Abstract
Background: Iodine 125 (125I) seed irradiation is an effective non-surgical treatment for unresectable hepatocellular carcinoma (HCC) patients. However, the safety and tolerability of 125I seed sequential irradiation therapy remain unclear, there is no unified standard of brachytherapy radiation dose, and further study on the basic radiobiology of continuous rate irradiation is necessary. Methods: Forty Kunming-mice (KM-mice, China) were injected with suspensions of human hepatocellular carcinoma cells (H22) to create an animal model and mimic 125I seed implantation. The survival rates of mice, curative effect, pathological impairments including apoptosis and necrosis were investigated. The mice were randomly divided into four groups, A, B, C and D. In group A, 0.78 mCi 125I seeds were implanted into the tumor focus. In groups B and C, 0.58 mCi and 0.38 mCi 125I seeds were inserted at the same location, respectively. Group D was a control group, without any treatment. After 28 days of therapy, the survival rates and the tumor size were measured, and pathological impairments was measured by light or electron microscopy. Results: The tumor volume inhibition rate was 68.21% ± 3.21%, 51.38% ± 4.96%, and 35.71% ± 2.79% after 0.78 mCi, 0.58 mCi, and 0.38 mCi 125I seeds irradiation, respectively. However, radiation-related side effects were also observed in the high-dose group. Pathological results showed that radiation effect was closely associated with radiation dose, as the increase of radiation dose, an increase in apoptosis and necrosis was detected. Significant cellular impairments were noted by pathological analysis under electron microscopy. Conclusions: Our results demonstrate that the Kunming-mouse is an ideal animal to study 125I brachytherapy, and the curative effect was closely associated with radiation dose. High-dose of brachytherapy may effectively increase apoptosis and necrosis in liver cells in KM-mice. A dose of 0.58 mCi 125I radioactive particles may be a safe, effective and minimally invasive therapeutic option for liver cancer.
Keywords: 125I seed, hepatocellular carcinoma, apoptosis, animal experimentation
Introduction
Hepatocellular carcinoma (HCC), the predominant form of primary liver cancer, is the third leading cause of global cancer deaths [1], with a dismal overall 5-year survival rate of only 5% is achieved, and the incidence and mortality continue to increase [2]. The difficulty of early diagnosis, the aggressive behaviors of HCC, and the poor effectiveness of conventional therapy, represent the reasons for the quite similar deaths per year and incidence number. Considering the fact that the diagnosis of HCC typically occurs in the advanced stages of the disease when the therapeutic options have only modest efficacy, the development of novel approaches for unresectable and non-transplantable HCC patients is therefore warranted. External-beam radiotherapy (EBRT) is one of the most common non-surgical approaches for the treatment of localized hepatocellular carcinoma [3]. Although technology advances in radiation oncology have rapidly expanded radiotherapy accuracy and precision to all parts of the body, however, EBRT requires high doses of irradiation for efficacy [4], and some complications occurs, such as radiation damages to the tissue around the tumor. Besides, the very radioresponsive organs surrounding the liver adversely affect the dose of radiation used to target the tumor on radiation treatment [5]. As a result, brachytherapy has been introduced as a new promising approach for the treatment of unresectable HCC to maximize local dose and minimize irradiation of the surrounding normal tissue [6,7].
Brachytherapy is a form of radiotherapy in which sealed sources of radioactive material are inserted permanently into body cavities or directly into tumors. Recently, 125I seed implantation, an efficient brachytherapy technique, has attracted increasing attention because of its specific advantages: 1) percutaneous implantation under the guidance of ultrasound or CT; 2) effective irradiation dose applied in a single procedure; 3) reduced radiation injury outside the target tumor; 4) extending the duration of killing tumor [8,9]. Because of its ability to offer high precision, little trauma, strong lethality, and fewer complications, 125I radioactive seed implantation has been widely applied in clinical practice for tumor treatment, such as prostate carcinoma [10], pancreatic cancer [11], recurrent colorectal cancer [12], head and neck carcinoma [13], and others [14-16]. However, the treatment of unresectable HCC with 125I radioactive seed implantation has not been widely applied in clinical practice, and the treatment effect and radiobiological effect of continuous rate irradiation has not been in-depth study. Therefore, further study on the basic radiobiology of continuous different dose rate irradiation is necessary, particularly to provide further clinical direction. Establishing a valuable animal model can help to find a method to overcome its complications. In the present study, A KM-mouse model was used to simulate HCC interstitial brachytherapy, and aimed to study the curative effect and cell-based radiation damage that was caused by 125I. The HCC model was exposed to 125I seeds at different doses of irradiation, and killing effect of tumor tissue in vivo were observed to reveal the radiobiological mechanism of 125I radioactive seed irradiation.
