Abstract
Background and purpose: Brain metastases (BMs) are typically associated with poor patient prognosis. Radiation therapy remains the primary treatment for BMs, and patient’s prognosis is affected by many factors. The aim of this study was to identify prognostic factors and to compare prognostic index scores in patients with BMs who received whole-brain radiotherapy (WBRT). Methods: A retrospective prognostic study was conducted in 125 patients with BMs who underwent WBRT between Jan 2008 and Jul 2011. The significance of prognostic variables with regard to survival was determined using univariate and multivariate analyses. A prognostic index (PI) was established based on Cox regression analysis and subgrouping values. The recursive partitioning analysis classes (RPA), basic score for brain metastases (BS-BM), Graded Prognostic Assessment index (GPA), and PI were assessed with regard to prognosis. Results: The median survival time was 213 days (7.1 months). In the univariate analysis of the test group, survival was significantly associated with Karnofsky performance status (KPS) score, the number of BMs, the presence of extracranial metastases, primary tumor status and the number of involved extracranial organs. The multivariate analysis showed that the KPS score (P = 0.002, Wald = 9.700), presence of extracranial metastases (P = 0.018, Wald = 5.604) and primary tumor status (P = 0.001, Wald = 10.212) were significantly correlated with overall survival. RPA, BS-BM and GPA were all closely related to prognosis, as determined using a log-rank test. In predicting the 3- and 6-month survival for patients, the PI was superior to the other three modes. Conclusions: The three indexes, RPA, BS-BM and GPA, are valid prognostic index models; however, the PI model was the most powerful.
Keywords: Neoplasms, brain metastases, whole-brain radiotherapy, prognosis, prognostic index scores
Introduction
Brain metastases (BMs) represent a major cause of morbidity and mortality in patients with cancer. Up to 40% of all cancer patients develop BMs during the course of their disease [1]. The prognosis of patients with BMs is poor, with a median survival time among untreated patients of approximately 1 month [2]. Whole-brain radiation therapy (WBRT) alone is the most common treatment for these patients, in particular for patients with multiple lesions, although other treatment approaches are available for selected patients with a small number of lesions [1,3].
In recent years, great efforts have been made to better understand the prognostic factors for patients with BMs. Theoretically, the results of such analyses could be used to create prognostic scores that might help select the appropriate treatment recommendations for the individual patient. Several scoring systems already exist [4-7], such as the recursive partitioning analysis classes (RPA), basic score for brain metastases (BS-BM), graded prognostic assessment index (GPA), Rotterdam score, Germany score, Diagnosis-Specific Graded Prognostic Assessment (DS-GPA) and others. The preceding three prognostic grading systems are the most widely used. The prognostic index (PI) discussed in this paper was established based on Cox regression analysis and subgrouping values of each prognostic factor. The purpose of the present study was to identify the efficacy of radiation therapy, to determine prognostic factors and to assess how the RPA, BS-BM, GPA and PI relate to patient prognosis.
Materials and methods
Patient population and eligibility criteria
A total of 140 cases of patients admitted to our department between Jan. 2008 and July 2011 met the selection criteria. Among the 140 patients, 15 cases were lost during follow-up. The selection criteria were as follows: pathological diagnosis of primary tumor type and imaging diagnosis [computed tomography (CT) and (or) magnetic resonance imaging (MRI)] of BM, received WBRT (complete treatment, TD ≥ 30 Gy) and did not receive BM surgical resection or stereotactic radiosurgery; no history of other malignant tumors; age ≤ 80 y; and KPS ≥ 50. By July 31, 2012, a total of 125 cases had complete follow-up data, among which 45 cases were males and 80 cases were females; the patient ages ranged between 20 and 80 years.
General information
Among all patients, 36 cases (29%) had a solitary BM, and 89 cases (71%) had multiple BMs; most of the lesions were located within the brain parenchyma. There were 70 cases (56%) of lung cancer, 39 cases (31%) of breast cancer, 8 cases (7%) of gastrointestinal tumors, 3 cases (2%) of gynecological tumors and 5 cases (4%) of other tumors. Eighty-five cases were accompanied by extracranial metastasis, which were commonly found in the lungs, bones, livers and lymph nodes. The clinical manifestations were primarily progressive increased intracranial pressure symptoms and local signs of nerve damage. Headache and dizziness were the most common symptoms of parenchymal metastatic tumor, followed by limb muscle strength changes, and then seizures.
