Abstract
IgA nephropathy (IgAN) is the most common glomerulonephritis and the etiology of which is complex and multiple, and the pathological damage of IgAN is diversified. MicroRNA is a kind of gene expression suppressor and recently, researchers have found that microRNAs may play an important role in the pathogenesis of IgAN. Herein, we found that miR-29b-3p not miR-29a or miR-29c was significantly down regulated in IgAN patients’ renal tissues. Predicted by bioinformatics tools and confirmed by dual luciferase assay and western blot, we found that the expression of CDK6 was repressed by miR-29b-3p directly. Subsequently, we found that miR-29b-3p down-regulation caused CDK6 overexpression can promote NF-κB signal by phosphorylating p65 which may enhance inflammation during IgAN pathogenesis.
Keywords: MicroRNA, IgA nephropathy, CDK6, p65, phosphorylation
Introduction
Immunoglobulin A nephropathy (IgAN) is the most common glomerulonephritis worldwide [1]. Prevalence of IgAN is extremely high, noted in 20%-40% of renal biopsies in Asian countries and South Europe [2,3]. Approximately 30-40% of IgAN patients progress to end-stage renal disease (ESRD) within 20 years [4]. At present, IgAN is thought to be a complex multifactorial disease; genetics and immunology mechanisms have been suggested to play a central role in the pathogenesis of IgAN.
The NF-κB family of transcription factors regulates the induction and resolution of inflammation. Two main pathways, classical and alternative, control the nuclear translocation of NF-κB. Classical NF-κB activation is usually a rapid and transient response to a wide range of stimuli whose main effector is RelA (p65)/p50. The alternative NF-κB pathway is a more delayed response to a smaller range of stimuli resulting in DNA binding of RelB/p52 complexes. Additional complexity in this system involves the posttranslational modification of NF-κB proteins and an ever-increasing range of co-activators, co-repressors, and NF-κB complex proteins. Collectively, NF-κB regulates the expression of numerous genes that play a key role in the inflammatory response during human and experimental kidney injury.
MicroRNAs (miRNAs) are short non-coding RNAs which modulate gene expression by binding to complementary segments present in the 3’UTR of the mRNAs of protein coding genes. MiRNAs play very important roles in maintaining normal human body physiology conditions, and abnormal miRNA expressions have been found related to many human diseases spanning from psychiatric disorders to malignant cancers [5-7].
Recently, some research has elucidated the connections between miRNAs and kidney disease, including IgAN [8,9]. Wang et al. reported that urinary expression of miR-200a, miR-200b, and miR-429 and intrarenal expression of miR-200c, miR-141, miR-205, and miR-192 were diversely regulated and correlated with disease severity and progression in patients with IgAN [9]. Tan and colleagues did a genome-wide microRNAs expression profiling in IgA nephropathy patients and found a total of 85 miRNAs that were differentially expressed in the six IgAN patients [10]. However, the potential role of miRNA in the IgAN pathogenesis has been poorly investigated.
In this study, we first compared the contents of miR-29 family members in IgAN patients’ tissues with control tissues and found that miR-29b-3p was down regulated significantly. Subsequently, we confirmed that CDK6 is one target gene of miR-29-3p and the inhibition of miR-29-3p can activate NF-κB pathway by phosphorylating p65.
Materials and methods
Clinical sample collection
From March to October 2012, we studied seven patients with IgAN (IgAN group), confirmed by kidney biopsy specimens in the Center Hospital of Putuo District, Shanghai. The kidney biopsy specimens demonstrated in all of the seven patients a histological grading of III according to the grading system of Lee et al. [11]. We excluded patients with other coexisting renal pathology and recurrent IgAN after kidney transplantation. The clinical research ethical committee of the Center Hospital of Putuo District approved the study. All patients provided their written informed consent. We studied the renal biopsy specimens with adjacent non-tumoral renal parenchyma from the nephrectomy specimens of seven patients with renal cell carcinoma (RCC) as controls for intrarenal miRNA expression study.
