Abstract
Previous studies demonstrated that systemic administration of lidocaine could induce central nervous toxicities, including behavioral convulsion as well as cognitive and cellular injury. Ketamine, a general anesthetic, is commonly used as an adjuvant in regional anesthesia with combination of lidocaine. The present was designed to investigate the effects of ketamine in central nervous toxicities of lidocaine. Ketamine (1.2 mg/kg, i.v.) was intravenously injected before and/or after administration of lidocaine in rats. After injection of lidocaine, convulsive behaviors of rats were scored according to the modified Racine scale. Cognitive functions and pathology of hippocampus CA3 pyramid neurons of these rats were evaluated on the one, three, five, and seven days after lidocaine induced convulsion. Both pre- and/or post-administration of ketamine (1.2 mg/kg) could significantly improve lidocaine induced convulsive behaviors of rats (P < 0.01). One, three, five, and seven days after lidocaine induced convulsion, cognitive function and pathology of hippocampus CA3 pyramid neurons of these rats were significantly impaired. In addition, cognitive functions and pathology of neurons of the rats that received ketamine (both pre- and/or post-) were further impaired, compared to the rats without ketamine. We conclude that both pre- and/or post-administration of ketamine could improve lidocaine induced convulsive behaviors. However, cognitive functions and pathology of neurons of these rats are further impaired, compared to the rats without ketamine. This result indicates that ketamine combined with lidocaine might be risky in regional anesthesia.
Keywords: Lidocaine, central nervous toxicities, ketamine, rats
Introduction
Lidocaine is an usual local anesthetic, commonly used in clinical regional anesthesia. Besides its regional anesthetic effect, lidocaine is also intravenously administrated for pain management and as general anesthetic adjuvant [1-3]. However, lidocaine could induce central nervous and cardiac toxicities when its blood concentrations reach to toxic thresholds [4,5]. Usually, central nervous toxicity of lidocaine result from the diffusion of lidocaine to systemic circulation after regional injection and the probability of systemic toxicities is even more frequent after intravenous injection.
By previous studies, systemic lidocaine could impair cognitive functions of rats and percentage of normal hippocampus CA3 pyramid neurons of these rats was significantly decreased [6,7]. These findings indicate that central nervous toxicities of lidocaine are not only represented as convulsive behaviors but also as impaired neurological functions and cellular morphology after convulsion.
Ketamine is a general anesthetic and commonly applied in clinic, especially in pediatric anesthesia [8-10]. Except for its general anesthetic effect, ketamine is also routinely used as an adjuvant in various regional anesthetic procedures, combined with local anesthetics [11-16]. By adding ketamine in local anesthetics, analgesic effects of local anesthetics might be improved, especially for post-operative analgesia [11-16]. Thus, ketamine and lidocaine would be administrated together in clinical setting. As a potent sedative agent, ketamine would improve convulsive behaviors induced by lidocaine [17]; however, the effect of ketamine in central nervous toxicity of lidocaine is still unclear. Whether ketamine would impair neurological outcomes of convulsion induced by lidocaine is an important concern about application of ketamine in regional anesthesia.
The present study was designed to evaluate the effects of ketamine in central nervous toxicities of lidocaine, including both convulsive behaviors and neurological outcomes after convulsion.
Methods
Animals and chemicals
With the approval of the Institutional Animal Experimental Ethics Committee of the Second Hospital of Fuzhou (Fuzhou, Fujian, China), male Wistar rats (weighing 200 to 300 g) were used in this study. All the rats were housed in cages with free access to food and water and were kept on a 12-hour light-dark cycle with room temperature maintained at 20-25°C. Lidocaine used in the present study was purchased from Guangzhou Baiyunshan Pharmaceutical Co., Ltd. (Guangdong, China). Ketamine was purchased from Jiangsu Hengrui Medicine Co., Ltd. (Jiangsu, China).
