Abstract
Papillary thyroid cancer (PTC) is the most rapidly increasing endocrine malignancy worldwide. Although less aggressive than the majority malignancies, PTC exhibits extensive cervical lymph node metastasis in early stage of PTC. However, the underlying molecular mechanism of this early-metastasis remains unknown. Toll like receptors (TLRs) constitute a crucial component of the innate immune response to bacterial and viral pathogens. Emerging evidence suggests that TLRs play important roles in cancer progression, invasion and immune evasion, whereas whether TLRs have any role in PTC remains to be clarified. In this study, we found that TLR3 was present in both PTC specimen and various thyroid cancer cell lines. Further IHC analysis of 63 PTC patients revealed that TLR3 expression was associated with cervical metastasis, but not correlated with patients’ TNM staging, extrathyroidal invasion. In addition, TLR3 promoted migration of K1 cells in vitro. Activation of TLR3 increased cancer stem cell marker and migration promoting CD44 expression in vitro, indicating that TLR3 might promote metastasis of PTC via modulating CD44 expression. Taken together, our data revealed that TLR3 is correlated with cervical metastasis of PTC and might be an essential prognostic indicator and target for PTC metastasis.
Keywords: Papillary thyroid cancer (PTC), metastasis, Toll-like receptor (TLR), CD44
Introduction
Papillary thyroid cancer (PTC) is the most common endocrine malignancy worldwide [1]. The incidence of which has increased dramatically over the past decades, being the “most rapidly growing” cancer among women [1,2]. Although indolent and less aggressive compared with other endocrine malignancies such as pancreatic carcinoma, extensive cervical lymph node metastasis occurs in early stage of this disease (stage I or stage II). Over 45% of PTC patients were found to have concurrent cervical metastasis when first diagnosed [3], which may contribute to the principle of routine central neck dissection. Moreover, the clinically undetectable “micro-metastasis” to the central or even lateral compartment of the neck still hinders the curability of this disease [4]. Pre-operational ultrasound screening is limited in differentiating inflammatory lymph nodes from “micro-metastatic” lymph nodes. Unfortunately however, molecular markers that are suitable for predicting “early metastasis” or “micro-metastasis” are still under exploration.
TLRs are enzymatically-inactive single membrane-spanning proteins that were first found on immune cells for recognizing pathogen-associated molecular patterns (PAMPs) and fighting against pathogen infection [5,6]. Recent reports revealed them “double-edge sword” as they might expressed on tumor cells and play important roles in promoting cancer proliferation, invasiveness and immune evasion [7]. Our previous study showed that TLR4 attenuates tumor inflammation by targeting pro-inflammatory cytokine IL-6 through MP contained miR-let-7b pathways, thus regulates the inflammatory microenvironment so as to better service the proliferation of hepatic cancer cells [8]. Moreover, TLR stimulated cancer cells up-regulate B7H1 and B7H2 and secrete substances to inhibit T cell proliferation and NK cell activity [9]. A growing body of evidence showed that TLR might also be involved in metastasis in breast, ovarian and colon cancer [10]. However, whether TLRs have any roles in carcinogenesis, development or metastasis of PTC is still unclear.
In this study, expression of TLR3 was first determined in PTC patients and cell lines. Then, its relationship with patients’ clinical data such as TNM staging, cervical lymph node metastasis and extrathyroidal invasion was analyzed, revealing its close relationship with cervical metastasis in PTC. Finally, in vitro assays using TLR3 specific agonist showed that upon stimulation, TLR3 promoted migration of PTC cell line, upon which metastasis associated molecule expression was modulated, partially revealed some underlying mechanism.
Materials and methods
Patients and control subjects
A total of 63 patients who underwent total thyroidectomy for PTC were included in this study from Dec, 2013 to Jun, 2014. Patient’s diagnosis was confirmed by pathological examination after surgical operation. The thyroid function of patients included were summarized in Table 1. The study was approved by the ethics committee of Tianjin Medical University, and was conducted according to the ethical guidelines of our institution. Informed consents were obtained from all participants.
Table 1.
Clinical characteristics of patient’s thyroid function (N=63)
| Thyroid function | TLR3 expression | P value | |
|---|---|---|---|
|
| |||
| −~++ | +++~++++ | ||
| TSH (mIU/L) | 2.66±2.01 | 2.85±1.09 | 0.632 |
| fT3 | 4.22±0.72 | 4.33±0.68 | 0.579 |
| fT4 | 18.2±3.32 | 17.5±3.64 | 0.328 |
| Tg (mIU/L) | 23.6±34.3 | 27.9±37.1 | 0.662 |
| A-Tg (IU/mL) | 47.8±88.3 | 54.8±101.6 | 0.776 |
| A-TPO (IU/mL) | 55.7±71.3 | 39.8±62.3 | 0.350 |
Data were expressed as mean ± standard deviation. fT3, free triiodothyronine; fT4, free tetraiodothyronine.