We evaluated the IgAN and control groups’ renal tissues using standard light microscopy to ensure that the specimens were mainly renal cortex. Specimens were snap-frozen in liquid nitrogen or deposited in RNAlater (QIAGEN, Hilden, Germany) and subsequently stored at -80°C.
Real-time reverse transcriptase quantitative PCR
Quantitive RT-PCR analysis was used to determine the relative expression level of miR-29a, miR-29b and miR-29c. Total RNA was extracted from tissues using Trizol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The expression levels of miR-29a, miR-29b and miR-29c were detected by TaqMan miRNA RT-Real Time PCR. Single-stranded cDNA was synthesized by using TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) and then amplified by using TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA, USA) together with miRNA-specific TaqMan MGB probes: miR-29a, miR-29b and miR-29c (Applied Biosystems, Foster City, CA, USA). The U6 snRNA was used for normalization. Each sample in each group was measured in triplicate and the experiment was repeated at least three times for the detection of miR-29a, miR-29b and miR-29c.
Cell culture
HEK293T cells were cultured in Dulbecco’s Modified Eagle Medium containing 10% fetal bovine serum (Hyclone, Logan, UT, USA), 100 IU/ml penicillin and 10 mg/mL streptomycin. All cells were maintained at 37°C under an atmosphere of 5% CO2.
3’-UTR luciferase reporter assays
To generate 3’-UTR luciferase reporter, a segment of 1023 bp 3’-UTR from CDK6 was cloned into the downstream of the firefly luciferase gene in pGL3-Control Vector (Promega). MiR-29b-3p mimic and miR-29b-3p inhibitor were synthesized by GenePharma Co., Ltd (Shanghai, China). pRL-TK vector containing Renilla luciferase was co-transfected for data normalization. For luciferase reporter assays, HEK293T cells were seeded in 48-well plates. Luciferase reporter vectors were co-transfected with miR-29b-3p mimic or inhibitor by using lipofectamine 2000 (Invitrogen, Carlsbad, CA USA). Two days later, cells were harvested and assayed with the Dual-Luciferase Assay (Promega, Madison, WI USA). Each treatment was performed in triplicate in three independent experiments. The results were expressed as relative luciferase activity (Firefly LUC/Renilla LUC).
Western blotting
Protein extracts were boiled in SDS/β-mercaptoethanol sample buffer, and 30 μg samples were loaded into each lane of 10% polyacrylamide gels. The proteins were separated by electrophoresis, and the proteins in the gels were blotted onto PVDF membranes (Amersham Pharmacia Biotech, St. Albans, Herts, UK) by electrophoretic transfer. Antibodies against the following proteins were used: tubulin, β-actin, CDK6 (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), P-Ser536 p65 (Cell Signaling Technology, Inc., Danvers, MA, USA).
Statistical analysis
Data were analyzed by using SPSS Statistical Package version 16. Independent two group’s analyses are used t-test. P < 0.05 was considered statistically significant.
Results
MiR-29b-3p was down-regulated in IgAN patients
In order to explore the roles of miR-29 family members in IgAN, the expression patterns of miR-29a, miR-29b and miR-29c in 7 kidney tissues of IgAN patients were detected by qRT-PCR, renal biopsy specimens with adjacent nontumoral renal parenchyma from the nephrectomy specimens of six patients with renal cell carcinoma (RCC) as controls (Figure 1). MiR-29b-3p level was significantly decreased in renal tissue of IgAN patients (P=0.01) compared with control group. The expression of miR-29a and miR-29c were reduced slightly but the difference was not significant (P=0.30, P=0.39).
Figure 1.