Convulsive model of lidocaine in rats
Experiments of convulsive behaviors were consisted of two parts: pre-injection and/or post-injection of ketamine. Based on preliminary experiments and previous study [18], dose of ketamine was set at 1.2 mg/kg (light sedation i.v.). All the systemic administration was conducted by intravenous injection through tail vein of rats. For the protocol of pre-injection of ketamine, 80 rats were randomly divided into two groups: respectively receiving ketamine (ketamine/lidocaine group) and saline (saline/lidocaine group) before injection of lidocaine. After injection of ketamine or saline, lidocaine at dose of 20 mg/kg was intravenously injected with single bolus. For the protocol of post-injection of ketamine, convulsion was induced by intravenous infusion of lidocaine at speed of 3.3 mg/min (1%, 20 ml/h). The convulsive behaviors of rats were scored according to a scale modified from Racine scale [19]: 0 = no convulsive behavior; 1 = sedation, less activity but revival of righting reflex; 2 = facial clonus or head nodding; 3 = forelimb clonus; 4 = rearing, animal in a standing posture aided by tail and laterally spread hind limbs showing increased tone; 5 = tonic-clonic seizure. Immediately after the appearance of tonic-conic seizure (score 5), the infusion of lidocaine was stopped and 80 rats were randomly divided into two groups: respectively receiving ketamine (lidocaine/ketamine group) and saline (lidocaine/saline group) to treat the convulsive behaviors. For all 80 rats, convulsive behaviors were scored at 30-second intervals from the appearance of tonic-clonic seizure to 20 minutes later.
Evaluation of cognitive function of rats after convulsion
Except for the above mentioned 160 rats (experienced convulsion), ten rats that not experiencing convulsions were tested (baseline group). For all the 160 lidocaine that experienced convulsion (4 groups, n = 40/group), the cognitive function of rats was tested respectively on the one, three, five, seven days after convulsion (ten rats/day). Cognitive function of rats was evaluated by Y-maze test as previously reported [20]. Briefly, Y-maze consisted of three arms: one start arm and two branch arms. Electric shock (40 ± 5 V) could be applied through metallic wires at the bottom of the three arms. Meanwhile, all the three arms could be illuminated by bulb. During the evaluation, rat was placed in the start arm and electric shock was applied to the start arm and one of the branch arms while the other branch arm was illuminated (For branch arms, electric shock or illumination was randomly). After electric shock, rat escaped to one of the two branch arms. When each trial finished, the rat was put back to start arm. For the rats, successful development of aversive memory was defined as the rat escaped into a non-shock (illuminated) arm in 9 of 10 successive trials.
Pathological evaluation of hippocampus CA3 neurons
All rats were sacrificed for pathological evaluation as previously described 6 after behavioral tests. In brief, after aortal infusion of paraformaldehyde, rats were decapitated and the brain of rats was removed and embedded in paraffin. Brain slices were obtained in the region of -2.3 to -3.8 mm from Bregma. Nissl’s staining (toluidine blue) was performed on the brain slices. Images of stained brain slices were taken by a BX51 microscope (Olympus, Japan) coupled with DPTO camera (Olympus, Japan) with magnification of × 400. For hippocampal cornu ammonis 3 (CA3) region, number of normal and abnormal pyramidal neurons was counted by a trained pathologist blinded to groups, based on the established standard [21]: distinguishable nucleus and nucleolus, uniform cytoplasma, and neuronal shape in accordance with the usual rules of stereological study of neuron identification.
Statistical analysis
Software SPSS 16.0 (SPSS Inc., Chicago, IL) was used for all statistical analyses except for particular mention. For some occasions, software Prism 5.0 (GraphPad, San Diego, CA) was used. Because of skew distribution, convulsive rates of the all groups were compared by nonparametric analysis: the whole “survival curves” of convulsive rate were compared by the Kaplan-Meier method with log-rank test. For comparison of convulsive scores, Kruskal-Wallis test followed by post hoc of Dunn test (software Prism 5.0) was applied among the four groups. Cognitive function of rats was represented by the number of trials in Y-maze that developed aversive memory. Number of trials was expressed as mean ± SD and one-way ANOVA followed by post hoc of LSD (normal distribution) or Games-Howell (skew distribution) test was applied. Percentage of normal to abnormal neurons in hippocampal CA3 region was expressed as mean ± SD. One-way ANOVA followed by post hoc tests with LSD (normal distribution) test or Games-Howell test (skew distribution) was applied for the comparison among the four groups. In all cases, P < 0.05 was considered statistically significant.