Cell lines
Human PTC cancer cell lines (K1, BCPAP), anaplastic thyroid cancer cell line (8505c, 8305c) and normal thyroid epithelium cell line (Nthy-ori 3-1) were obtained from the Cell Bank of the Chinese Academy of Medical Sciences (Beijing, China) and cultured according to their guidelines.
RT-PCR and real-time PCR
Total RNA was isolated(TRIzol, Invitrogen), reverse transcribed (TOYOBO) and subjected to polymerase chain reaction (PCR) for 30 cycles at 94°C for 0.5 min, 55°C for 0.5 min, and 72°C for 1 min. Primers were as follows: for human TLR3, forward: 5’-GCCAACTTCACAAGGTATAG-3’, reverse: 5’-GACAAGCCATTATGAGACAG-3’; human CD44, forward: 5’-CTACTTCAGACAACCACAAG-3’, reverse: 5’-TCCATATCCATCCTTCTTCC-3’; human GAPDH, forward: 5’-ACCACAGTCCATGCCATCAC-3’, reverse: 5’-TCCACCACCCTGTTGCTGTA-3’; human CCR4, forward: 5’-GAAATTCTGTGGTGGTTCTG-3’, reverse: 5’-TGCCAGGTATCTATCAATGC-3’; human ICAM-1, forward: 5’-GCCAGCTTATACACAAGAAC-3’, reverse: 5’-CCACAGTGATGATGACAATC-3’; human SGK1, forward: 5’-ATGGTGGAGAGTTGTTCTAC-3’, reverse: 5’-GAGGCTTGTTCAGAATGTTG-3’. Real-time PCR was performed with a FastStart Universal SYBR Green Master Kit (Roche, USA) on an ABI 7900 system. mRNA levels were normalized to GAPDH (glyceraldehyde 3-phosphate dehydrogenase). Protocols of real-time PCR were described previously [8].
Immunohistochemistry
Tissues were fixed in 4% paraformaldehyde in 0.2 M phosphate buffer (pH 7.4) for 48 h, followed by embedding into paraffin sections (4 mm). The endogenous peroxidase activity was inactivated in a solution containing 3% hydrogen peroxide (H2O2) in methanol. Then, the sections were blocked for 2 h at room temperature with 1.5% blocking serum. Sections were incubated with anti-TLR3 (1:100, Abcam) antibody overnight at 4°C. Labelled horseradish peroxidase was applied for 30 min at room temperature, followed by application of diaminobenzidine solution until color developed. Slides were counterstained with haematoxylin. Negative control slides were performed without primary antibody. The staining intensity was graded as follows: 0 (no staining), 1 (weak staining), 2 (moderate staining), 3 (intense staining).
Cell migration assay
Migration assays were performed in Transwell (Corning Inc., 8.0 μm pore size). For migration assay, 3×104 K1 cells in serum-free medium (with 10 μg/mL poly I:C, or 1 μg/mL LPS or PBS) were added to the upper wells. Media containing 10% FBS (with 10 μg/mL poly I:C, or 1 μg/mL LPS or control PBS) were added to the lower wells. Cells that migrated through the filter after 6 or 12 h were stained with 0.2% crystal violet and counted by phase microscopy.
Western blot
Cell lysates and pre-stained molecular weight markers were separated by SDS-PAGE followed by transfer onto nitrocellulose membranes. The membranes were blocked in TBST (Tris-buffered saline with 0.5% of Triton X-100) containing 5% nonfat milk, and probed with antibodies. After incubation with the secondary antibody conjugated with horseradish peroxidase, membranes were extensively washed, and the immunoreactivity was visualized by enhanced chemiluminescence according to the manufacturer’s protocol (ECL kit, Santa Cruz, Santa Cruz, CA). Antibody β-actin (SC-47778) was purchased from Santa Cruz, CD44 (ab157107) from Abcam.
Statistical analysis
Data are expressed as the mean ± standard error of the mean (SEM) and interpreted by Mann-Whitney Rank Sum Test or repeated measure ANOVA. Significant levels were defined as p values less than 0.05.