Materials and methods
Radiation source and reagents
Brachytherapy seeds iodine-125 (125I) and the 125I seed implantation instrument were purchased from Ningbo Junan Pharmaceutical Technology Company (Ningbo, China). A single seed is 0.84 mm in diameter, 4.5 mm long; three different apparent radioactivities were included: 0.38 mCi/seed, 0.58 mCi/seed and 0.78 mCi/seed, and the half life were 59.43 days. The 125I seeds were selected randomly for activity testing to confirm the seed container integrity and apparent activity of the seeds.
Establishment of the animal models
All the animal experiments were approved by the Ethics Committee of Laboratory Animals of Tumor Hospital of Guangxi Medical University. Fifty female KM-mice (age, 4-6 weeks; weight, 18.0-20.0 g) were used for the experiment. The animals were provided and raised by the Medical Animal Test Center of Guangxi Medical University (China). The animals were acclimatized for at least 48 h before experimentation, and fed with a standard diet. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health of China (document number 55, 2001) and the Animal Experiment Ethics Review of Guangxi Medical University.
In this study, for preparation of H22 tumor cell suspension, the H22 tumor cells were injected into the KM-mice abdominal cavity to culture. Human H22 hepatocellular carcinoma cell lines were obtained from the American Type Culture Collection (Manassas, VA). Following implantation, the carcinomatous ascites developed rapidly. In order to reduce the potential difference of tumor growth, H22 tumor cell suspension was obtained from the same tumor donor mouse. The suspension was mechanically homogenized, and finally, the viable cells were adjusted to a concentration of 7 × 106 cells/ml.
To establish an animal model, 0.2 ml H22 tumor cell suspension was subcutaneously injection at the right axillary space using an 18G needle. With B-ultrasound monitoring the inoculated position of each mouse, we chose the mice with only one tumor implanted in the armpit of right forelimb. Treatments were started after 14-16 days of the implantation. The mice with tumor diameter of ~1.0 cm were selected and this day was designated as “Day 0”.
Grouping and 125I seeds irradiation
Of the 50 mice, 40 (in which the tumor diameter reached 1.0 cm) were randomly divided into 4 groups, with 10 mice in each group. The mice were treated with B-ultrasound-guided 125I seed brachytherapy at different doses. The mice in group A were treated with 0.78 mCi, the mice in group B and group C were treated with 0.58 mCi and 0.38 mCi, respectively. The mice in the group D (control group) were not treated. All brachytherapy seeds were inserted into the same location.
Physical condition assessment of model animals
All mice were labeled and continuous observed in 28 days, the numbers of live mice in each group were record, and reactions of tumors and its surrounding skin were noted. The tumors were measured every 4 days with calipers during the period of study, and the tumor volume and tumor inhibit rate were calculated. The tumor volume was calculated by the following formula: V = (A × B2)/2, where V is tumor volume, A is the tumor measurement at the longest point and B is the tumor dimension at the widest diameter. The tumor inhibit rate was calculated by the formula: R = (V1-V2)/V1 × 100%. Where V1 is the average tumor volume of control group, V2 is the average tumor volume of treatment group. Each animal was weighed at the time of administration. At 28 days after implantation, the mice were sacrificed after anesthetized and the tumor mass was harvest and weighed, and made for pathological assessment.