Results
Ketamine improved lidocaine induced convulsive behaviors
Pre-injection of ketamine (1.2 mg/kg, i.v.) could significantly prevent lidocaine induced convulsive state (Figure 1). For convulsive rate (Figure 1A), ketamine/lidocaine group was significantly improved than saline/lidocaine group (P < 0.01 by Kaplan-Meier test). Meanwhile, for convulsive scores (Figure 1B), ketamine/lidocaine group was also significantly improved than saline/lidocaine group (P < 0.01 by Kruskal-Wallis test). For post-injection of ketamine, when convulsion was already induced by lidocaine, convulsive behaviors of rats were also significantly improved both in convulsive rate and convulsive scores (lidocaine/saline group vs. lidocaine/ketamine group, P < 0.01). These results indicated that pre-injection of ketamine could significantly prevent lidocaine induced convulsive state and already induced convulsion could also be treated by post-injection of ketamine.
Figure 1.
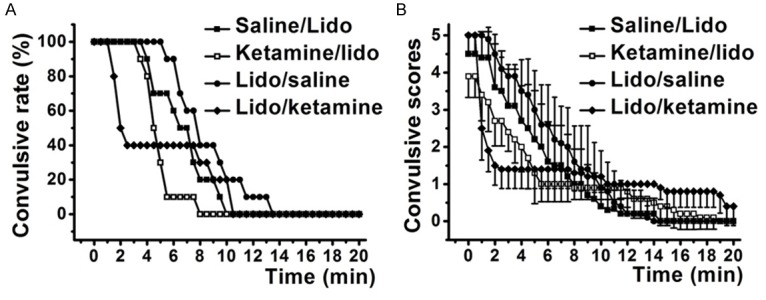
Lidocaine induced convulsive behaviors were significantly improved by both pre- and/or post-injection of ketamine. Ketamine/lidocaine group indicated the pre-injection of ketamine before administration of lidocaine and Saline/lidocaine group was used as control; Lidocaine/ketamine group indicated the post-injection of ketamine after lidocaine induced tonic-clonic seizure and Lidocaine/saline group was used as control. A. Positive convulsion was defined as convulsive score up to 2 according to the Racine scale. B. Convulsive scores of rats were decreased by ketamine.
Ketamine further impaired cognitive function of rats after convulsion
Comparing to baseline group, systemic injection of lidocaine could impair cognitive function of rats (saline/lidocaine and lidocaine/saline groups vs. baseline, P < 0.05, Figure 2). For the rats in the ketamine/lidocaine and lidocaine/ketamine groups, number of Y-maze trials that rats develop aversive memory was significantly increased, comparing to saline groups (P < 0.05 by One-way ANOVA).
Figure 2.
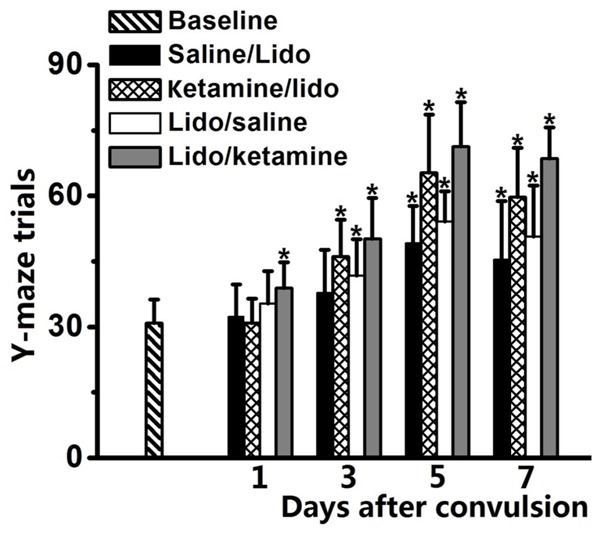
Cognitive function of rats after lidocaine induced convulsion was evaluated by Y-maze test and result was represented as number of trials in Y-maze test. Increased trials to develop aversive memory represented impairment of cognitive function. Ketamine/lidocaine group indicated the pre-injection of ketamine before administration of lidocaine and Saline/lidocaine group was used as control; Lidocaine/ketamine group indicated the post-injection of ketamine after lidocaine induced tonic-clonic seizure and Lidocaine/saline group was used as control. *P < 0.05, comparing to baseline group.