Treatment
6 MV of X-ray irradiation was utilized for this treatment. Patients received whole-brain, parallel, opposed bilateral field irradiation, with a tumor dose of 3000-4000 cGy/2-4 weeks. Additional doses of 1000-2000 cGy/1-2 weeks were added at the tumor bed for patients with a single metastatic lesion. Mannitol and glucocorticoid dehydration treatment was performed during radiotherapy.
Methods
The primary endpoint of the study was the overall survival (OS) for all patients meeting the selection criteria. Survival was determined from the starting date of BM radiotherapy. The following items were analyzed: 1. the effect of WBRT on patient symptoms, reduction in intracranial tumor size and patient survival; 2. the differences in the survival of BM patients with different pathological tumor types; 3. the various prognostic factors affecting survival, including age, gender, KPS, number of lesions, extracranial metastases, pathology, primary lesion, total dose, and number of involved extracranial organs; univariate analysis was performed first, and factors with significance were then subjected to multivariate analysis; 4. the relationship between 3 prognosis index scoring systems (RPA, BS-BM and GPA) and prognosis; the patients were grouped according to the three scoring systems to compare the survivals among the 3 groups; 5. the prediction ability and comparison of the different models.
Statistical analyses
In the test group, univariate analyses of survival were performed with the Kaplan-Meier method and the log-rank test. Those prognostic factors that were significant in the univariate analysis (P < 0.05) were additionally evaluated in a multivariate analysis, which was performed with the Cox proportional hazards model. The PI was established based on the sum of the product of the coefficient of variation of the Cox model and subgrouping value of each prognostic factor. Analyses were performed using IBM Statistics Data Editor, version 19.0. Using MedCalc software and the receiver operating characteristic curve (ROC), the area under the curve (AUC) of the PI and three other scoring modes were compared regarding the prediction of the 3- and 6-month survival for patients. An AUC of 0.5 indicates no diagnostic value of the detection index; a diagnosis of a low, medium, and high value refer to scores of 0.5-0.7, 0.7-0.9 and 0.9 or more, respectively.
Results
General information
The selected patients all had treatment efficacy information available, and 125 cases had complete follow-up data, among which 45 cases were males and 80 cases were females. The patient ages ranged from 20-80 years, with a median age of 56 years; 98 cases had KPS ≥ 70, and 27 cases had KPS < 70. All patients received WBRT treatment. At 3 months after radiotherapy completion, cranial MRI or CT was repeated and viewed (compared with the imaging results before the radiotherapy). A total of 12% CR (complete response), 68% PR (partial response), 10% SD (stable disease), and 10% PD (progressive disease) were seen in patients with brain metastases. By the end of follow-up, there were 116 cases of deaths and 9 cases of survival among the 125 cases of patients. Kaplan-Meier analysis results showed that the median survival of the whole group was 213 days (7.1 months). The 3- and 6-month survival rates were 80.8% and 55.2%, respectively. The 1- and 2-year survival rates were 34.4% and 6% (the survival curves are shown in Figure 1).
Figure 1.
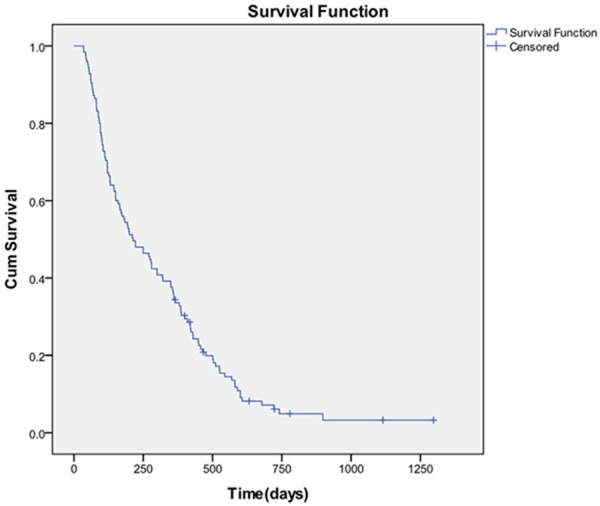
Survival curves of patients receiving radiotherapy for brain metastases.
Effect of pathological type on survival
The data showed no significant difference in the survival of BM patients with different pathological tumor types (χ 2 = 8.218, P = 0.084).
Prognostic univariate and multivariate survival analyses
In the univariate analysis of the test group, survival was significantly associated with KPS score, the number of brain metastases, presence of extracranial metastases, primary tumor status and the number of involved extracranial organs (P < 0.05; Table 1). The multivariate analysis showed that KPS score (P = 0.002, Wald = 9.700), the presence of extracranial metastases (P = 0.018, Wald = 5.604) and primary tumor status (P = 0.001, Wald = 10.212) significantly correlated with overall survival (Table 2).