Pathological assessment
After 28 days of treatment, the mice were sacrificed after anesthetized by cervical dislocation method and varied indexes were observed. Two experienced researchers (Qing-Hong Qin and Bai-Sha Huang) conducted an assessment with the naked eye on the extent of the tumor and adjacent tissue, and changes in the skin tissue. Histopathological examination included cross-sectional assessed by transmission-type electron microscope and light microscope. For each sample, tumor tissues were divided into two portions, one was fixed in 2.5% glutaraldehyde, and then a histological slice (thickness of < 0.1 μm) was made to observe under a transmission-type electron microscope (JEM-1200 EX, JEOL Company, Japan). The other tumor tissue was stained with hematoxylin and eosin (H&E), with a slice thickness of 5 μm, where the researchers used a light microscope to assess the pathological impairments of tumor tissue.
Data analysis
Standard statistical software (SPSS version 16.0; SPSS, Inc., Chicago, IL, USA) was used for data analysis. The data were reported as means ± standard deviation (SD). Statistical significance was analyzed using the analysis of variance (ANOVA). Differences between experimental groups were considered significant when the P-value was less than 0.05 (P < 0.05).
Results
Survival rate of tumor-bearing mice after different dose rate irradiation
Data showed that tumor-killing effects were related to dose rate. In the 0.78 mCi group (group A), only 70% of mice (7 cases) survived after 28 days, while those of 125I seed 0.58 mCi and 0.38 mCi dose rate irradiation were both 90% (9 cases). In the control group that did not receive any treatment, only 50% of mice (5 cases) survived. The tumor-bearing mice of control group were mainly died of Chest wall, lung metastasis and cachexia caused by malignant ascites, while mice in treatment group were did not found any signs of metastasis. Some mice in group A exhibited signs of pain and sickness. Hair loss in the right front leg and clumsy tail movement were observed in two mice from group A, while the other groups of animals did not appear significant side effects.
In vivo anti-tumor effect of 125I
Tumor volume and weight are important indicators for evaluating antitumor efficacy of the different therapy regimens. As shown in Figure 1, the variation on tumor volume and body weight with different administration were monitored and recorded respectively. Figure 1A shows the tumor size of different groups. Figure 1B shows the tumor volume has no obvious differences between the treatment group and control group after 4 days of treatment. However, after 12 days of treatment, tumor growth in control group was significantly faster than the treatment groups. Moreover, the antitumor efficacy of 0.78 mCi dose group was significantly higher than that of 0.58 mCi dose and 0.38 mCi dose group (P < 0.05). Moreover, the results show that the tumor volume inhibition rate was 68.21% ± 3.21%, 51.38% ± 4.96% and 35.71% ± 2.79% after 0.78 mCi, 0.58 mCi and 0.38 mCi 125I seeds irradiation, respectively, indicating the dose-dependent antitumor efficacy in vivo (P < 0.05). To quantitatively evaluate the tumor regression effect of 125I brachytherapy, the excised tumor of sacrificed mice were weighed. In accordance with the results of tumor volume, the tumor weight of mice after treatment with high-dose rate irradiation was 1.49 ± 0.13 g, which was significantly smaller than that of moderate-dose (2.18 ± 0.32 g) and low-dose (2.94 ± 0.29 g), as show in Figure 1C (P < 0.01), respectively. The body weights of mice were monitored as an index of systemic toxicity, and the body weight variations of mice was shown in Figure 1D. During the experiment period, the body weight of mice treated with different dose rate irradiation did not show significant difference (P = 0.42), displaying a significantly lower systematic toxicity. Overall, these results indicated that 125I brachytherapy could generate a synergistic antitumor efficacy in vivo and reduce the systematic toxicity in murine HCC model.
Figure 1.