Ketamine increased percentage of normal neurons in hippocampus CA3 after convulsion
Percentage of normal neurons in hippocampus CA3 region was significantly decreased after lidocaine induced convulsion at one, three, five and seven days (saline/lidocaine and lidocaine/saline groups vs. baseline, P < 0.01, Figure 3). For the rats in the ketamine/lidocaine and lidocaine/ketamine groups, percentage of normal neurons in hippocampus CA3 region was further decreased, comparing to saline/lidocaine and lidocaine/saline (P < 0.01). Of note, comparing to ketamine/lidocaine group, percentage of normal neurons in lidocaine/ketamine group was further decreased (ketamine/lidocaine group vs. lidocaine/ketamine group, P < 0.01). Morphological samples of hippocampus CA3 pyramidal neurons in all the four groups were revealed in Figure 4.
Figure 3.
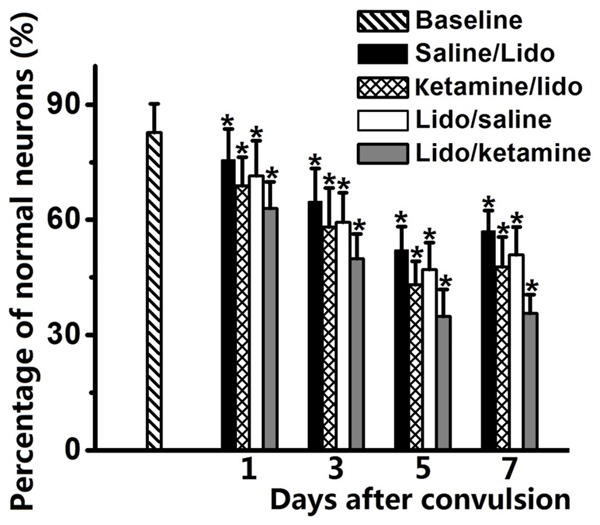
Percentage of normal hippocampus CA3 pyramid neurons was significantly decreased after lidocaine induced convulsion. Both pre- and/or post-injection of ketamine could further decrease the percentage of normal neurons. Ketamine/lidocaine group indicated the pre-injection of ketamine before administration of lidocaine and Saline/lidocaine group was used as control; Lidocaine/ketamine group indicated the post-injection of ketamine after lidocaine induced tonic-clonic seizure and Lidocaine/saline group was used as control. *P < 0.05, comparing to baseline group.
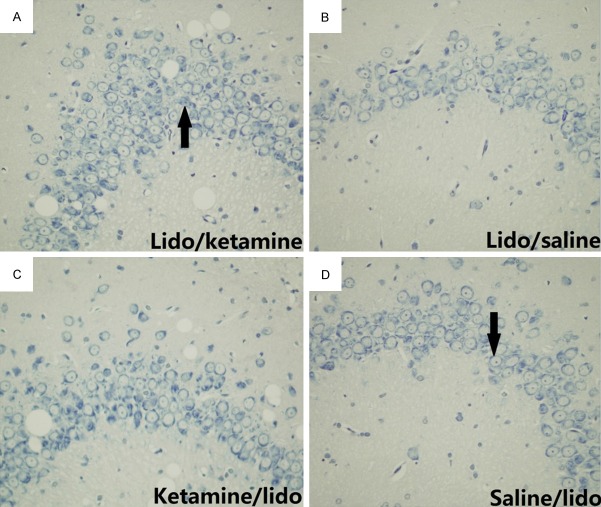
Figure 4.