Table 1.
Univariate survival analysis (log-rank test)
| Factor | n (%) | P value | χ 2 value |
|---|---|---|---|
| Age/year | 0.927 | .008 | |
| < 65 | 100 (80) | ||
| ≥ 65 | 25 (20) | ||
| Gender | 0.241 | 1.373 | |
| Male | 45 (36) | ||
| Female | 80 (64) | ||
| KPS | 0.000 | 25.068 | |
| ≥ 70 | 98 (78) | ||
| < 70 | 27 (22) | ||
| No. of lesions | 0.002 | 12.489 | |
| 1 | 36 (29) | ||
| 2-3 | 40 (32) | ||
| > 3 | 49 (39) | ||
| Extracranial metastases | 0.000 | 22.472 | |
| Yes | 85 (68) | ||
| No | 40 (32) | ||
| Pathology | 0.084 | 8.218 | |
| Lung | 70 (56) | ||
| Breast | 39 (31) | ||
| Gastrointestinal | 8 (7) | ||
| Gynecological | 3 (2) | ||
| Other | 5 (4) | ||
| Primary lesion | 0.000 | 21.376 | |
| Controlled | 79 (63) | ||
| Uncontrolled | 46 (37) | ||
| Total dose | 0.516 | 1.323 | |
| 30 | 11 (9) | ||
| > 30, ≤ 40 | 92 (74) | ||
| > 40 | 22 (17) | ||
| No. of involved extracranial organs | 0.008 | 27.340 | |
| 0 | 40 (32) | ||
| 1 | 35 (28) | ||
| 2 | 28 (22) | ||
| ≥ 3 | 22 (18) |
Table 2.
Multivariate COX regression analysis
| Factor | B | SE | Wald | P value | 95.0% CI |
|---|---|---|---|---|---|
| KPS | -0.757 | 0.235 | 10.369 | 0.001 | 0.296-0.744 |
| Extracranial metastases | -0.790 | 0.218 | 13.079 | 0.000 | 0.296-0.696 |
| Control of primary tumor | -0.706 | 0.199 | 12.608 | 0.000 | 0.334-0.729 |
Maximum likelihood estimation of forward stepwise regression method (Forward: LR).
The relationship between 3 scoring models and prognosis
A log-rank test suggested that all 3 scoring models, RPA, BS-BM and GPA, were associated with prognosis. The median survival for patients of RPA level I, level II and level III in our study was 17.5 months, 6.6 months and 3.2 months, respectively. The median survival of patients with a BS-BM score of 0, 1, 2 or 3 was 2 months, 4.3 months, 12.2 months and 19.3 months, respectively. The median survival of patients with a GPA score of 0-1.0, 1.5-2.0, 2.5-3.0 or 3.5-4 was 3.2 months, 7 months, 14.3 months and 17.5 months, respectively (Figure 2).
Figure 2.
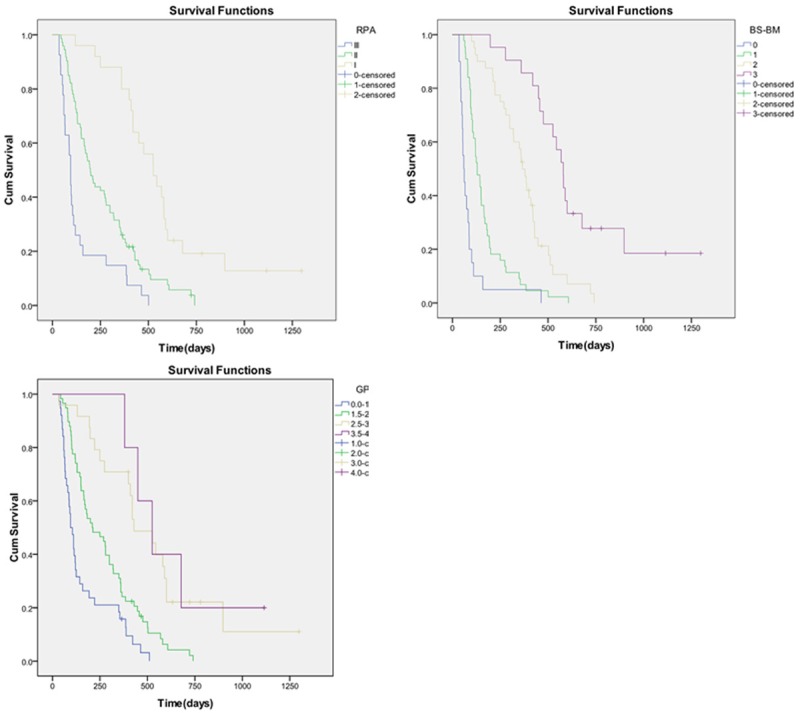
Survival curves for three prognostic index models.