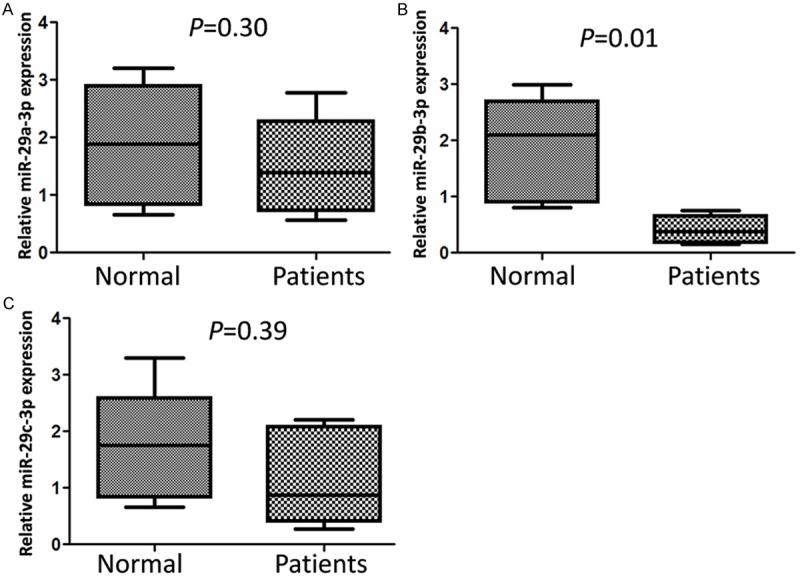
Down-regulated miR-29b-3p was found in IgAN patients’ renal tissues. The expression level of miR-29a-3p (A), miR-29b-3p (B) and miR-29c-3p (C) in individual sample was detected by RT-qPCR. Statistical analyses were performed to analyze the overall trend of miRNA in all IgAN patients’ renal tissues and control tissues. U6 snRNA serves as an internal reference among different samples and helps normalize for experimental error.
MiR-29b-3p repressed CDK6 expression by directly targeting CDK6 mRNA 3’UTR
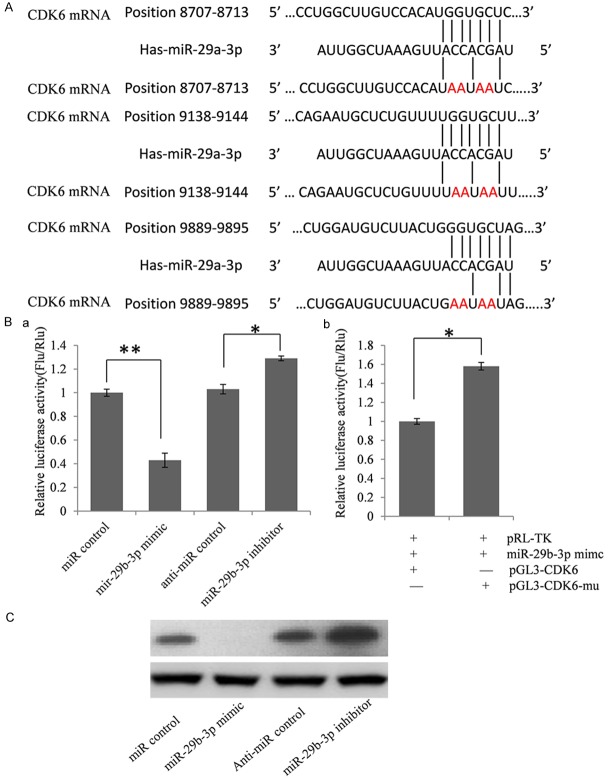
MiRNA is a kind of important post transcription negative regulators for protein coding genes. To explore the role of reduced miR-29b-3p in IgAN pathogenesis, we first predicted miR-29b-3p target genes by using online bioinformatics tools: TargetScan and miRanda. We find CDK6 may be one target gene of miR-29b-3p and there are 3 miR-29b-3p binding sites in the 3’UTR of CDK6 mRNA. There are reports that CDK6 play an important role in inflammation [12,13], so we first confirmed CDK6 was a target gene of miR-29b-3p by using dual luciferase assay and western blot.