Abnormality of CA3 hippocampal neurons caused by systemic lidocaine was revealed by Nissl’s staining. Ketamine/lidocaine group indicated the pre-injection of ketamine before administration of lidocaine and Saline/lidocaine group was used as control; Lidocaine/ketamine group indicated the post-injection of ketamine after lidocaine induced tonic-clonic seizure and Lidocaine/saline group was used as control. The arrow in part D indicted typical normal neuron while the arrow in part A indicated abnormal neuron, based on the standard as previously described [6].
Discussion
In the present study, we found that both pre-injection and/or post-injection of ketamine could significantly improve lidocaine induced convulsive behaviors. However, neural outcomes after convulsion including cognitive function of rats and normality of hippocampus CA3 neurons were further impaired by ketamine than lidocaine alone. For clinical setting, the combined administration of lidocaine and ketamine are routinely both in general and regional anesthesia and ketamine is a commonly used adjuvant in regional anesthesia combined with local anesthetics [11-16]. By previous studies, ketamine in regional anesthesia could enhance analgesic effects of local anesthetics and prolonged post-operative analgesia, especially useful in IVRA [11-16]. However, based on the results of the present study, the combination of ketamine and lidocaine might be risky because ketamine could enhance neural toxicities of lidocaine, inducing worse neural outcomes. In addition, for sedative potency of ketamine, lidocaine induced convulsive behaviors might be attenuated when ketamine is administered with lidocaine, thus, the underlying risk could be underestimated.
The dose of intravenous ketamine in the present study was set at 1.2 mg/kg because this is a relevant dose of ketamine both for light sedation [18] and regional anesthetic adjuvant [11-16]. Ketamine at dose of 1-2 mg/kg (i.v.) is a commonly used dose for hypnosis [18]. For treatment of convulsion, the dose at light sedation is sufficient [7], thus, we chose the dose of ketamine at light sedation in rats. In addition, as regional anesthetic adjuvant, ketamine is used from about 0.5-1 mg/kg in IVRA [11,14,15]. Therefore, based on this study and previous studies, ketamine at regional anesthetic relevant doses is already risky.
By previous studies, the mechanisms of lidocaine to induce convulsion might be from activation of limbic structures such as hippocampus and amygdala 6 or from depressing cortical inhibitory neurons [22]. Hippocampal region is the most important brain region where memory information processed. And CA3 network of the hippocampus is the most critical part for spatial memory storage [23]. Previous studies reported that hippocampal CA3 region is susceptible to chemical agents and ischemia induced injury [24]. Systemic injection of lidocaine has been demonstrated to impair normal hippocampus CA3 pyramid neurons even at sub-convulsive doses [6]. By in vitro study, activity of hippocampal neurons and caspase-3 expression in hippocampal slices were significantly affected by lidocaine [25]. In addition, cerebral injection of lidocaine could alter cognitive functions that relevant to caudate nucleus and hippocampus [26,27]. In the present study, cognitive function of rats and normality of neurons were significantly impaired after lidocaine induced convulsion and ketamine could aggravate these injuries. Ketamine is a potent NMDA (N-methyl-D-aspartic acid receptor) receptor antagonist [28] and neural activities as well as cellular concentrations of calcium ion might be affect by ketamine. In addition, NMDA receptor has been demonstrated to be involved in learning and memory [29-31]. However, it is still unknown whether ketamine enhances central nervous toxicities of lidocaine by affecting calcium homeostasis.
There are still some limitations in the present study: firstly, only one dose of ketamine was tested in the present study for the reason to control animal use. If dose-response of ketamine in central nervous toxicities of lidocaine was determined, it would be very valuable for clinical application of ketamine in regional anesthesia. Secondly, cognitive function impairment and abnormalities of CA3 neurons were most significant on the fifth and seventh day after convulsion. We did not determine whether these neural injures would resolve spontaneously or even aggravate.
In summary, the present study demonstrated that both pre- and/or post-injection of ketamine could improve lidocaine-induced convulsive behaviors in rats, but neural outcomes of the rats were further impaired comparing to lidocaine alone. This finding indicates that combined application of ketamine and lidocaine might be risky in some occasions.
Disclosure of conflict of interest
None.