Evaluation of the prediction ability of different models
Our study used a Cox multivariate regression model to perform prognosis analysis and showed that 3 factors, KPS, the presence of extracranial metastases and primary tumor status, affect overall survival. The prognostic prediction PI model was established based on our group data. Based on PI value = group value of prognostic factor 1* the coefficient of variation 1 (β1) + group value of prognostic factor 2* the coefficient of variation 2 (β2) +…, the PI value in our group = -0.757* KPS group (1 or 2) -0.790 * the presence of extracranial metastases (0 or 1) -0.706* primary tumor status (0 or 1). This equation was used to calculate the individual PI value of the 125 patients. ROC curves were used to show the prediction results after comparing 4 prognostic index scores: PI, RPA, BS-BM and GPA (Figure 3). The pairwise comparison of the AUC of the 3-month survival rate between the PI value and RPA (P < 0.0001), as well as between the PI value and GPA (P = 0.0012), showed significant differences (P < 0.0001), while the PI value showed no significant difference compared with BS-BM (P = 0.1571). The pairwise comparison of the AUC of prediction of the 6-month survival rate respectively between the PI value and RPA (P < 0.0001), BS-BM (P = 0.0273) and GPA (P = 0.0002) all showed significant differences. These results showed that with regard to predicting the 3- and 6-month survival rates of patients in our study, the PI was superior to the other scoring models (Table 3). The PI value predicted that the AUC of the 3- and 6-month survival rates were 0.914 and 0.900, respectively (Table 3). Therefore, the four indexes are all valid prognostic index models, and the PI model is the most powerful.
Figure 3.
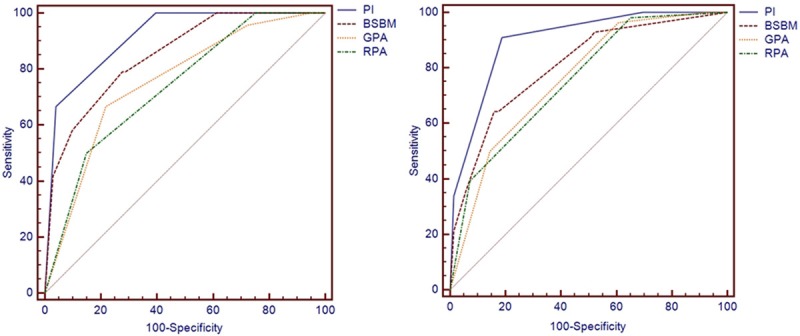
ROC curves of the prognostic index predicting the 3- and 6-month survival rates.
Table 3.
AUC of each scoring model to predict survival
| AUC | P value | 95% CI | |
|---|---|---|---|
| Three-month survival | |||
| PI | 0.914 | < 0.0001 | 0.851-0.957 |
| BS-BM | 0.850 | < 0.0001 | 0.775-0.908 |
| GPA | 0.755 | < 0.0001 | 0.670-0.828 |
| RPA | 0.738 | < 0.0001 | 0.651-0.812 |
| Six-month survival | |||
| PI | 0.900 | < 0.0001 | 0.834-0.947 |
| BS-BM | 0.808 | < 0.0001 | 0.727-0.873 |
| GPA | 0.761 | < 0.0001 | 0.677-0.833 |
| RPA | 0.758 | < 0.0001 | 0.673 to 0.830 |
Discussion
Many patients with BMs have a relatively poor survival prognosis, whereas some patients may live for a few years. It is important to be able to estimate patient survival to determine the best therapy for each individual case. Traditionally, radiation therapy has constituted the primary treatment for brain metastases. WBRT is administered to address both visible disease and presumed micro deposits of tumor cells in the brain. A total dose of 30 Gy to 40 Gy is typically delivered to the patient in daily fractions of 2 Gy to 3 Gy [8]. In general, surgical resection of the BM is a treatment option considered primarily in patients with a single, large lesion, and stereotactic radiosurgery is reserved for lesions whose maximum diameter is 3 cm or less, with better tumor control rates observed in smaller lesions [8]. To reduce the risk of a selection bias, only patients who received WBRT alone were included in this study. Patients generally in good condition were given a booster dose to local lesions in the brain after WBRT.