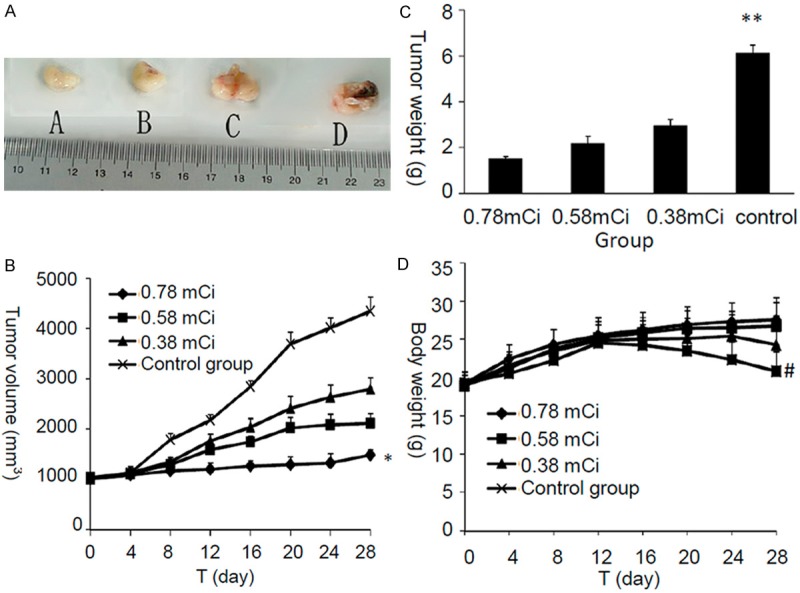
Antitumor effects of different activities of 125I seed brachytherapy on H22 tumor-bearing mice after inserted administration; A. Tumor specimens; B. Tumor volume; C. Tumor weight; D. Body weight. *P < 0.05, tumor volume was statistically significant difference between four groups; **P < 0.01, tumor weight was statistically significant difference between four groups; #P = 0.42, the body weight of mice in different groups did not show significant difference.
Histopathology of HCC after 125I seed implantation
Representative HE sections were obtained from the 0.78 mCi (Figure 2A), 0.58 mCi (Figure 2B), 0.38 mCi (Figure 2C) and 0 mCi (Figure 2D) groups 28 d after 125I seed implantation. In the 0.78 mCi and 0.58 mCi groups, a large area of coagulative necrosis was observed around the 125I seed. Significant necrosis was noted in the area that l~2 mm away from the particle source, and cell structures were obviously damaged. Small-area of necrosis was also found in 3~5 mm distance, and it was characterized by chronic inflammatory-cell infiltration and increased histologic reaction. However, the necrosis and growth inhibition in cancer cells were more obvious in 0.78 mCi group than in 0.58 mCi group. In the 0.38 mCi group, significant necrosis was not noted under the microscope, and the morphological structure remained in normal condition as well. In the control group, there were no significant necrotic or damaged regions, full view of tumor cells were observed in the tumor tissue, the tumor cells were densely arranged in a disorderly fashion, with large, darkly stained nuclei with obvious fission. These suggested that 125I seed implantation caused the necrosis and growth inhibition of cancer cells and enlargement of irradiation dose could enhance the beneficial effect.
Figure 2.
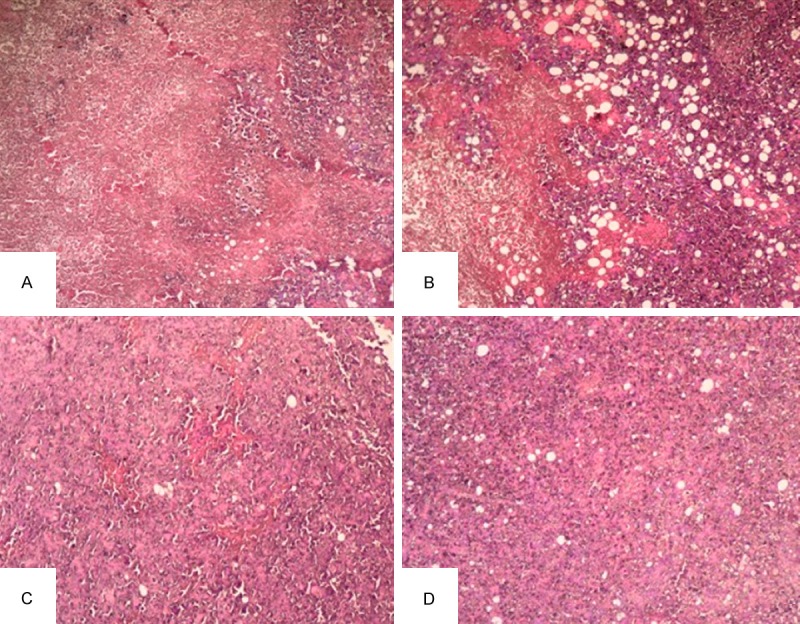
Histopathology of 125I implanted HCC cancer (× 100). Representative HE stained sections from the 0.78 mCi (A), 0.58 mCi (B), 0.38 mCi (C) and 0 mCi (D) groups 28 days after 125I seed implantation were prepared as described in the materials and methods section.