References
- 1.Aouad MT, Sayyid SS, Zalaket MI, Baraka AS. Intravenous lidocaine as adjuvant to sevoflurane anesthesia for endotracheal intubation in children. Anesth Analg. 2003;96:1325–1327. doi: 10.1213/01.ANE.0000061586.63978.DE. [DOI] [PubMed] [Google Scholar]
- 2.Challapalli V, Tremont-Lukats IW, McNicol ED, Lau J, Carr DB. Systemic administration of local anesthetic agents to relieve neuropathic pain. Cochrane Database Syst Rev. 2005:CD003345. doi: 10.1002/14651858.CD003345.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith LJ, Shih A, Miletic G, Miletic V. Continual systemic infusion of lidocaine provides analgesia in an animal model of neuropathic pain. Pain. 2002;97:267–273. doi: 10.1016/S0304-3959(02)00028-3. [DOI] [PubMed] [Google Scholar]
- 4.Chiang YY, Tseng KF, Lih YW, Tsai TC, Liu CT, Leung HK. Lidocaine-induced CNS toxicity-a case report. Acta Anaesthesiol Sin. 1996;34:243–246. [PubMed] [Google Scholar]
- 5.Steen PA, Michenfelder JD. Neurotoxicity of anesthetics. Anesthesiology. 1979;50:437–453. doi: 10.1097/00000542-197905000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Blas-Valdivia V, Cano-Europa E, Hernandez-Garcia A, Ortiz-Butron R. Hippocampus and amygdala neurotoxicity produced by systemic lidocaine in adult rats. Life Sci. 2007;81:691–694. doi: 10.1016/j.lfs.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Zhou C, Huang H, Liu J, Wang X, Chen X, Zhang W. Emulsified isoflurane increases convulsive thresholds of lidocaine and produces neural protection after convulsion in rats. Anesth Analg. 2014;118:310–317. doi: 10.1213/ANE.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 8.Cho HK, Kim KW, Jeong YM, Lee HS, Lee YJ, Hwang SH. Efficacy of ketamine in improving pain after tonsillectomy in children: meta-analysis. PLoS One. 2014;9:e101259. doi: 10.1371/journal.pone.0101259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doctor K, Roback MG, Teach SJ. An update on pediatric hospital-based sedation. Curr Opin Pediatr. 2013;25:310–316. doi: 10.1097/MOP.0b013e328360bb92. [DOI] [PubMed] [Google Scholar]
- 10.Schnabel A, Poepping DM, Kranke P, Zahn PK, Pogatzki-Zahn EM. Efficacy and adverse effects of ketamine as an additive for paediatric caudal anaesthesia: a quantitative systematic review of randomized controlled trials. Br J Anaesth. 2011;107:601–611. doi: 10.1093/bja/aer258. [DOI] [PubMed] [Google Scholar]
- 11.Abdel-Ghaffar HS, Kalefa MA, Imbaby AS. Efficacy of ketamine as an adjunct to lidocaine in intravenous regional anesthesia. Reg Anesth Pain Med. 2014;39:418–422. doi: 10.1097/AAP.0000000000000128. [DOI] [PubMed] [Google Scholar]
- 12.Bosenberg A. Adjuvants in pediatric regional anesthesia. Pain Manag. 2012;2:479–486. doi: 10.2217/pmt.12.51. [DOI] [PubMed] [Google Scholar]
- 13.Elmetwaly KF, Hegazy NA, Aboelseoud AA, Alshaer AA. Does the use of ketamine or nitroglycerin as an adjuvant to lidocaine improve the quality of intravenous regional anesthesia? Saudi J Anaesth. 2010;4:55–62. doi: 10.4103/1658-354X.65122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorgias NK, Maidatsi PG, Kyriakidis AM, Karakoulas KA, Alvanos DN, Giala MM. Clonidine versus ketamine to prevent tourniquet pain during intravenous regional anesthesia with lidocaine. Reg Anesth Pain Med. 2001;26:512–517. doi: 10.1053/rapm.2001.27857. [DOI] [PubMed] [Google Scholar]