In our study, after receiving WBRT, 12% of patients with intracranial tumors showed CR, 68% showed PR, 10% showed SD, and 10% showed PD. Kaplan-Meier analysis results showed that the median survival in the whole group was 7.1 months, which is approximately 4 months longer than what has been reported in the literature [4,9,10]. The 3- and 6-month survival rates were 80.8% and 55.2%, respectively; the 1- and 2-year survival rates were 34.4% and 6%, respectively. However, improved intracranial symptoms in patients do not necessarily indicate improved survival. The effects on survival are difficult to determine due to the existence of lesions at other sites. Not all of the causes of death in patients are associated with brain lesions; therefore, the analysis of prognostic factors is necessary.
As for BM patients, studying and discussing on how to improve life quality and prolong survival, as well as analyzing related factors that affect survival, play important roles in guiding individualized clinical treatment. Clinical variables associated with survival have been well studied over the years. There are many non-treatment factors that affect the prognosis of patients with BMs. The literature showed that significant prognostic factors include age, KPS score, primary lesion control, the presence of extracranial metastasis, the presence of multiple metastases, etc. [4-7]. Several scoring systems have been established based on the combination of the screened prognostic factors. The RPA, BS-BM and GPA scoring systems are widely used in BM studies outside of China. Gaspar et al. [4], for the first time, proposed the BM prognostic index scoring model RPA. In 1997, RTOG performed regression analysis of various factors on 1200 cases of BM patients receiving radiotherapy (some patients first had surgical resection), which suggested that age, KPS score and disease conditions are associated with prognosis. The RPA can be divided into 3 levels based on scores: level I is age ≤ 65 years old, KPS ≥ 70, and patients with primary tumor lesion control and no extracranial metastasis; level III includes patients with KPS score < 70; and the other cases are considered level II. The results showed that patients in the different levels had significantly different prognoses; the median survival of patients in level I, II and III was 7.1, 4.2 and 2.3 months, respectively [4]. The RPA provides a standard for the clinical comparison of BM patients, and the reliability of this standard has been demonstrated by many clinical studies [2,11-13]. Due to the wide range of patients grouped into level II, Yamamoto et al [14] further divided the RPA level II patients. Subsequently, well-known pro-gnostic index scores, such as the BS-BM and GPA, were established. Lorenzoni et al. [5] proposed a simplified grouping evaluation survival method, known as the BS-BM. The BS-BM uses 3 prognostic factors that are each assigned a value of 1 or 0: KPS score; primary lesion control; and the existence of extracranial metastasis. The scores are added, and the values range between 0 (the worst) and 3 (the best). The median survival of patients with a score of 0, 1, 2 or 3 is 1.9 months, 3.3 months, 13.1 months and 32.0 months, respectively (P < 0.0001). None of the score 0 patients have lived longer than 4 months. Studies have also compared 3 evaluation survival methods (RPA levels, SIR and BS-BM). In Cox analysis, it was shown that the SIR and BS-BM are closely associated with survival (P = 0.031 and P = 0.043, respectively). Because it is simple to use, the BS-BM is desirable, and it has been shown to be effective for clinical use [15]. Sperduto found in an RTOG9508 [6] study that BM numbers may affect patient prognosis and also believed that the primary tumor control score has some subjectivity; therefore, Sperduto eliminated the primary tumor control score from the RPA and added BM numbers to the RPA to generate the GPA score system. The GPA system generates a value between 0 and 4. A higher score indicates a better prognosis. In clinical studies, Sperduto summarized RTOG data from 5 clinical studies with a total of 1960 cases and demonstrated that the GPA is a good prognostic index. Thus the GPA has been widely used in the clinic [2,16-19].
The multivariate analysis in our study suggested that KPS, the presence of extracranial metastases and primary lesion control are major prognostic factors. Recently, other studies suggested that pathological type and the extracranial metastasis organ number are also associated with prognosis. It has been suggested that prognostic factors and the applicability of prognostic systems will vary by primary diagnosis and that site-specific prognostic systems should be developed. A study by Sperduto identified specific diagnostic prognostic factors and developed the diagnosis-specific GPA (DS-GPA) indexes [7,20,21]. Among the studies, different types of BM tumors have different prognostic factors and different GPA calculation methods. For example, pathological subtype factors were added to breast cancer prognostic factors, while gastrointestinal tumor prognosis was only associated with KPS [20]. Retrospective data suggest that certain subpopulations of patients with BMs, such as those with ERBB2-positive breast cancer (formerly known as HER2) or those with NSCLC caused by mutations in the epidermal growth factor receptor gene, EGFR, survive longer than comparable patients with wild-type tumors [22,23].
