Abstract
Although many epidemiologic studies have investigated obesity and thyroid cancer risk, definite conclusions cannot be drawn. To clarify the effects of obesity on the risk of thyroid cancer, a meta-analysis was performed. Related studies were identified from PubMed, Springer Link, Ovid, Chinese Wanfang Data Knowledge Service Platform, Chinese National Knowledge Infrastructure (CNKI), and Chinese Biology Medicine (CBM) till 16 Aug 2014. Pooled RRs and 95% CIs were used to assess the strength of the associations. A total of 16 studies including 12616154 subjects were involved in this meta-analysis. A significantly elevated thyroid cancer risk was found in overall analysis (RR = 1.29, 95% CI 1.20-1.37, P < 0.00001). In the gender subgroup analyses, a statistically significant association was found in male patients (RR = 1.35, 95% CI 1.16-1.58, P = 0.0001) and in female patients (RR = 1.29, 95% CI 1.19-1.40, P < 0.00001). When we limited the meta-analysis to studies that controlled for age (RR = 1.34, 95% CI 1.24-1.44, P < 0.00001), smoke (RR = 1.36, 95% CI 1.22-1.52, P < 0.00001), alcohol use (RR = 1.40, 95% CI 1.15-1.71, P = 0.0009), and history of benign thyroid disease (RR = 1.51, 95% CI 1.24-1.83, P < 0.0001), a significant association between obesity and thyroid cancer risk remained. This meta-analysis provides the evidence that obesity may contribute to the thyroid cancer development.
Keywords: Thyroid cancer, BMI, obesity, association, meta-analysis
Introduction
Obesity is due to excessive fat accumulation that may impair health resulting from social behaviour and environmental and genetic factors [1]. During the last 20 years, obesity has rapidly become a global pandemic health problem: catastrophic data come from America and from Europe where ~35% and ~20% of the population, respectively, are obese [2]. Globally, the World Health Organization (WHO) has predicted that, in 2015, ~2.3 billion of adults will be overweight; 700 million will be obese, while ~200 million of school aged children will be obese/overweight (http://www.IASO.org/).
Thyroid cancer is the most common endocrine cancer, traditionally classified into two major groups based on morphologic and clinical features: differentiated carcinoma and undifferentiated carcinoma [3]. The worldwide incidence of thyroid cancer has been rapidly increasing over the last three decades [4]. Dieringer et al. found that obesity was significantly associated with larger thyroid cancer size and marginally significantly associated with advanced thyroid cancer stage [5]. In addition, Kim et al. showed that a higher body mass index (BMI) was associated with more aggressive tumor features, such as lymph node metastasis, lymphatic invasion, and tumor multiplicity [6]. Therefore, obesity might have a critical role in the thyroid cancer development.
A series of studies have investigated the association between the obesity and thyroid cancer susceptibility, but provided controversial or inconclusive results [7-22]. Thus, we performed this meta-analysis to assess the relationship of obesity with risk of thyroid cancer.
Materials and methods
Publication search
We searched databases containing PubMed, Springer Link, Ovid, Chinese Wanfang Data Knowledge Service Platform, Chinese National Knowledge Infrastructure (CNKI), and Chinese Biology Medicine (CBM) up to 16 Aug 2014, using the following Mesh terms: (“thyroid Neoplasms” [MeSH] or “thyroid cancer” or “thyroid tumor” or “thyroid carcinoma” or “carcinoma of thyroid”) and (“obesity” or “body mass index”). The references from retrieved articles were also searched.
Inclusion criteria and data extraction
Studies included in this meta-analysis have to meet the following criteria: (1) case-control study or cohort study studying on association between obesity and risk of thyroid cancer; (2) all patients with the diagnosis of thyroid cancer confirmed by pathological or histological examination; (3) sufficient published data about sample size, risk ratio (RR), and their 95% confidence interval (CI). Studies were excluded when they were: (1) not case-control study or cohort study; (2) duplicate of previous publication; (3) based on incomplete data; (4) meta-analyses, letters, reviews, or editorial articles.
Data were independently extracted by two reviewers using a standardized data extraction form. Discrepancies were resolved by discussion and if consensus was not achieved the decision was made by all the reviewers. The title and abstract of all potentially relevant articles were screened to determine their relevance. Full articles were also scrutinized if the title and abstract were ambiguous. The following information was collected from each study: authors, year of publication, age, sex, sample size, covariants.