- 15.Kumar A, Sharma D, Datta B. Addition of ketamine or dexmedetomidine to lignocaine in intravenous regional anesthesia: A randomized controlled study. J Anaesthesiol Clin Pharmacol. 2012;28:501–504. doi: 10.4103/0970-9185.101941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laskowski K, Stirling A, McKay WP, Lim HJ. A systematic review of intravenous ketamine for postoperative analgesia. Can J Anaesth. 2011;58:911–923. doi: 10.1007/s12630-011-9560-0. [DOI] [PubMed] [Google Scholar]
- 17.Matsumura N, Nakaki T. Isobolographic analysis of the mechanisms of action of anticonvulsants from a combination effect. Eur J Pharmacol. 2014;741:237–246. doi: 10.1016/j.ejphar.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Shimoyama M, Shimoyama N, Inturrisi CE, Elliott K. Oral ketamine produces a dose-dependent CNS depression in the rat. Life Sci. 1997;60:PL9–14. doi: 10.1016/s0024-3205(96)00592-9. [DOI] [PubMed] [Google Scholar]
- 19.Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 20.Wan Y, Xu J, Ma D, Zeng Y, Cibelli M, Maze M. Postoperative impairment of cognitive function in rats: a possible role for cytokine-mediated inflammation in the hippocampus. Anesthesiology. 2007;106:436–443. doi: 10.1097/00000542-200703000-00007. [DOI] [PubMed] [Google Scholar]
- 21.West MJ. Regionally specific loss of neurons in the aging human hippocampus. Neurobiol Aging. 1993;14:287–293. doi: 10.1016/0197-4580(93)90113-p. [DOI] [PubMed] [Google Scholar]
- 22.Schurr A, Spears B, Reid KH, West CA, Edmonds HL Jr, Rigor BM. Lidocaine depresses synaptic activity in the rat hippocampal slice. Anesthesiology. 1986;64:501–503. doi: 10.1097/00000542-198604000-00015. [DOI] [PubMed] [Google Scholar]
- 23.Lisman JE. Relating hippocampal circuitry to function: recall of memory sequences by reciprocal dentate-CA3 interactions. Neuron. 1999;22:233–242. doi: 10.1016/s0896-6273(00)81085-5. [DOI] [PubMed] [Google Scholar]
- 24.Papp G, Witter MP, Treves A. The CA3 network as a memory store for spatial representations. Learn Mem. 2007;14:732–744. doi: 10.1101/lm.687407. [DOI] [PubMed] [Google Scholar]
- 25.Dahmani S, Rouelle D, Gressens P, Mantz J. The effects of lidocaine and bupivacaine on protein expression of cleaved caspase 3 and tyrosine phosphorylation in the rat hippocampal slice. Anesth Analg. 2007;104:119–123. doi: 10.1213/01.ane.0000249048.56863.08. [DOI] [PubMed] [Google Scholar]
- 26.Khakpour-Taleghani B, Lashgari R, Motamedi F, Naghdi N. Effect of reversible inactivation of locus ceruleus on spatial reference and working memory. Neuroscience. 2009;158:1284–1291. doi: 10.1016/j.neuroscience.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol Learn Mem. 1996;65:65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- 28.Browne CA, Lucki I. Antidepressant effects of ketamine: mechanisms underlying fast-acting novel antidepressants. Front Pharmacol. 2013;4:161. doi: 10.3389/fphar.2013.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morris RG. NMDA receptors and memory encoding. Neuropharmacology. 2013;74:32–40. doi: 10.1016/j.neuropharm.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 30.Paoletti P. Molecular basis of NMDA receptor functional diversity. Eur J Neurosci. 2011;33:1351–1365. doi: 10.1111/j.1460-9568.2011.07628.x. [DOI] [PubMed] [Google Scholar]
- 31.Yan J, Jiang H. Dual effects of ketamine: neurotoxicity versus neuroprotection in anesthesia for the developing brain. J Neurosurg Anesthesiol. 2014;26:155–160. doi: 10.1097/ANA.0000000000000027. [DOI] [PubMed] [Google Scholar]