In our data analysis, the survival of BM patients with different pathological tumor types did not show a significant difference, which could be due to the small sample size. One study found that the number of involved extracranial organs was an independent prognostic factor in patients with BM, which has not been previously demonstrated. Patients with involvement of only one extracranial organ had a significantly better survival prognosis than patients with involvement of two or more organs. Additionally, the type of extracranial organ involved by metastatic disease had no significant impact on the survival prognosis. Patients with bone metastasis did not have a better prognosis than those patients with lung metastasis, liver metastasis, or other metastasis. The retrospective nature of the data should be taken into account when interpreting these results [24,25]. In our study, the number of organs with extracranial metastasis showed a correlation with prognosis in univariate analysis but did not show a correlation in multivariate analysis, which requires further analysis using larger sample sizes.
The log-rank test in our study suggested that the 3 prognostic index scores, RPA, BS-BM and GPA, were all associated with patient prognosis. Our study results are similar to other corresponding literature studies [4,5,9,20]. The median survival of patients of RPA level I, level II and level III in our study was 17.5 months, 6.6 months and 3.2 months, respectively; the median survival of patients with BS-BM scores 0, 1, 2 and 3 was 2 months, 4.3 months, 12.2 months and 19.3 months, respectively. The median survival of patients with GPA scores 0-1.0, 1.5-2.0, 2.5-3.0 and 3.5-4 was 3.2 months, 7 months, 14.3 months and 17.5 months, respectively. For RPA class III patients, the median OS of 3.2 months in our series was higher than that obtained from the Antoni and Gaspar research [4,9]. With regard to RPA class I patients, the median OS times in our series and Antoni were substantially higher than that of Gaspar [4,9]. The BS-BM can be described the same way. The BS-BM scores 2 patients had a better outcome than those described in the study by Antoni and Lorenzoni et al. [5,9]. For patients with GPA scores 2.5-3.0, our OS times compared favorably to those established in the Antoni and Sperduto analysis [9,20]. The results from the above studies suggested that patients belonging to RPA category III, BS-BM 0 or GPA 0-1 had very poor median survival (Table 4).
Table 4.
Median survival time in months, stratified by RPA, BS-BM, and GPA scores in our series and compared with the literature
| RPA score | Our | Antoni | Gaspar | BS-BM score | Our | Antoni | Lorenzoni | GPA score | Our | Antoni | Sperduto |
|---|---|---|---|---|---|---|---|---|---|---|---|
| I | 17.5 | 20.1 | 7.1 | 0 | 2 | 1.6 | 2.2 | 0-1 | 3.2 | 2.5 | 3.1 |
| II | 6.6 | 5.1 | 4.2 | 1 | 4.3 | 3.7 | 3.4 | 1.5-2.0 | 7 | 4.4 | 5.4 |
| III | 3.2 | 1.3 | 2.3 | 2 | 12.2 | 8.9 | 5.1 | 2.5-3.0 | 14.3 | 9.0 | 9.6 |
| 3 | 19.3 | 20.6 | 7.0 | 3.5-4.0 | 17.5 | 19.1 | 16.7 |
Our study used ROC curves to compare the 3- and 6-month survival rate predication ability. The results suggested that the AUC of predicting the 3- and 6-month survival rate of the PI, 0.914 and 0.900, respectively, are higher than those of the other 3 prognostic indexes. The results are satisfying. The reason for its superior prediction effect is that the PI is based on the weighted values given to each influence factor in our study; in comparison, the other scoring systems assign the same weight to different factors.
The treatment of BM patients in our study was simple radiotherapy and auxiliary symptomatic treatment. Recent studies have reported that the combination of WBRT with TMZ for BMs from various solid tumors showed an overall MST of 8-13 months and mild side effects [26]. With regard to lung cancer, tyrosine kinase inhibitor (TKI) treatment for BMs in lung cancer patients showed encouraging results, and treatment efficacy is related to EGFR mutation status. A small clinical trial showed that erlotinib combined with WBRT has better efficacy compared with erlotinib alone, and toxicity can be tolerated [27,28]; the median survival reached 11.8 months.
In summary, BM is a late manifestation of malignant tumors and the common cause of death and disability; radiotherapy is one of the main treatments of BM. The survival of BM patients is affected by many factors. Our study suggests that KPS, the presence of extracranial metastases and primary lesion control are the primary prognostic factors. Our study provides clinical evidence for prognostic evaluation of BM patients. The existing 3 prognostic scoring systems are all suitable for analyzing the data in our study; however, the PI model, which is based on our data, has a better performance than the other three systems. The study results require further verification by prospective studies with larger sample sizes. In addition, WBRT combined with chemotherapy and targeted therapy should also be investigated. It is hoped that prospective trials will help clarify and guide treatment selection for patients to help optimize clinical outcomes.