Results
TLR3 is present in patients with PTC and PTC cell lines
To ascertain whether TLR3 is expressed in PTC, fresh PTC specimens and normal thyroid tissues were obtained and subjected to reverse-transcriptase PCR for the detection of TLR3. Representative expression patterns were shown in Figure 1A. TLR3 was differentially but specifically expressed in PTC specimens, whereas little or no TLR3 expression was found in normal thyroid tissues. Then, TLR3 expression status was further determined in vitro in PTC cell line K1 and BCPAP, anaplastic thyroid cancer cell line 8505 c, 8305 c and normal thyroid epithelial cell line Nthy-ori 3-1 (Figure 1B). In accordance with the fresh tissues, RT-PCR and quantitative real-time PCR showed that TLR3 is expressed in all cancerous cell lines but normal thyroid epithelial cell line Nthy-ori 3-1, suggesting its specificity for malignancies.
Figure 1.
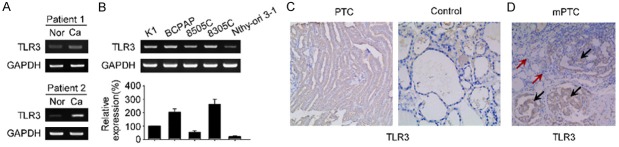
TLR3 is present in patients with PTC and PTC cell lines. (A and B) mRNA expression of TLR3 in thyroid cancer tissue (A) and cell lines (B). Fresh specimens were obtained within 3 hours of surgical operation, homogenized to isolate RNAs and subjected to reverse-transcriptase PCR (A and upper panel of B) or quantitative real-time PCR (lower panel of B) analysis for TLR3 determination. (C) Representative IHC analysis of TLR3 in paraffin embedded PTC and control thyroid tissue. (D) IHC analysis of heterogeneous TLR3 staining in a representative mPTC. The black arrows show staining of TLR3 in the cancerous nests, yet red arrows indicate no staining of TLR3 in the normal thyroid epithelium in the same individual. Nor, normal thyroid tissue; Ca, thyroid cancer; PTC, papillary thyroid cancer; mPTC, multifocal PTC.
IHC analysis of TLR3 further confirmed its presence. Although varied in staining degree, TLR3 expressed only in PTC samples but not normal thyroid controls (Figure 1C). Of note, TLR3 staining was homogenous within the cancerous lesion in single loci PTC (one cancerous lesion in two thyroid lobes) specimen; whereas an obvious heterogeneous expression pattern of TLR3 was observed across the multifocal PTC (mPTC, ≥ two cancerous lesions in two thyroid lobes) (Figure 1D). TLR3 staining was most prominent and specifically within the scattered cancerous loci, whereas interestingly, the surrounding normal parenchymal thyroid tissue is free of TLR3 staining, thus providing convincing evidence showing its specificity in differentiating benign and malignant origins (Figure 1D).
TLR3 is correlated with cervical metastasis in patients with PTC
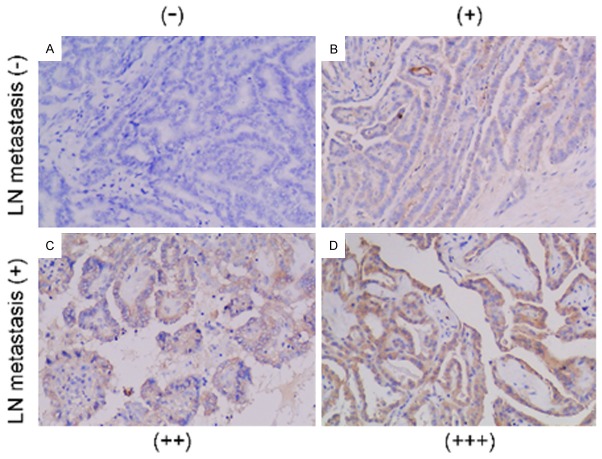
Since TLR3 is present specifically in PTC, we thus further asked whether this expression pattern correlates with patients’ clinical data. A total of 63 patients were included in this study. The clinical characteristics of these patients were summarized in Table 1. TLR3 staining varied in degree in all these PTC patients. Representative pictures showing various degree of TLR3 were shown in Figure 2. We found that TLR3 expression was closely correlated with cervical metastasis in PTC patients (Table 2). The TLR3 staining was strong in 73.3% (22/30) of PTC patients with cervical lymph node metastasis, whereas only 42.4% (14/33) of patients without cervical lymph node metastasis showed strong staining of TLR3. Thus, higher expression of CD44 was more frequently detected in patients with cervical lymph node metastasis in contrast to those who without. However, TLR3 expression was not correlated with extrathyroidal invasion, TNM staging, gender or age, demonstrating that TLR3 activation might contribute to only metastasis but not aggressiveness in patients with PTC.