Electron microscopic observations
Electron microscopy (EM) was performed for subcellular identification. Under the electrical microscope, cytopathological changed obviously, cellular organelles were unclear, obvious impairment was found for most organelles. There were many apoptotic cells in tumors that treated with 0.78 mCi irradiation, some of apoptotic cells appear as different changes of cell apoptosis such as cell shrinking, nucleus concentrating and fragmentation, chromatin concentrated and multiple cytoplasm swelled, as show in Figure 3A. Some apoptotic cells also appear as nuclear chromatin dissolved disappear, and only a small amount of heterochromatin scattered in the edge of the nuclear membrane, and there are a large number of dark cells and multiple apoptotic body being swallowed (Figure 3B). In the tumor cells of group B and C, the nucleus was irregular, some tumor cells broke apart and disappeared, seedless circular corpuscles were observed, organelles’ structure was unclear, the mitochondria intumesced a little and the lysosome increased (Figure 3C and 3D). Under low magnification, there were many apoptotic cells scattered distribution between integral tumor cells. The cells of control group are very tumor cells in appearance, with integral nuclear membrane, and chromatin evenly distributed, and there were a little of apoptosis cell (Figure 3E).
Figure 3.
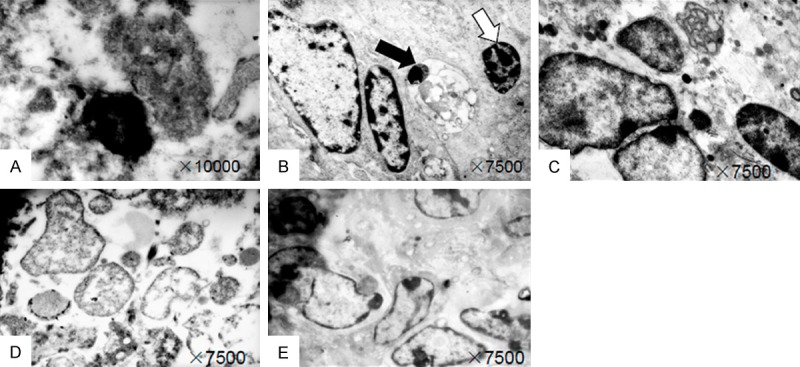
Electron-microscopic observation: A. Tumor cell shrinkage and nucleus concentrating; B. Appearance of apoptotic body (black arrow) and dark cell (white arrow); C and D. Shrinkage and swelling of tumor cells, organelles’ structure was unclear; E. Tumor cells with integral nuclear membrane, and chromatin evenly distributed.
Discussion
125I brachytherapy was introduced into radiation therapy since 1970s [17], this method takes advantage of the simplest physical properties of radiation, it has resulted in positive clinical treatment of many tumors, and has been proven to be effective for the inhibition of cancer progression [18]. Particularly, 125I brachytherapy has become the first choice for patients with prostate cancer treatment, because of its less side effects and significant curative effect, and recently the American Society for Radiation Oncology (ASTRO) further standardizes the 125I particles for the treatment of prostate cancer [19]. Nevertheless, compared with springing up of 125I radioactive seeds interstitial implantation, the application in the treatment of hepatocellular carcinoma notably absent, and the radiobiological effect of 125I seed low dose rate irradiation remains unclear. In the present study, we successfully established a Kunming-mouse model to study the radiobiological effect and pathological impairments induced by interstitial implantation of 125I seeds in HCC. In this study, all the geometric data related with radiation dosimetry were measured and calculated carefully, which guaranteed the radiation accuracy for this whole research. After 28 days of follow-up, the survival rate of mice in treatment group is significantly higher than the control group. The tumor-bearing mice of control group were mainly died of chest wall, lung metastasis and cachexia caused by malignant ascites, while mice in treatment group were did not found any signs of metastasis, suggesting that 125I brachytherapy is a useful and safe treatment. Different doses of 125I seed implantation could effectively decreased tumor volume, while the treating effect in the 0.78 mCi group was more obvious than the other groups.