To validate whether CDK6 is indeed the target gene of miR-293, a 1023 bp segment of CDK6 3’-UTR containing miR-29b-3p binding sites was cloned into the downstream of the firefly luciferase reporter gene in the pGL3 control vector (designated as pGL3-CDK6) for the dual luciferase assay. HEK293T cells were co-transfected with pGL3-CDK6 and miR-29b-3p mimics or inhibitor (Figure 2B). Compared with the miRNA control, the luciferase activity was significantly suppressed by the miR-29b-3p, about 56.8% (P < 0.01). Furthermore, the luciferase activity was significantly up-regulated by the miR-29b-3p inhibitor compared with the anti-miR control, about 27.7% (P < 0.05). These results indicate that miR-29b-3p targets the 3’-UTR of CDK6, leading to the change of firefly luciferase translation.
Figure 2.
MiR-29b-3p suppressed CDK6 expression by targeting 3’UTR. A. Predicted miR-29b-3p binding sites in CDK6 3’UTR. Ba. Confirmation of the target gene of miR-29b-3p. HEK293T cells were co-transfected with miRNA control, miR-29b-3p mimic, anti-miR control or miR-29b-3p inhibitor and pGL3-CDK6 for dual-luciferase assay. PRL-TK containing Renilla luciferase was co-transfected for data normalization. Bb. Mutation analysis of the miR-29b-3p binding sites. When 4 nucleotides of these binding sites of miR-29b-3p in the 3’-UTR of CDK6 was mutated (pGL3-CDK6-Mu), the luciferase activity was significantly decreased in HEK293T cells co-transfected with miR-29b-3p mimics and pGL3-CDK6 compared with pGL3-CDK6-Mu. C. CDK6 protein level in miR-29b-3p mimic or inhibitor-treated HEK293T cells was detected by western blot.
Seed sequence mutation clone was also used to further confirm the binding site for miR-29b-3p (Figure 2A). The vector contains putative miR-29b-3p binding regions in the 3’-UTR of CDK6 with 4 mutant nucleotides each (designated as pGL3-CDK6-Mu) was used and wild type CDK6 vector were used as control. The histogram in Figure 2B (right) showed that the enzyme activity was reduced about 36.7% in cells co-transfected with miR-29b-3p mimics and pGL3-CDK6 compared with pGL3-CDK6-Mu (P < 0.05). These data indicate that miR-29b-3p may suppress CDK6 expression through binding to seed sequence at the 3’-UTR of CDK6, and CDK6 may be a direct target of miR-29b-3p.
MiR-29b-3p regulates endogenous CDK6 expression in HEK293T cells
Although CDK6 was identified as a target gene for miR-29b-3p, it was unknown whether miR-29b-3p could regulate endogenous CDK6 expression. HEK293T cells were transfected with miR-29b-3p mimics or inhibitor to see whether the dysregulation of miR-29b-3p expression affected endogenous CDK6 expression. Compared with corresponding control, the level of CDK6 protein was significantly suppressed by miR-29b-3P mimics and up-regulated by miR-29b-3p inhibitor (Figure 2C).
Inhibited miR-29b-3p increased CDK6 expression and TNF induced p65 phosphorylation
CDK6 is a p65 NF-κB kinase, so we used western blot to determine the influence of reduced miR-29b-3p expression on TNF-induced p65 phosphorylation. In HEK293T cells, miR-29b-3p inhibitor significantly up-regulated CDK6 expression and increased cytokine-triggered p65 Ser536 phosphorylation (Figure 3A).
Figure 3.