Figure 2.
TLR3 expression is correlated with cervical micro-metastasis in papillary thyroid cancer patients. (A) Examples of different degrees of staining in tissue sections: − (A), + (B), ++ (C), and +++ (D). Magnification: (A-D) 100× magnification. (B) TLR3 expression correlates strongly with cervical metastasis in PTC patients.
Table 2.
Clinical characteristics of patient’s clinicopathological factors (N=63)
| Clinicopathological factors | No. of patients | TLR3 expression | P value | |
|---|---|---|---|---|
|
| ||||
| −~++ | +++~++++ | |||
| Gender | ||||
| Female | 47 | 21 | 26 | 0.6161 |
| Male | 16 | 6 | 10 | |
| Age | ||||
| <45 years | 32 | 16 | 16 | 0.2444 |
| ≥45 years | 31 | 11 | 20 | |
| TNM staging | ||||
| I-II | 41 | 20 | 21 | 0.1946 |
| III-IV | 22 | 7 | 15 | |
| Extrathyroidal invasion | ||||
| Yes | 19 | 6 | 13 | 0.2346 |
| No | 44 | 21 | 23 | |
| Lymph nodes metastasis | ||||
| Yes | 30 | 8 | 22 | 0.0133 |
| No | 33 | 19 | 14 | |
Activation of TLR3 promotes migration of PTC in vitro
Next, to further confirm whether TLR3 plays any direct role in promoting cancer metastasis in PTC, in vitro migration assay was thus performed using TLR3 specific agonist poly (I:C) at indicated time period. As shown in Figure 3A and 3B, TLR3 activation significantly increased the K1 cell’s mobility. The mean migrated cell increased from 75±9 to 145±11 cells per sight at 6 h, and 110±10 to 230±30 cells at 12 h, respectively (P<0.01), while control or TLR4 activation had no such effect.
Figure 3.
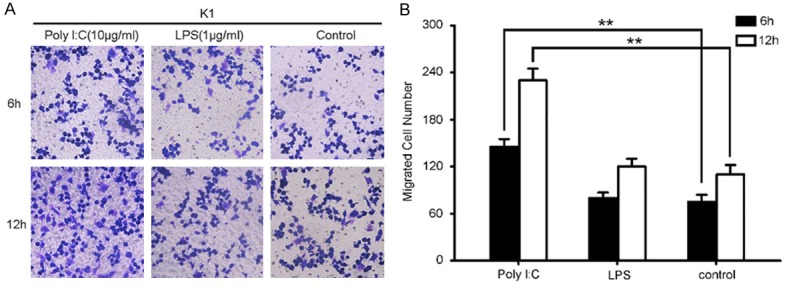
Activation of TLR3 promotes migration of PTC in vitro. A. Representative Transwell migration assay shows significant improvements in cell mobility upon activation of TLR3. 3×104 K1 cells were implanted into the upper chamber of the well and treated with 10 μg/mL poly I:C, 1 μg/mL LPS or PBS as controls. B. Quantization of Transwell migration assay was counted in a blinded fashion by light microscopy at 20× magnification. Data is representative of three independent experiments. **, P<0.01.
TLR3 activation resulted in up-regulation of CD44 in PTC cells
Since TLR3 activation promotes migration of PTC cells, it is reasonable to hypothesize that migration and adhesion associated molecular events might play a role in this process. In this setting, the expression of CD44, ICAM-1, SGK1 and CCR4 upon the stimulation of TLR3 was tested via RT-PCR. As shown in Figure 4A, ICAM-1 and CCR4 showed no significant change. However, CD44 RNA expression were increased with respect to the poly I:C stimulation, which reached a maximal effect on the 50 μg/mL. On the other hand, SGK1 expression was also up-regulated but not correlated with the degree of TLR3 stimulation (Figure 4A). Western blot analysis of CD44 further confirmed the above result (Figure 4B), indicating that up-regulation of CD44 might be responsible for this migration promoting effect.
Figure 4.