Statistical analysis
Statistical analysis was conducted by using STATA statistical package (version 11, STATA, College Station, TX). The association of polymorphisms of obesity and risk of thyroid cancer was estimated by RR with 95% CI. The heterogeneity was tested by the Q-statistics with P-values < 0.1, and its possible sources of heterogeneity were assessed by Galbraith plot. Dependent on the results of heterogeneity test among individual studies, the fixed effect model (Mantel-Haenszel) or random effect model (DerSimonian and Laird) was selected to summarize the combined OR and their 95% CI. The significance of the pooled OR was determined by the z test. Publication bias was investigated with the funnel plot, in which the Standard Error (SE) of log OR of each study was plotted against its OR. Funnel-plot asymmetry was further assessed by the method of Egger’s linear regression test. All the P values were two sided. P value less than 0.05 was considered statistically significant.
Results
Eligible studies
In this current study, a total of 16 eligible studies met the inclusion criteria [7-22]. Eight articles reported two cohorts, and each cohort was considered as a study. There were 11 studies performed using male, 12 studies using female, and 1 studies using mixed populations. A total of 12616154 subjects were included in this meta-analysis. The characteristics of each study included in this meta-analysis are presented in Table 1.
Table 1.
Characteristics of the studies
| First author/Year | Age | Male (%) | No. of subjects | Adjustment of covariants |
|---|---|---|---|---|
| Samanic a/2004 | 52 | 100 | 3668486 | Age, calendar year |
| Samanic b/2004 | 47 | 100 | 832214 | Age, calendar year |
| Oh/2005 | ≥20 | 100 | 781283 | Age, smoking status, average amount of alcohol consumed per day, frequency of regular exercise for more than 30 minutes during a week, family history of cancer, residency area at baseline |
| Rapp/2005 | 42 | 0 | 78484 | Smoking, occupational group at baseline |
| Engeland a/2006 | 62 | 100 | 963523 | Age, year of birth, height |
| Engeland b/2006 | 58 | 0 | 1037424 | Age, year of birth, height |
| Samanic/2006 | 34 | 100 | 362552 | Age, calendar year, smoking status, and relative to normal weight subjects |
| Song/2008 | 56 | 0 | 170481 | Age, height, smoking status, alcohol intake, physical exercise, pay level at study entry |
| Leitzmann/2009 | 62 | 0 | 484326 | Age, sex, physical activity, race, education, smoking status, current alcohol use; oral contraceptive use among women |
| Meinhold a/2009 | 43 | 100 | 21207 | Birth year, smoking status, body mass index, number of personal radiographs to the head or neck, cumulative occupational radiation dose, medical history of benign thyroid conditions |
| Meinhold b/2009 | 39 | 0 | 69506 | Birth year, smoking status, body mass index, number of personal radiographs to the head or neck, cumulative occupational radiation dose, medical history of benign thyroid conditions |
| Clavel-Chapelon/2010 | 49 | 0 | 91909 | Age, stratified on year of birth, history of goiter or thyroid nodules, smoking status, iodine |
| Almquist a/2011 | 43 | 100 | 289866 | Age, smoking |
| Almquist b/2011 | 44 | 0 | 288834 | Age, smoking |
| Kitahara/2011 | 58 | 74 | 848932 | Education, race, marital status, smoking, alcohol intake, sex |
| Kabat/2012 | 44 | 0 | 144319 | Age, education, pack-years of smoking, alcohol intake, history of benign thyroid disease |
| Rinaldi a/2012 | 52 | 100 | 150000 | Center, age, smoking |
| Rinaldi b/2012 | 51 | 0 | 370000 | Center, age, smoking |
| Han a/2013 | 51 | 100 | 9275 | Age, smoking status, TSH levels |
| Han b/2013 | 50 | 0 | 8138 | Age, smoking status, TSH levels |
| Farfel a/2014 | 16-19 | 100 | 1145865 | Year of birth, country of origin, years of schooling |
| Farfel b/2014 | 16-19 | 0 | 478445 | Year of birth, country of origin, years of schooling |
| Kitahara a/2014 | 7-13 | 100 | 162632 | Birth cohort |
| Kitahara b/2014 | 7-13 | 0 | 158453 | Birth cohort |
Quantitative synthesis
The main results of this meta-analysis and the heterogeneity test were shown in Table 2. A significantly elevated thyroid cancer risk was found in overall analysis (RR = 1.29, 95% CI 1.20-1.37, P < 0.00001, Figure 1). In the gender subgroup analyses, a statistically significant association was found in male patients (RR = 1.35, 95% CI 1.16-1.58, P = 0.0001) and in female patients (RR = 1.29, 95% CI 1.19-1.40, P < 0.00001). When we limited the meta-analysis to studies that controlled for age (RR = 1.34, 95% CI 1.24-1.44, P < 0.00001), smoke (RR = 1.36, 95% CI 1.22-1.52, P < 0.00001), alcohol use (RR = 1.40, 95% CI 1.15-1.71, P = 0.0009), and history of benign thyroid disease (RR = 1.51, 95% CI 1.24-1.83, P < 0.0001), a significant association between obesity and thyroid cancer risk remained.