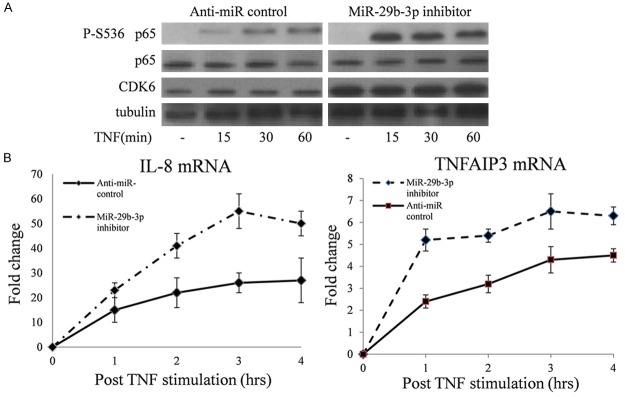
miR-29b-3p inhibition can up-regulate CDK6 expression and enhance NF-κB transcription. A. HEK293T cells were treated for 24 h with miR-29b-3p or adequate scrambled controls followed by treatment with TNFα as indicated. Total cell extracts were analyzed for phosphorylation of p65 and expression of the indicated proteins by immunoblotting. B. HEK293T cells were treated with TNFα after overnight. Expression of IL-8 and TNFAIP3was quantified by using qRT-CR, and samples were normalized to GAPDH levels. Fold change values represent comparison with un-stimulated cells in.
NF-κB target genes expression were up-regulated by miR-29b-3p inhibitor
To test a possible impact of overexpressed CDK6 on NF-kB-triggered gene transcription, HEK293T cells were transiently transfected with miR-29b-3p inhibitor or anti-miRNA control. 24 hours after transfection, cells were treated with TNF (10 ng/mL). The expression of IL-8 and TNFAIP3 were detected using qRT-PCR at five time points after TNF stimulation. As shown in Figure 3B, miR-29b-3p can increase IL-8 and TNFAIP3 expression significantly compared with anti-miRNA control.
Discussion
Primary IgAN has a hereditary propensity whose pathogenic factor is complex and multiple, and the pathological damage of IgAN is diversified. There are reports that microRNA, as an important of gene expression regulator, participated in the pathogenesis of IgAN.
Serino et al. report a comprehensive microarray screening of miRNA in IgAN and reveal a novel pathphysiological mechanism whereby the microRNA miR-418b regulates the levels of miRNA encoding C1β3GALT1 in IgAN patients. The C1β3GALT1 single nucleotide polymorphism (SNP) variant is associated with genetic susceptibility to IgAN and miR-418b binding to the C1β3GALT1 mRNA can be modulated by SNP (rs1047763) 1365G/A [14]. Tan and colleagues did a genome-wide microRNAs expression profiling in IgA nephropathy patients and found a total of 85 miRNAs that were differentially expressed in the six IgAN patients [10]. In this study, we first detect miR-29a, miR-29b and miR-29c expression by qRT-PCR and found that miR-29b-3p is apparently high in renal tissues of IgAN patients. After that, we confirmed that the expression of CDK6 was regulated by miR-29b-3p. MiR-29b-3p expression down-regulation can increase NF-κB activation when stimulated by cytokines the result of which may enhance inflammation. In a word, we can regard miR-29b-3p as potential diagnostic biomarkers and probable factors that are involved in the pathogenesis of IgAN. Further investigation is needed to explore this subject.
Acknowledgements
This study was supported by Construct Program of the Key Discipline of State Administration of Traditional Chinese Medicine of People’s Republic of China, Key Project of Shanghai Committee of Education (14ZZ118) and Key Medical Discipline Project of Shanghai Municipal Health Bureau (ZK2012A34). Budgetary funds of Shanghai University of Traditional Chinese Medicine (2014YSN65).
Disclosure of conflict of interest
None.