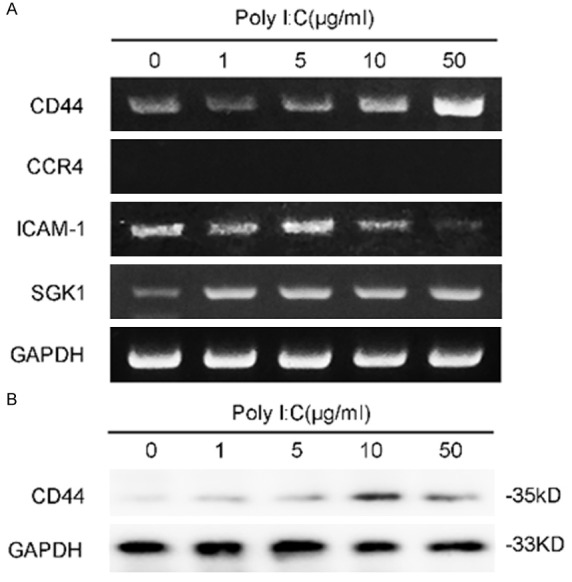
Expression of selected migration associated molecules upon stimulation of TLR3. A. Reverse-transcriptase PCR shows the expression of CD44, ICAM-1, SGK1 and CCR4. K1 cells were treated with various concentration of poly I:C (as indicated) or PBS for 24 h and then subjected to total RNA isolation and PCR. B. Western blot shows the expression of CD44 at 36 h when treated with poly I:C.
Discussion
TLRs were first found on immune cells responsible for initiating innate and acquired immune responses [11]. Then, tumor TLRs were identified and was being indicated as a major culprit of cancer progression and immune evasion [12]. Our study provides evidence that the TLR3 specifically expressed in PTC tissue, even a heterogeneous expression pattern was observed within multifocal PTC specimen that showing both strong staining cancerous tissue and non-staining normal thyroid tissue, suggesting a potential helpful diagnostic tool in making difficult pathological diagnosis.
Early lymph node metastasis is one of the characteristics of PTC biological behavior and accounts for the majority of the cancer related death throughout the course [13]. Even the “microcarcinomas of thyroid” (less than 1cm in diameter) have a tendency to metastasis in 45% of all papillary thyroid microcarcinomas (PTMCs) [14]. Although remarkable progress has been made in understanding molecular pathogenesis of thyroid cancer, yet only a few studies have shed light on thyroid cancer early metastasis. González-Reyes et al reported that tumor with high TLR3 expression was significantly associated with higher probability of metastasis in breast cancer [15]. Moreover, LPS may potentiate the ability of colon cancer cells to metastasis via Nox enzyme mediated redox signaling in colon cancer [16]. Recently, Fabbri et al reported that tumor-secreted miR-21 and miR-29a also can function by binding as ligands to receptors of the murine TLR7 and human TLR8 in immune cells, triggering a TLR-mediated pro-metastatic inflammatory response that may ultimately lead to tumor growth and metastasis [17]. Our data provides direct evidence that TLR3 expression is correlated with PTC cervical lymph node metastasis. It is of more clinical significance to predict whether there is lymph node metastasis in the lateral compartment. In this setting, clinical strategy taken TLR3 expression into consideration might be of great importance in surgery management decision.
In our study, we demonstrated in vitro that TLR3 promoted metastasis of PTC cells, although the underlying mechanisms and molecular events require further demonstration. Interestingly, upon stimulation of TLR3, SGK1 as well as CD44 expression was up-regulated. CD44 and its variants are glycosylated transmembrane glycoproteins that are implicated in tumor growth and metastasis [18,19]. Bourguignon et al reported that CD44 phosphorylates Rho GTPase, thus further phosphorylates myosin light chain II (MLC II) to promote tumor progression [20]. In our previous study, we found that TLR4 signaling directly activated MyD88/myosin light chain II (MLC II) pathway and transformed cytoskeleton in H22 cancer cells [8]. It is thus reasonable to hypothesize that TLR3 promotes metastasis via up-regulation of CD44 expression. Moreover, since human CD44 has various isoforms, which subtype of CD44 variants plays a predominant role still needs to be unfolded.