Table 2.
Main results of this meta-analysis
| No. of studies | RR (95% CI) | P Value | I 2 (%) | P heterogeneity | |
|---|---|---|---|---|---|
| Overall | 24 | 1.29 (1.20-1.37) | < 0.00001 | 21 | 0.18 |
| Man | 11 | 1.35 (1.16-1.58) | 0.0001 | 23 | 0.23 |
| Women | 12 | 1.29 (1.19-1.40) | < 0.00001 | 0 | 0.77 |
| Adjusted for | |||||
| Age | 18 | 1.34 (1.24-1.44) | < 0.00001 | 3 | 0.42 |
| Smoke | 16 | 1.36 (1.22-1.52) | < 0.00001 | 37 | 0.07 |
| Alcohol use | 5 | 1.40 (1.15-1.71) | 0.0009 | 64 | 0.02 |
| History of benign thyroid disease | 4 | 1.51 (1.24-1.83) | < 0.0001 | 0 | 0.75 |
Figure 1.
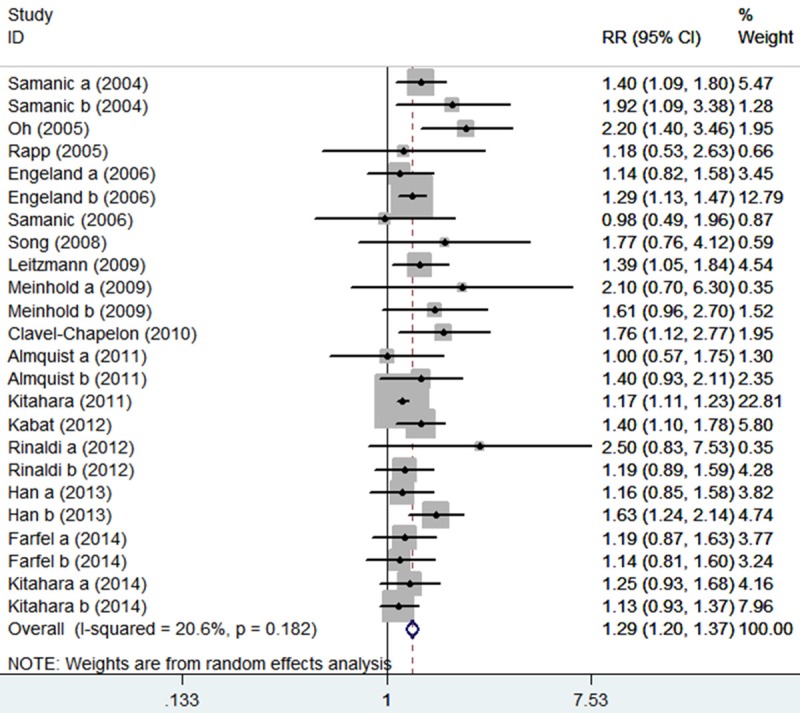
The association between obesity and thyroid cancer risk.
The Galbraith plot was used to find the source of the heterogeneity. As shown in Figure 2, two studies were the outliers. After excluding these studies, the between-study heterogeneity effectively decreased and there was no obvious heterogeneity among the remaining studies (I2 = 0%). Besides, the result was still statistically significant (RR = 1.21, 95% CI 1.16-1.26, P < 0.00001).
Figure 2.
