumors which have lower ADC values [31,32]. Hydatid fluid contains the scoleces, sodium chloride, protein, glycose, ion, lipid and polysaccharide content [33,34]. Some studies demonstrated that the ADC values of hydatid cysts were significantly lower than those of simple cysts [16,18]. We believe that the ADC values of hydatid cysts lessen because of the scoleces, sodium chloride, protein, glycose, ion, lipid and polysaccharide content.
Sonmez et al. [18] the mean ADC values of the type-1 HCs and the simple cysts were 2.27 × 10-3 s/mm2, 2.67 ± 0.04 × 10-3 s/mm2 respectively. A statistically significant difference was found between the ADC values of the simple cysts and the type 1 HCs (P = 0.01). When the cut-off value was accepted as 2.5 × 10-3 s/mm2, sensitivity, specificity, PPV, NPV and accuracy rates were 50%, 95%, 88%, 72% and 76%, respectively. Oruc et al. [17] the mean ADC value of simple cysts was 3.08 ± 0.17 × 10-3 s/mm2, whereas that of type 1 HCs was 2.84 ± 0.38 × 10-3 s/mm2. There was no statistically significant difference between the ADC values of simple cysts and those of type 1 HCs (P > 0.05). Inan et al. [16] the mean ADC value of simple cysts was 3.5 ± 0.5 × 10-3 s/mm2, whereas that of type 1 HCs was 2.5 ± 0.9 × 10-3 s/mm2. There was statistically significant difference between the ADC values of simple cysts and those of type 1 HCs (P = 0.012).
In the current study the mean ADC values of type 1 hydatid cyst and residual cavity groups were nearly the same values. A statistically significant difference was not determined between the groups. Our results emphasized that ADC measurements might not be suitable to differentiate the postoperative residual cavity from the type 1 hydatid cyst in the liver due to similarity of ADC values between postoperative residual cavity and type 1 hydatid cyst. The cause of decreased ADC values of post-operative cavities may be due to postoperative hemorrhage and infection within this cavity.
Certain limitations of the present study should be considered. First of all, a sample size was relatively small because of the rarity of the disease. The second limitation was the reduction in signal-to-noise ratio (S/N ratio) of artifacts associated with cardiac movements. The third limitation was the reduction in S/N ratio of artifacts associated with respiratory movements due to no used respiratory triggering in most patients. Another limitation is that although we paid attention not to include patients with hemorrhagic or infected fluid collection in postoperative period; to find out and exclude all of the patients in this group might not be clinically possible. Future studies would be improved with a larger number of patients by the application of pulse triggering, respiratory triggering, and particularly the use of 3T MRI to increase the S/N ratio.
Conclusion
This is the first report to evaluate the ADC values of patients with residual cavities, and also the first one to compare patients with type 1 hydatid cyst. Although DWI offers rapid, noninvasive, and quantitative assessment of these conditions, especially these results highlighted that using DWI might not be rational in determining recurrence of hydatid cysts.
Disclosure of conflict of interest
None.