Acknowledgements
This work is Funded by National Natural Science Foundation of China (81402518; 81472920), Jiangsu Provincial Special Program of Medical Science (BL2012046), Changzhou Social Development Project (CE20125026; CE20135050) and Changzhou Scientific Program (ZD201315; CY20130017).
Disclosure of conflict of interest
None.
References
- 1.Dziggel L, Segedin B, Podvrsnik NH, Oblak I, Schild SE, Rades D. Validation of a survival score for patients treated with whole-brain radiotherapy for brain metastases. Strahlenther Onkol. 2013;189:364–6. doi: 10.1007/s00066-013-0308-3. [DOI] [PubMed] [Google Scholar]
- 2.Viani GA, da Silva LG, Stefano EJ. Prognostic indexes for brain metastases: which is the most powerful? Int J Radiat Oncol Biol Phys. 2012;83:e325–30. doi: 10.1016/j.ijrobp.2011.12.082. [DOI] [PubMed] [Google Scholar]
- 3.Gállego Pérez-Larraya J, Hildebrand J. Brain metastases. Handb Clin Neurol. 2014;121:1143–57. doi: 10.1016/B978-0-7020-4088-7.00077-8. [DOI] [PubMed] [Google Scholar]
- 4.Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, McKenna WG, Byhardt R. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745–751. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 5.Lorenzoni J, Devriendt D, Massager N, David P, Ruíz S, Vanderlinden B, Van Houtte P, Brotchi J, Levivier M. Radiosurgery for treatment of brain metastases: estimation of patient eligibility using three stratification systems. Int J Radiat Oncol Biol Phys. 2004;60:218–24. doi: 10.1016/j.ijrobp.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 6.Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys. 2008;70:510–14. doi: 10.1016/j.ijrobp.2007.06.074. [DOI] [PubMed] [Google Scholar]
- 7.Sperduto PW, Chao ST, Sneed PK, Luo X, Suh J, Roberge D, Bhatt A, Jensen AW, Brown PD, Shih H, Kirkpatrick J, Schwer A, Gaspar LE, Fiveash JB, Chiang V, Knisely J, Sperduto CM, Mehta M. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010;77:655–61. doi: 10.1016/j.ijrobp.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 8.Lu-Emerson C, Eichler AF. Brain metastases. Continuum (Minneap Minn) 2012;18:295–311. doi: 10.1212/01.CON.0000413659.12304.a6. [DOI] [PubMed] [Google Scholar]
- 9.Antoni D, Clavier JB, Pop M, Schumacher C, Lefebvre F, Noël G. Institutional, retrospective analysis of 777 patients with brain metastases: treatment outcomes and diagnosis-specific prognostic factors. Int J Radiat Oncol Biol Phys. 2013;86:630–7. doi: 10.1016/j.ijrobp.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 10.Windsor AA, Koh ES, Allen S, Gabriel GS, Yeo AE, Allison R, van der Linden YM, Barton MB. Poor outcomes after whole brain radiotherapy in patients with brain metastases: results from an international multicentre cohort study. Clin Oncol (R Coll Radiol) 2013;25:674–80. doi: 10.1016/j.clon.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Kondziolka D, Parry PV, Lunsford LD, Kano H. The accuracy of predicting survival in individual patients with cancer. J Neuro Surg. 2014;120:24–30. doi: 10.3171/2013.9.JNS13788. [DOI] [PubMed] [Google Scholar]
- 12.Saito EY, Viani GA, Ferrigno R, Nakamura RA, Novaes PE, Pellizzon CA, Fogaroli RC, Conte MA, Salvajoli JV. Whole brain radiation therapy in management of brain metastasis: results and prognostic factors. Radiat Oncol. 2006;1:20. doi: 10.1186/1748-717X-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chidel MA, Suh JH, Reddy CA, Chao ST, Lundbeck MF, Barnett GH. Application of recursive partitioning analysis and evaluation of the use of whole brain radiation among patients treated with stereotactic radiosurgery for newly diagnosed brain metastases. Int J Radiat Oncol Biol Phys. 2000;47:993–9. doi: 10.1016/s0360-3016(00)00527-7. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto M, Sato Y, Serizawa T, Kawabe T, Higuchi Y, Nagano O, Barfod BE, Ono J, Kasuya H, Urakawa Y. Subclassification of recursive partitioning analysis Class II patients with brain metastases treated radiosurgically. Int J Radiat Oncol Biol Phys. 2012;83:1399–405. doi: 10.1016/j.ijrobp.