125I brachytherapy irradiation creates a radiated area around the seeds in a columnar or round shape, depending on the type of seed used and the generating power [20]. This novel technique ensures protracted cell killing over a period of several months via targeted delivery of high-dose radiation. However, similar to other thermal methods, such as external-beam radiotherapy for local tumor treatment, tumor size and the different shapes and characteristics of the tumors significantly affects the outcome of the procedure [21,22]. In the present study, our results revealed that the therapeutic effect of 125I seed implantation in tumors with different shapes was discrepant, regardless of the similar tumor size. Those approximately spherical tumors was significantly inhibited, while tumors with irregular shape, such as long and narrow and flat type, the tissue close around the radioactive particles were obvious necrosis, but residual tumor tissue is often observed at the tumor margin, since the shape of the brachytherapy region is not round. Studies have shown that around a single 125I particles, cell apoptosis rate could fell sharply from 100% to 0% only a few millimeters distance [23]. Thus, when 125I radioactive particles was applied to tumor treatment, the spatial location of radioactive particle should be designed reasonable, in order to ensure uniform distribution of dose rate and avoid dose “cold spots” as much as possible.
Our results indicated that apoptosis may play a central role regarding the observed killing effects when cells were exposed to 125I seed irradiation. We analyzed the ratios of apoptosis and necrosis with H&E staining under a light microscope. The results showed that the tumor necrosis rate of 0.78 mCi group was significantly higher than those of 0.58 mCi group and 0.38 mCi group, which indicates that high-dose irradiation therapy increases the tumor necrosis rate, which is in agreement with the fact that cancer treatment is more effective at 0.78 mCi group. However, the mice exhibited behavioral signs of radiation damages, as the dose enhanced gradually. Some mice in 0.78 mCi group exhibited signs of pain and sickness. Hair loss in the right front leg and clumsy tail movement were observed in two mice from group A. That may be explaining that why the survival rate in group A lower than that in group B and group C.
Electron-microscopic observation gave us further and deep understanding about the extent of pathological impairments. Our results demonstrated that the ratios of apoptosis and necrosis in H22 cells increased significantly as the dose of radiation increased. Similar irradiation-induced apoptosis patterns were also observed in the other cancer cell lines [24]. Radiation works by directly damaging the DNA content of tumor cells and producing free radicals, which further damage the tumor cell DNA [25]. This can lead to immediate cell death or cause mutations. Therefore, the 125I irradiation-induced apoptosis is a key mechanism underlying the therapeutic effect of 125I seed implantation in HCC. It was reported that vascular fibrosis and closure is a second mechanism of radiation injury [26]. Obvious cellular organelles impairment was found in the area closely around particle sources, and the morphological structure of cells remained in normal condition as well in the distance of 3 ~ 5 mm away from the particle sources. Our results indicated that suitable dosage of 125I seed brachytherapy is a safe, minimally invasive and promising therapeutic option for liver cancer due to the eradication of local tumor cells.
In summary, our study successfully establishes the Kunming-mouse HCC model and provides a beneficial exploration of radiobiology of continuous different dose 125I seed irradiation in the treatment of HCC. 125I seed implantation effectively inhibited tumor growth and reduced tumor volume, and the dose and duration of brachytherapy requires careful calculation. 125I irradiation-induced apoptosis is the key mechanisms underlying the therapeutic effect of 125I seed implantation. Although many issues remain to be addressed, we believe that, with further development of fundamental research, the application of 125I seed implantation in clinical practice will continue to be improved.
Acknowledgements
This work was supported by Science and Technology Research Fund from Guangxi Provincial Science and Technology Department (No. 10124001A-3).