References
- 1.Mcmanus DP, Zhang W, Li J, Bartley PB. Echinococcosis. Lancet. 2003;18:1295–304. doi: 10.1016/S0140-6736(03)14573-4. [DOI] [PubMed] [Google Scholar]
- 2.Craig PS, McManus DP, Lightowlers MW, Chabalgoity JA, Garcia HH, Gavidia CM, Gilman RH, Gonzalez AE, Lorca M, Naquira C, Nieto A, Schantz PM. Prevention and control of cystic echinococcosis. Lancet Infect Dis. 2007;7:385–94. doi: 10.1016/S1473-3099(07)70134-2. [DOI] [PubMed] [Google Scholar]
- 3.Rothlin M, Decurtins M, Largiader F. Results of surgery in Hepatic echinococcosis. Helv Chir Acta. 1994;60:587–92. [PubMed] [Google Scholar]
- 4.Scmidt-Matthiesen A, Schott O, Encke A. Surgery and longterm Follow-up of hepatic Echinococcosis outside endemic Regions. Z Gastroenterol. 2002;40:51–7. doi: 10.1055/s-2002-20208. [DOI] [PubMed] [Google Scholar]
- 5.Pitt HA, Korzellus J, Tompkins RK. Management of hepatic Echinococcosis in Southern California. Am J Surg. 1986;152:110–5. doi: 10.1016/0002-9610(86)90159-5. [DOI] [PubMed] [Google Scholar]
- 6.Dawson JL, Stamatakis JD, Stringer MD, Williams R. Surgical treatment of hepatic hydatid disease. Br J Surg. 1988;75:946–50. doi: 10.1002/bjs.1800751004. [DOI] [PubMed] [Google Scholar]
- 7.Langer JC, Rose DB, Keystone JS, Taylor BR, Langer B. Diagnosis and management of hydatid disease of the liver. A 15-Year North American experience. Ann Surg. 1984;199:412–7. doi: 10.1097/00000658-198404000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erdem E, Nessar M, Sungurtekin U, Ozden A, Tetik C. The management of hepatic hydatid cysts: review of 94 cases. J Hepatobil Pancreat Surg. 1998;5:179–83. doi: 10.1007/s005340050030. [DOI] [PubMed] [Google Scholar]
- 9.Bozkurt B, Soran A, Karabeyoglu M, Unal B, Coskun F, Cengiz O. Follow-up problems and changes in obliteration of the residual cystic cavity after treatment for hepatic hydati-dosis. J Hepatobiliary Pancreat Surg. 2003;10:441–5. doi: 10.1007/s00534-003-0853-8. [DOI] [PubMed] [Google Scholar]
- 10.Pandolfo I, Blandino G, Scribano E, Longo M, Certo A, Chirico G. CT findings in hepatic involvement by Echinococcus granulosus. J Comput Assist Tomogr. 1984;8:839–45. doi: 10.1097/00004728-198410000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Suwan Z. Sonographic findings in hydatid disease of the liver: comparison with other imaging methods. Ann Trop Med Parasitol. 1995;89:261–9. doi: 10.1080/00034983.1995.11812951. [DOI] [PubMed] [Google Scholar]
- 12.Sielaff TD, Taylor B, Langer B. Recurrence of hydatid disease. World J Surg. 2001;25:83–6. doi: 10.1007/s002680020011. [DOI] [PubMed] [Google Scholar]
- 13.Eckert J, Deplazes P. Biological, epidemiological and clinical aspects ofechinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev. 2004;17:107–35. doi: 10.1128/CMR.17.1.107-135.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Lavai-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology. 1986;161:401–7. doi: 10.1148/radiology.161.2.3763909. [DOI] [PubMed] [Google Scholar]
- 15.Ceçe H, Gündoğan M, Karakaş O, Karakaş E, Boyacı FN, Yıldız S, Ozgönül A, Karakaş EY, Cullu N, Seker A. The role of diffusion-weighted magnetic resonance imaging in the classification of hepatic hydatid cysts. Eur J Radiol. 2013;82:90–4. doi: 10.1016/j.ejrad.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 16.Inan N, Arslan A, Akansel G, Anik Y, Sarisoy HT, Ciftci E, Demirci A. Diffusion-weighted imaging in the differential diagnosis of simple and hydatid cysts of the liver. AJR Am J Roentgenol. 2007;189:1031–6. doi: 10.2214/AJR.07.2251. [DOI] [PubMed] [Google Scholar]