References
- 1.D’Amico G. The commonest glomerulonephritis in the world: IgA nephropathy. Q J Med. 1987;64:709–727. [PubMed] [Google Scholar]
- 2.Gharavi AG, Yan Y, Scolari F, Schena FP, Frasca GM, Ghiggeri GM, Cooper K, Amoroso A, Viola BF, Battini G, Caridi G, Canova C, Farhi A, Subramanian V, Nelson-Williams C, Woodford S, Julian BA, Wyatt RJ, Lifton RP. IgA nephropathy, the most common cause of glomerulonephritis, is linked to 6q22-23. Nat Genet. 2000;26:354–357. doi: 10.1038/81677. [DOI] [PubMed] [Google Scholar]
- 3.Zuo L, Wang M. Current burden and probable increasing incidence of ESRD in China. Clin Nephrol. 2010;74(Suppl 1):S20–S22. [PubMed] [Google Scholar]
- 4.D’Amico G. Natural history of idiopathic IgA nephropathy: Role of clinical and histological prognostic factors. Am J Kidney Dis. 2000;36:227–237. doi: 10.1053/ajkd.2000.8966. [DOI] [PubMed] [Google Scholar]
- 5.Maes OC, Chertkow HM, Wang E, Schipper HM. MicroRNA: Implications for Alzheimer Disease and other Human CNS Disorders. Curr Genomics. 2009;10:154–168. doi: 10.2174/138920209788185252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu J, Li Y, Wang F, Wang X, Cheng B, Ye F, Xie X, Zhou C, Lu W. Suppressed miR-424 expression via upregulation of target gene Chk1 contributes to the progression of cervical cancer. Oncogene. 2013;32:976–987. doi: 10.1038/onc.2012.121. [DOI] [PubMed] [Google Scholar]
- 7.Farazi TA, Hoell JI, Morozov P, Tuschl T. MicroRNAs in human cancer. Adv Exp Med Biol. 2013;774:1–20. doi: 10.1007/978-94-007-5590-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai Y, Sui W, Lan H, Yan Q, Huang H, Huang Y. Microarray analysis of micro-ribonucleic acid expression in primary immunoglobulin a nephropathy. Saudi Med J. 2008;29:1388–1393. [PubMed] [Google Scholar]
- 9.Wang G, Kwan BC, Lai FM, Chow KM, Kam-Tao LP, Szeto CC. Expression of microRNAs in the urinary sediment of patients with IgA nephropathy. Dis Markers. 2010;28:79–86. doi: 10.3233/DMA-2010-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan K, Chen J, Li W, Chen Y, Sui W, Zhang Y, Dai Y. Genome-wide analysis of microRNAs expression profiling in patients with primary IgA nephropathy. Genome. 2013;56:161–169. doi: 10.1139/gen-2012-0159. [DOI] [PubMed] [Google Scholar]
- 11.Lee HS, Lee MS, Lee SM, Lee SY, Lee ES, Lee EY, Park SY, Han JS, Kim S, Lee JS. Histological grading of IgA nephropathy predicting renal outcome: Revisiting H. S. Lee’s glomerular grading system. Nephrol Dial Transplant. 2005;20:342–348. doi: 10.1093/ndt/gfh633. [DOI] [PubMed] [Google Scholar]
- 12.Buss H, Handschick K, Jurrmann N, Pekkonen P, Beuerlein K, Muller H, Wait R, Saklatvala J, Ojala PM, Schmitz ML, Naumann M, Kracht M. Cyclin-dependent kinase 6 phosphorylates NF-kappaB P65 at serine 536 and contributes to the regulation of inflammatory gene expression. PLoS One. 2012;7:e51847. doi: 10.1371/journal.pone.0051847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hennessy EJ, Sheedy FJ, Santamaria D, Barbacid M, O’Neill LA. Toll-like receptor-4 (TLR4) down-regulates microRNA-107, increasing macrophage adhesion via cyclin-dependent kinase 6. J Biol Chem. 2011;286:25531–25539. doi: 10.1074/jbc.M111.256206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serino G, Sallustio F, Cox SN, Pesce F, Schena FP. Abnormal miR-148b expression promotes aberrant glycosylation of IgA1 in IgA nephropathy. J Am Soc Nephrol. 2012;23:814–824. doi: 10.1681/ASN.2011060567. [DOI] [PMC free article] [PubMed] [Google Scholar]