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Center MM, Jemal A. International trends in liver cancer incidence rates. Cancer Epidemiol Biomarkers Prev. 2011;20:2362–2368. doi: 10.1158/1055-9965.EPI-11-0643. [DOI] [PubMed] [Google Scholar]
- 3.Dawson LA, McGinn CJ, Normolle D, Ten Haken RK, Walker S, Ensminger W, Lawrence TS. Escalated focal liver radiation and concurrent hepatic artery fluorodeoxyuridine for unresectable intrahepatic malignancies. J. Clin. Oncol. 2000;18:2210–2218. doi: 10.1200/JCO.2000.18.11.2210. [DOI] [PubMed] [Google Scholar]
- 4.Klein J, Dawson LA. Hepatocellular carcinoma radiation therapy: review of evidence and future opportunities. Int J Radiat Oncol Biol Phys. 2013;87:22–32. doi: 10.1016/j.ijrobp.2012.08.043. [DOI] [PubMed] [Google Scholar]
- 5.Bujold A, Dawson L. Stereotactic radiation therapy and selective internal radiation therapy for hepatocellular carcinoma. Cancer/Radiothérapie. 2011;15:54–63. doi: 10.1016/j.canrad.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Bush DA, Kayali Z, Grove R, Slater JD. The safety and efficacy of high-dose proton beam radiotherapy for hepatocellular carcinoma: a phase 2 prospective trial. Cancer. 2011;117:3053–3059. doi: 10.1002/cncr.25809. [DOI] [PubMed] [Google Scholar]
- 7.Ricke J, Wust P, Stohlmann A, Beck A, Cho CH, Pech M, Wieners G, Spors B, Werk M, Rosner C. CT-guided interstitial brachytherapy of liver malignancies alone or in combination with thermal ablation: phase I-II results of a novel technique. Int J Radiat Oncol Biol Phys. 2004;58:1496–1505. doi: 10.1016/j.ijrobp.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 8.Meigooni AS, Hayes JL, Zhang H, Sowards K. Experimental and theoretical determination of dosimetric characteristics of IsoAid ADVANTAGE™ 125I brachytherapy source. Med Phys. 2002;29:2152–2158. doi: 10.1118/1.1500395. [DOI] [PubMed] [Google Scholar]
- 9.Nath R, Melillo A. Dosimetric characteristics of a double wall 125I source for interstitial brachytherapy. Med Phys. 1993;20:1475–1483. doi: 10.1118/1.597158. [DOI] [PubMed] [Google Scholar]
- 10.Nag S, Beyer D, Friedland J, Grimm P, Nath R. American Brachytherapy Society (ABS) recommendations for transperineal permanent brachytherapy of prostate cancer. Int J Radiat Oncol Biol Phys. 1999;44:789–799. doi: 10.1016/s0360-3016(99)00069-3. [DOI] [PubMed] [Google Scholar]
- 11.Zhongmin W, Yu L, Fenju L, Kemin C, Gang H. Clinical efficacy of CT-guided iodine-125 seed implantation therapy in patients with advanced pancreatic cancer. Eur Radiol. 2010;20:1786–1791. doi: 10.1007/s00330-009-1703-0. [DOI] [PubMed] [Google Scholar]
- 12.Martínez-Monge R, Nag S, Martin EW. Three different intraoperative radiation modalities (electron beam, high-dose-rate brachytherapy, and iodine-125 brachytherapy) in the adjuvant treatment of patients with recurrent colorectal adenocarcinoma. Cancer. 1999;86:236–247. doi: 10.1002/(sici)1097-0142(19990715)86:2<236::aid-cncr7>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 13.Nag S, Cano ER, Demanes DJ, Puthawala AA, Vikram B. The American Brachytherapy Society recommendations for high-dose-rate brachytherapy for head-and-neck carcinoma. Int J Radiat Oncol Biol Phys. 2001;50:1190–1198. doi: 10.1016/s0360-3016(01)01567-x. [DOI] [PubMed] [Google Scholar]