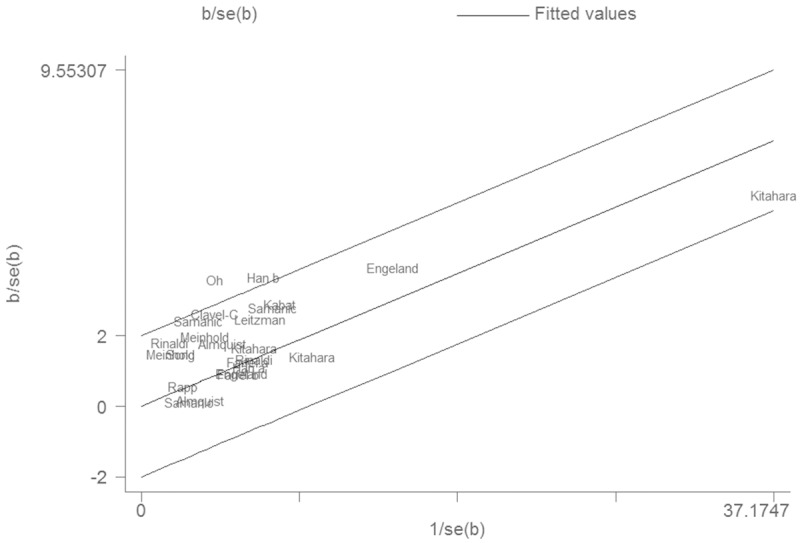
Galbraith plot of the association between obesity and thyroid cancer risk.
Publication bias was examined by the funnel plot. The shape of the funnel plot was symmetrical (Figure 3). Egger’s test indicated no significant publication bias (P = 0.427).
Figure 3.
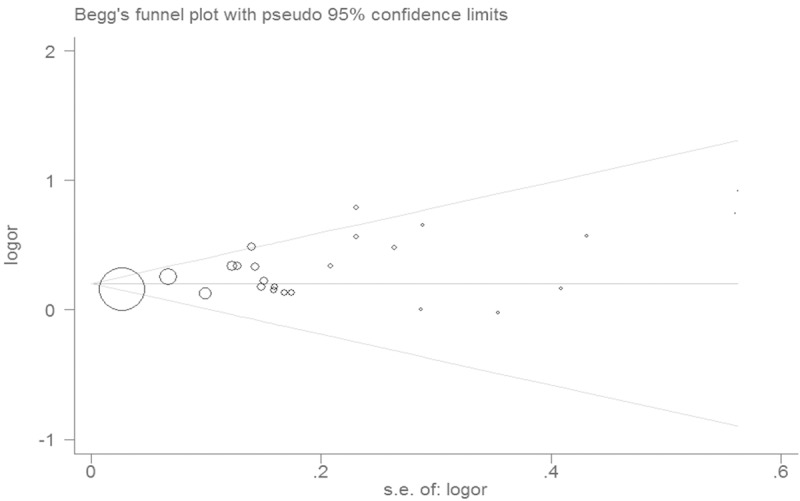
Funnel plot for the association between obesity and thyroid cancer risk.
Discussion
Although many studies analyzing the research results about obesity and the risk of thyroid cancer, definite conclusions cannot be drawn. Therefore, we did this meta-analysis to estimate the relationship between obesity and susceptibility to thyroid cancer. We found that obesity individuals showed an increased risk of thyroid cancer. The subgroup analysis based on sex found that obesity showed increased thyroid cancer risk in female patients and in male patients.
Obesity is a serious problem which heightens the risk of several chronic illnesses including cancer development. Potential mechanisms linking obesity and thyroid cancer risk include elevated TSH levels, insulin resistance, and adipokines effect [23]. TSH and insulin influence the growth and differentiation of follicular cells [24]. Adipokines such as adiponectin, leptin, and hepatocyte growth factor may regulate cancer cell proliferation and may be related to cancer progression [23]. Increased expression of leptin and its receptor in thyroid cancer were reported [25]. Its association with tumor aggressiveness and biological behavior was also demonstrated [25].
We had to mention the importance of heterogeneity and publication bias, which might influence the results of meta-analysis. In our study, significant heterogeneity was observed. We used Galbraith plot to explore the sources of heterogeneity. We found that I2 value was decreased after excluding the outliers. The results suggested that the two outlying studies might be the major source of the heterogeneity. However, heterogeneity did not seem to influence the results, because the significance of the result was not altered after excluding the outliers. Additionally, funnel plots and Egger’s tests were used to find potential publication bias. The results indicated that there was no significant publication bias.