In sum, we found TLR3 expression is closely related with cervical lymph node metastasis. And, in vitro activation promoted migration of PTC cells, which suggests a potential prognostic and intervention target for the management of PTC metastasis.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC), NO. 81402392, 81272782, 81472580, 31200574; Tianjin Research Program of Application Foundation and Advanced Technology, NO. 13JCQNJC10200; TJMUCH Foundation for Young Investigators, NO. B1317.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014;140:317–322. doi: 10.1001/jamaoto.2014.1. [DOI] [PubMed] [Google Scholar]
- 3.Popadich A, Levin O, Lee JC, Smooke-Praw S, Ro K, Fazel M, Arora A, Tolley NS, Palazzo F, Learoyd DL, Sidhu S, Delbridge L, Sywak M, Yeh MW. A multicenter cohort study of total thyroidectomy and routine central lymph node dissection for cN0 papillary thyroid cancer. Surgery. 2011;150:1048–1057. doi: 10.1016/j.surg.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Qubain SW, Nakano S, Baba M, Takao S, Aikou T. Distribution of lymph node micrometastasis in pN0 well-differentiated thyroid carcinoma. Surgery. 2002;131:249–256. doi: 10.1067/msy.2002.120657. [DOI] [PubMed] [Google Scholar]
- 5.Chew V, Abastado JP. Immunomodulation of the tumor microenvironment by Toll-like receptor-3 (TLR3) ligands. Oncoimmunology. 2013;2:e23493. doi: 10.4161/onci.23493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. 2010;28:367–388. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- 7.Huang B, Zhao J, Unkeless JC, Feng ZH, Xiong H. TLR signaling by tumor and immune cells: a double-edged sword. Oncogene. 2008;27:218–224. doi: 10.1038/sj.onc.1210904. [DOI] [PubMed] [Google Scholar]
- 8.Li D, Cai W, Gu R, Zhang Y, Zhang H, Tang K, Xu P, Katirai F, Shi W, Wang L, Huang T, Huang B. Th17 cell plays a role in the pathogenesis of Hashimoto’s thyroiditis in patients. Clin Immunol. 2013;149:411–420. doi: 10.1016/j.clim.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Huang B, Zhao J, Li H, He KL, Chen Y, Chen SH, Mayer L, Unkeless JC, Xiong H. Toll-like receptors on tumor cells facilitate evasion of immune surveillance. Cancer Res. 2005;65:5009–5014. doi: 10.1158/0008-5472.CAN-05-0784. [DOI] [PubMed] [Google Scholar]
- 10.Ridnour LA, Cheng RY, Switzer CH, Heinecke JL, Ambs S, Glynn S, Young HA, Trinchieri G, Wink DA. Molecular pathways: toll-like receptors in the tumor microenvironment--poor prognosis or new therapeutic opportunity. Clin Cancer Res. 2013;19:1340–1346. doi: 10.1158/1078-0432.CCR-12-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7:179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 12.Hennessy EJ, Parker AE, O’Neill LA. Targeting Toll-like receptors: emerging therapeutics? Nat Rev Drug Discov. 2010;9:293–307. doi: 10.1038/nrd3203. [DOI] [PubMed] [Google Scholar]
- 13.McLeod DS, Sawka AM, Cooper DS. Controversies in primary treatment of low-risk papillary thyroid cancer. Lancet. 2013;381:1046–1057. doi: 10.1016/S0140-6736(12)62205-3. [DOI] [PubMed] [Google Scholar]
- 14.Randolph GW, Duh QY, Heller KS, LiVolsi VA, Mandel SJ, Steward DL, Tufano RP, Tuttle RM American Thyroid Association Surgical Affairs Committee’s Taskforce on Thyroid Cancer Nodal Surgery. The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid. 2012;22:1144–1152. doi: 10.1089/thy.2012.0043. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez-Reyes S, Marin L, Gonzalez L, Gonzalez LO, del Casar JM, Lamelas ML, Gonzalez-Quintana JM, Vizoso FJ. Study of TLR3, TLR4 and TLR9 in breast carcinomas and their association with metastasis. BMC Cancer. 2010;10:665. doi: 10.1186/1471-2407-10-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Leary DP, Bhatt L, Woolley JF, Gough DR, Wang JH, Cotter TG, Redmond HP. TLR-4 signalling accelerates colon cancer cell adhesion via NF-kappaB mediated transcriptional up-regulation of Nox-1. PLoS One. 2012;7:e44176. doi: 10.1371/journal.pone.0044176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R, Lovat F, Fadda P, Mao C, Nuovo GJ, Zanesi N, Crawford M, Ozer GH, Wernicke D, Alder H, Caligiuri MA, Nana-Sinkam P, Perrotti D, Croce CM. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci U S A. 2012;109:E2110–2116. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4:33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 19.Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528–539. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 20.Bourguignon LY. Hyaluronan-mediated CD44 activation of RhoGTPase signaling and cytoskeleton function promotes tumor progression. Semin Cancer Biol. 2008;18:251–259. doi: 10.1016/j.semcancer.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]