- 14.Lee W, Daly BD, DiPetrillo TA, Morelli DM, Neuschatz AC, Morr J, Rivard MJ. Limited resection for non-small cell lung cancer: observed local control with implantation of I-125 brachytherapy seeds. Ann Thorac Surg. 2003;75:237–242. doi: 10.1016/s0003-4975(02)04098-5. [DOI] [PubMed] [Google Scholar]
- 15.Gutin PH, Leibel SA, Wara WM, Choucair A, Levin VA, Philips TL, Silver P, Da Silva V, Edwards MS, Davis RL. Recurrent malignant gliomas: survival following interstitial brachytherapy with high-activity iodine-125 sources. J Neurosurg. 1987;67:864–873. doi: 10.3171/jns.1987.67.6.0864. [DOI] [PubMed] [Google Scholar]
- 16.Halligan JB, Stelzer KJ, Rostomily RC, Spence AM, Griffin TW, Berger MS. Operation and permanent low activity 125I brachytherapy for recurrent high-grade astrocytomas. Int J Radiat Oncol Biol Phys. 1996;35:541–547. doi: 10.1016/s0360-3016(96)80017-4. [DOI] [PubMed] [Google Scholar]
- 17.Charyulu K, Block N, Sudarsanam A. Preoperative extended field radiation with I-125 seed implant in prostatic cancer: a preliminary report of a randomized study. Int J Radiat Oncol Biol Phys. 1979;5:1957–1961. doi: 10.1016/0360-3016(79)90945-3. [DOI] [PubMed] [Google Scholar]
- 18.Wallner K, Roy J, Zelefsky M, Fuks Z, Harrison L. Short-term freedom from disease progression after I-125 prostate implantation. Int J Radiat Oncol Biol Phys. 1994;30:405–409. doi: 10.1016/0360-3016(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 19.Rosenthal SA, Bittner NH, Beyer DC, Demanes DJ, Goldsmith BJ, Horwitz EM, Ibbott GS, Lee WR, Nag S, Suh WW. American Society for Radiation Oncology (ASTRO) and American College of Radiology (ACR) Practice Guideline for the Transperineal Permanent Brachytherapy of Prostate Cancer. Int J Radiat Oncol Biol Phys. 2011;79:335–341. doi: 10.1016/j.ijrobp.2010.08.045. [DOI] [PubMed] [Google Scholar]
- 20.Selbach HJ, Bambynek M, Aubineau-Lanièce I, Gabris F, Guerra AS, Toni MP, de Pooter J, Sander T, Schneider T. Experimental determination of the dose rate constant for selected 125I- and 192Ir-brachytherapy sources. Metrologia. 2012;49:S219. [Google Scholar]
- 21.Lo SS, Dawson LA, Kim EY, Mayr NA, Wang JZ, Huang Z, Cardenes HR. Stereotactic body radiation therapy for hepatocellular carcinoma. Discov Med. 2010;9:404–410. [PubMed] [Google Scholar]
- 22.Skinner HD, Sharp HJ, Kaseb AO, Javle MM, Vauthey JN, Abdalla EK, Delclos ME, Das P, Crane CH, Krishnan S. Radiation treatment outcomes for unresectable hepatocellular carcinoma. Acta Oncol. 2011;50:1191–1198. doi: 10.3109/0284186X.2011.592147. [DOI] [PubMed] [Google Scholar]
- 23.Kirov AS, Williamson JF. Monte Carlo-aided dosimetry of the Source Tech Medical Model STM1251 I-125 interstitial brachytherapy source. Med Phys. 2001;28:764–772. doi: 10.1118/1.1367280. [DOI] [PubMed] [Google Scholar]
- 24.Zhuang HQ, Wang JJ, Liao AY, Wang JD, Zhao Y. The biological effect of 125I seed continuous low dose rate irradiation in CL187 cells. J Exp Clin Canc Res. 2009;28:12. doi: 10.1186/1756-9966-28-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang SM, Harari PM. Modulation of radiation response after epidermal growth factor receptor blockade in squamous cell carcinomas: inhibition of damage repair, cell cycle kinetics, and tumor angiogenesis. Clinl Cancer Res. 2000;6:2166–2174. [PubMed] [Google Scholar]
- 26.Wilcox JN, Waksman R, King SB, Scott NA. The role of the adventitia in the arterial response to angioplasty: the effect of intravascular radiation. Int J Radiat Oncol Biol Phys. 1996;36:789–796. doi: 10.1016/s0360-3016(96)00299-4. [DOI] [PubMed] [Google Scholar]