This meta-analysis has limitations that must be acknowledged. First, the numbers of published studies were not sufficient for a comprehensive analysis, particularly for smoker. Second, lacking of the original data of the eligible studies limited the evaluation of the effects of the gene-obesity interactions in thyroid cancer.
In summary, this meta-analysis suggested that obesity was associated with the risk of thyroid cancer. However, large and well-designed studies are warranted to validate our findings.
Disclosure of conflict of interest
None.
References
- 1.Friedman JM. Obesity in the new millennium. Nature. 2000;404:632–4. doi: 10.1038/35007504. [DOI] [PubMed] [Google Scholar]
- 2.Berghöfer A, Pischon T, Reinhold T, Apovian CM, Sharma AM, Willich SN. Obesity prevalence from a European perspective: a systematic review. BMC Public Health. 2008;8:200. doi: 10.1186/1471-2458-8-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dal Maso L, Bosetti C, La Vecchia C, Franceschi S. Risk factors for thyroid cancer: an epidemiological review focused on nutritional factors. Cancer Causes Control. 2009;20:75–86. doi: 10.1007/s10552-008-9219-5. [DOI] [PubMed] [Google Scholar]
- 4.Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R. Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol. 2013;2013:965212. doi: 10.1155/2013/965212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dieringer P, Klass EM, Caine B, Smith-Gagen J. Associations between body mass and papillary thyroid cancer stage and tumor size: a population-based study. J Cancer Res Clin Oncol. 2015;141:93–8. doi: 10.1007/s00432-014-1792-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim SH, Park HS, Kim KH, Yoo H, Chae BJ, Bae JS, Jung SS, Song BJ. Correlation between obesity and clinicopathological factors in patients with papillary thyroid cancer. Surg Today. 2014 doi: 10.1007/s00595-014-0984-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Samanic C, Gridley G, Chow WH, Lubin J, Hoover RN, Fraumeni JF Jr. Obesity and cancer risk among white and black United States veterans. Cancer Causes Control. 2004;15:35–43. doi: 10.1023/B:CACO.0000016573.79453.ba. [DOI] [PubMed] [Google Scholar]
- 8.Oh SW, Yoon YS, Shin SA. Effects of excess weight on cancer incidences depending on cancer sites and histologic findings among men: Korea National Health Insurance Corporation Study. J. Clin. Oncol. 2005;23:4742–54. doi: 10.1200/JCO.2005.11.726. [DOI] [PubMed] [Google Scholar]
- 9.Rapp K, Schroeder J, Klenk J, Stoehr S, Ulmer H, Concin H, Diem G, Oberaigner W, Weiland SK. Obesity and incidence of cancer: a large cohort study of over 145,000 adults in Austria. Br J Cancer. 2005;93:1062–7. doi: 10.1038/sj.bjc.6602819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engeland A, Tretli S, Akslen LA, Bjørge T. Body size and thyroid cancer in two million Norwegian men and women. Br J Cancer. 2006;95:366–70. doi: 10.1038/sj.bjc.6603249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samanic C, Chow WH, Gridley G, Jarvholm B, Fraumeni JF Jr. Relation of body mass index to cancer risk in 362,552 Swedish men. Cancer Causes Control. 2006;17:901–9. doi: 10.1007/s10552-006-0023-9. [DOI] [PubMed] [Google Scholar]
- 12.Song YM, Sung J, Ha M. Obesity and risk of cancer in postmenopausal Korean women. J. Clin. Oncol. 2008;26:3395–402. doi: 10.1200/JCO.2007.15.7867. [DOI] [PubMed] [Google Scholar]
- 13.Leitzmann MF, Brenner A, Moore SC, Koebnick C, Park Y, Hollenbeck A, Schatzkin A, Ron E. Prospective study of body mass index, physical activity and thyroid cancer. Int J Cancer. 2010;126:2947–56. doi: 10.1002/ijc.24913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meinhold CL, Ron E, Schonfeld SJ, Alexander BH, Freedman DM, Linet MS, Berrington de González A. Nonradiation risk factors for thyroid cancer in the US Radiologic Technologists Study. Am J Epidemiol. 2010;171:242–52. doi: 10.1093/aje/kwp354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clavel-Chapelon F, Guillas G, Tondeur L, Kernaleguen C, Boutron-Ruault MC. Risk of differentiated thyroid cancer in relation to adult weight, height and body shape over life: the French E3N cohort. Int J Cancer. 2010;126:2984–90. doi: 10.1002/ijc.25066. [DOI] [PubMed] [Google Scholar]