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 15.Nieder C, Pawinski A, Molls M. Prediction of short survival in patients with brain metastases based on three different scores: a role for 'triple-negative' status? Clin Oncol (R Coll Radiol) 2010;22:65–9. doi: 10.1016/j.clon.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Sperduto CM, Watanabe Y, Mullan J, Hood T, Dyste G, Watts C, Bender GP, Sperduto P. A validation study of a new prognostic index for patients with brain metastases:The graded prognostic assessment. J Neurosurg. 2008;109(Suppl):87–9. doi: 10.3171/JNS/2008/109/12/S14. [DOI] [PubMed] [Google Scholar]
- 17.Sperduto P, Sneed P, Bhatt A. A multi-institutional validation study of a new prognostic index (graded prognostic assessment, GPA) for patients with brain metastases. Int J Radiat Oncol Biol Phys. 2008;72:S51. [Google Scholar]
- 18.Nieder C, Bremnes RM, Andratschke NH. Prognostic scores in patients with brain metastases from non-small cell lung cancer. J Thorac Oncol. 2009;4:1337–41. doi: 10.1097/JTO.0b013e3181b6b6f4. [DOI] [PubMed] [Google Scholar]
- 19.Golden DW, Lamborn KR, McDermott MW, Kunwar S, Wara WM, Nakamura JL, Sneed PK. Prognostic factors and grading systems for overall survival in patients treated with radiosurgery for brain metastases: variation by primary site. J Neurosurg. 2008;109(Suppl):77–86. doi: 10.3171/JNS/2008/109/12/S13. [DOI] [PubMed] [Google Scholar]
- 20.Sperduto PW, Kased N, Roberge D. Summary report on the graded prognostic assessment: An accurate and facile diagnosisspecific tool to estimate survival for patients with brain metastases. J. Clin. Oncol. 2012;30:419–25. doi: 10.1200/JCO.2011.38.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dziggel L, Segedin B, Podvrsnik NH, Oblak I, Schild SE, Rades D. A survival score for patients with brain metastases from less radiosensitive tumors treated with whole-brain radiotherapy alone. Strahlenther Onkol. 2014;190:54–8. doi: 10.1007/s00066-013-0394-2. [DOI] [PubMed] [Google Scholar]
- 22.Eichler AF, Kahle KT, Wang DL, Joshi VA, Willers H, Engelman JA, Lynch TJ, Sequist LV. EGFR mutation status and survival after diagnosis of brain metastasis in nonsmall cell lung cancer. Neuro Oncol. 2010;12:1193–9. doi: 10.1093/neuonc/noq076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eichler AF, Kuter I, Ryan P, Schapira L, Younger J, Henson JW. Survival in patients with brain metastases from breast cancer: the importance of HER-2 status. Cancer. 2008;112:2359–2367. doi: 10.1002/cncr.23468. [DOI] [PubMed] [Google Scholar]
- 24.Rades D, Gerdan L, Segedin B, Nagy V, Khoa MT, Trang NT, Schild SE. Brain metastasis. Prognostic value of the number of involved extracranial organs. Strahlenther Onkol. 2013;189:996–1000. doi: 10.1007/s00066-013-0442-y. [DOI] [PubMed] [Google Scholar]
- 25.Gerdan L, Segedin B, Nagy V, Khoa MT, Trang NT, Schild SE, Rades D. Brain metastasis from non-small cell lung cancer (NSCLC): prognostic importance of the number of involved extracranial organs. Strahlenther Onkol. 2014;190:64–7. doi: 10.1007/s00066-013-0439-6. [DOI] [PubMed] [Google Scholar]
- 26.Kyritsis AP, Markoula S, Levin VA. A systematic approach to the management of patients with brain metastases of known or unknown primary site. Cancer Chemother Pharmacol. 2012;69:1–13. doi: 10.1007/s00280-011-1775-9. [DOI] [PubMed] [Google Scholar]
- 27.Welsh JW, Komaki R, Amini A, Munsell MF. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J. Clin. Oncol. 2013;31:895–902. doi: 10.1200/JCO.2011.40.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhuang H, Wang J, Zhao L. The theoretical foundation and research progress for WBRT combined with erlotinib for the treatment of multiple brainmetastases in patients with lung adenocarcinoma. Int J Cancer. 2013;133:2277–83. doi: 10.1002/ijc.28290. [DOI] [PubMed] [Google Scholar]