- 17.Oruç E, Yıldırım N, Topal NB, Kılıçturgay S, Akgöz S, Savcı G. The role of diffusion-weighted MRI in the classification of liver hydatid cysts and differentiation of simple cysts and abscesses from hydatid cysts. Diagn Interv Radiol. 2010;16:279–87. doi: 10.4261/1305-3825.DIR.2807-09.2. [DOI] [PubMed] [Google Scholar]
- 18.Sonmez G, Sivrioglu AK, Mutlu H, Ozturk E, Incedayı M, Karaman B, Basekim CC. Is it possible to differentiate between hydatid and simple cysts in the liver by means of diffusion-weighted magnetic resonance imaging? Clin Imaging. 2012;36:41–5. doi: 10.1016/j.clinimag.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Schipper HG, Kager PA. Diagnosis and treatment of hepatic echinococcosis, an overview. Scand J Gastroenterol Suppl. 2004;241:50–5. doi: 10.1080/00855920410011004. [DOI] [PubMed] [Google Scholar]
- 20.Bourée P. Hydatidosis: dynamics of transmission. World J Surg. 2001;25:4–9. doi: 10.1007/s002680020001. [DOI] [PubMed] [Google Scholar]
- 21.Arambulo P. Public health importance of cystic echinococcosis in Latin America. Acta Trop. 1997;67:113–24. doi: 10.1016/s0001-706x(97)00049-1. [DOI] [PubMed] [Google Scholar]
- 22.Eckert J, Thompson RC. Echinococcus strains in Europe. Trop Med Parasitol. 1988;39:1–8. [PubMed] [Google Scholar]
- 23.Jenkins DJ. Hydatidosis: a zoonosis of unrecognised increasing importance? J Med Microbiol. 1998;47:281–2. doi: 10.1099/00222615-47-4-281. [DOI] [PubMed] [Google Scholar]
- 24.Euzeby J. The epidemiology of hydatidosis with reference to the Mediterranean area. Parasitotogica. 1991;33:25–39. [PubMed] [Google Scholar]
- 25.Seiferth T, Endsberger G, Stolte M. Echinococcosis-current status of diagnosis and therapy. Leber Magen Darm. 1993;23:161–4. [PubMed] [Google Scholar]
- 26.Biava MF, Dao A, Fortier B. Laboratory diagnosis of cystic hydatid disease. World J Surg. 2001;25:10–4. doi: 10.1007/s002680020002. [DOI] [PubMed] [Google Scholar]
- 27.Farmer PM, Chatterley S, Spier N. Echinococcal cyst of the liver: diagnosis and surgical management. Ann Clin Lab Sci. 1990;20:385–91. [PubMed] [Google Scholar]
- 28.Khuroo MS, Wani NA, Javid G, Khan BA, Yattoo GN, Shah AH, Jeelani SG. Percutaneous drainage compared with surgery for hepatic hydatid cysts. N Engl J Med. 1997;337:881–7. doi: 10.1056/NEJM199709253371303. [DOI] [PubMed] [Google Scholar]
- 29.Kilickesmez O, Bayramoglu S, Inci E, Cimilli T. J Value of apparent diffusion coefficient measurement for discrimination of focal benign and malignant hepatic masses. J Med Imaging Radiat Oncol. 2009;53:50–5. doi: 10.1111/j.1754-9485.2009.02036.x. [DOI] [PubMed] [Google Scholar]
- 30.Negru D, Fotea V, Grigoraş M, Moisii L, Daniil C. MRI in the differential diagnosis of liver focal lesions. Rev Med Chir Soc Med Nat Iasi. 2006;110:548–54. [PubMed] [Google Scholar]
- 31.Taouli B, Koh DM. Diffusion-weighted MR imaging of the liver. Radiology. 2010;254:47–66. doi: 10.1148/radiol.09090021. [DOI] [PubMed] [Google Scholar]
- 32.Kim T, Murakami T, Takahashi S, Hori M, Tsuda K, Nakamura H. Diffusion-weighted single-shot echoplanar MR imaging for liver disease. AJR Am J Roentgenol. 1999;173:393–8. doi: 10.2214/ajr.173.2.10430143. [DOI] [PubMed] [Google Scholar]
- 33.Pedrosa I, Saíz A, Arrazola J, Ferreirós J, Pedrosa CS. Hydatid disease: radiologic and pathologic features and complications. Radiographics. 2000;20:795–817. doi: 10.1148/radiographics.20.3.g00ma06795. [DOI] [PubMed] [Google Scholar]
- 34.Volders WK, Gelin G, Stessens RC. Best cases from the AFIP. Hydatid cyst of the kidney: radiologic-pathologic correlation. Radiographics. 2001;21:255–60. doi: 10.1148/radiographics.21.suppl_1.g01oc16s255. [DOI] [PubMed] [Google Scholar]