- 16.Almquist M, Johansen D, Björge T, Ulmer H, Lindkvist B, Stocks T, Hallmans G, Engeland A, Rapp K, Jonsson H, Selmer R, Diem G, Häggström C, Tretli S, Stattin P, Manjer J. Metabolic factors and risk of thyroid cancer in the Metabolic syndrome and Cancer project (Me-Can) Cancer Causes Control. 2011;22:743–51. doi: 10.1007/s10552-011-9747-2. [DOI] [PubMed] [Google Scholar]
- 17.Kitahara CM, Platz EA, Freeman LE, Hsing AW, Linet MS, Park Y, Schairer C, Schatzkin A, Shikany JM, Berrington de González A. Obesity and thyroid cancer risk among U. S. men and women: a pooled analysis of five prospective studies. Cancer Epidemiol Biomarkers Prev. 2011;20:464–72. doi: 10.1158/1055-9965.EPI-10-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kabat GC, Kim MY, Thomson CA, Luo J, Wactawski-Wende J, Rohan TE. Anthropometric factors and physical activity and risk of thyroid cancer in postmenopausal women. Cancer Causes Control. 2012;23:421–30. doi: 10.1007/s10552-011-9890-9. [DOI] [PubMed] [Google Scholar]
- 19.Rinaldi S, Lise M, Clavel-Chapelon F, Boutron-Ruault MC, Guillas G, Overvad K, Tjønneland A, Halkjær J, Lukanova A, Kaaks R, Bergmann MM, Boeing H, Trichopoulou A, Zylis D, Valanou E, Palli D, Agnoli C, Tumino R, Polidoro S, Mattiello A, Bueno-de-Mesquita HB, Peeters PH, Weiderpass E, Lund E, Skeie G, Rodríguez L, Travier N, Sánchez MJ, Amiano P, Huerta JM, Ardanaz E, Rasmuson T, Hallmans G, Almquist M, Manjer J, Tsilidis KK, Allen NE, Khaw KT, Wareham N, Byrnes G, Romieu I, Riboli E, Franceschi S. Body size and risk of differentiated thyroid carcinomas: findings from the EPIC study. Int J Cancer. 2012;131:E1004–14. doi: 10.1002/ijc.27601. [DOI] [PubMed] [Google Scholar]
- 20.Han JM, Kim TY, Jeon MJ, Yim JH, Kim WG, Song DE, Hong SJ, Bae SJ, Kim HK, Shin MH, Shong YK, Kim WB. Obesity is a risk factor for thyroid cancer in a large, ultrasonographically screened population. Eur J Endocrinol. 2013;168:879–86. doi: 10.1530/EJE-13-0065. [DOI] [PubMed] [Google Scholar]
- 21.Farfel A, Kark JD, Derazne E, Tzur D, Barchana M, Lazar L, Afek A, Shamiss A. Predictors for Thyroid Carcinoma in Israel: A National Cohort of 1,624,310 Adolescents Followed for up to 40 Years. Thyroid. 2014;24:987–93. doi: 10.1089/thy.2013.0173. [DOI] [PubMed] [Google Scholar]
- 22.Kitahara CM, Gamborg M, Berrington de González A, Sørensen TI, Baker JL. Childhood height and body mass index were associated with risk of adult thyroid cancer in a large cohort study. Cancer Res. 2014;74:235–42. doi: 10.1158/0008-5472.CAN-13-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paes JE, Hua K, Nagy R, Kloos RT, Jarjoura D, Ringel MD. The relationship between body mass index and thyroid cancer pathology features and outcomes: a clinicopathological cohort study. J Clin Endocrinol Metab. 2010;95:4244–50. doi: 10.1210/jc.2010-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parameswaran R, Brooks S, Sadler GP. Molecular pathogenesis of follicular cell derived thyroid cancers. Int J Surg. 2010;8:186–93. doi: 10.1016/j.ijsu.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Uddin S, Bavi P, Siraj AK, Ahmed M, Al-Rasheed M, Hussain AR, Ahmed M, Amin T, Alzahrani A, Al-Dayel F, Abubaker J, Bu R, Al-Kuraya KS. Leptin-R and its association with PI3K/AKT signaling pathway in papillary thyroid carcinoma. Endocr Relat Cancer. 2010;17:191–202. doi: 10.1677/ERC-09-0153. [DOI] [PubMed] [Google Scholar]


