Abstract
Immune regulation plays important but as-yet-unclear roles in the development of preeclampsia. This study explored potential contributions to immune regulation by dendritic cells (DCs) derived from peripheral blood of preeclampsia patients on the differentiation of Th1 and Th17 cells. Pregnant women with preeclampsia (n = 73) and healthy pregnant women (n = 80) were included in the study. Peripheral blood samples were collected from each participant, and DCs were derived from peripheral blood mononuclear cells in vitro. The phenotypes of DCs, identified by CD14, CD80, CD83, and CD86 expression, were detected by flow cytometry, and secretion of interleukin-23 (IL-23) into the culture medium by DCs was measured by ELISA. CD4 + T cells were separated by the magnetic beads and subjected to flow cytometry to determine their ability to differentiate to Th1 or Th17 cells. Compared with DCs derived from healthy pregnant women, DCs derived from preeclampsia patients expressed higher levels of CD83, CD80, and CD86 (P < 0.05). Additionally, secretion of IL-23 was higher in DCs derived from the preeclampsia group than from the control group (P < 0.001). DCs derived from preeclampsia patients also had a stronger ability to promote the differentiation of CD4 + T cells into Th1/Th17 cells when cultured with different cytokines (P < 0.01). Thus, altered phenotypes and functions of DCs may promote the abnormal balance of Th1 and Th17 in the development of preeclampsia.
Keywords: Preeclampsia, dendritic cell, Th1 cell, Th17 cell
Introduction
Preeclampsia (PE) is a condition in which women whose blood pressure is normal before pregnancy present with hypertension and proteinuria after 20 weeks of gestation [1]. It is an idiopathic disorder that affects the systemic small arteries. One of the five hypertensive disorders in pregnancy, preeclampsia can be life-threatening for both mothers and fetuses due to risks of cerebral hemorrhage, coma, and placental abruption. Preeclampsia occurs in approximately 3%-5% of pregnancies [2,3]. In developing countries, preeclampsia is contributing factor to preterm labor; although, medically, this is generally beneficial to mothers, it affects the health of babies [3]. Despite its global impacts, the causes of preeclampsia remain unknown, leading to a paucity of effective preventive methods.
In 1976, James proposed the immune maladaptation hypothesis, leading to many studies uncovering the important role of the immune system in the etiology of preeclampsia [4]. With the development of molecular biology, newer hypotheses have emerged to describe its origins: immunological, abnormalities of vascular regulatory substances, placental ischemia, insulin resistance, nutritional deficiency, and heredity. Current concepts in reproductive immunology describe pregnancy as a successful natural semiallograft, depending on the dynamic balance between the maternal immune system and the fetal immune system [5]. Since embryos are developed from fertilized eggs with matrilineal and patrilineal tissue-specific antigens, preeclampsia may be a disease of incompatibility between maternal immunity and fetal immunity. Normal pregnancy is not rejected by the maternal body, which shows that a dynamic balance is maintained between the maternal immune system and the fetal immune system. In other words, there is a completely tolerant immune system; however, once the balance is disrupted, immune rejection can result in adverse events like miscarriage and preeclampsia. Thus, the symptoms of the immune rejection will improve following the discharge of the fetus from the mother. This immunological hypothesis has been bolstered by recent studies uncovering a correlation between preeclampsia and the balance of Th1/Th17 T-cell subsets [6,7]. Th1 and Th17 cells produce cytokines that are correlated with the pathophysiological process of hypertensive disorders in pregnancy [8,9].
The antigen-presenting cells known as dendritic cells (DCs) are present in the blood and exposed to the tissues. Their role is to regulate the innate and acquired immune responses to environmental stimuli. As antigen-presenting cells, DCs can activate initial T cells to become the antigen-presenting cells of effector cells. The expressions of their mature state and surface costimulatory molecules are a key link to producing immune responses in the body [10,11]. Initial CD4+ T lymphocytes are activated by antigen-presenting cells to differentiate into four cell subgroups-Treg, Th1, Th2, and Th17 cells-with different biological functions [12]. These cells can be transformed into other types under certain conditions, thereby keeping the immune regulation in a dynamic balance [13]. Only DCs can activate initial T lymphocytes [14]. Although DCs play a key role in regulating the immune system, they can induce both immune tolerance and immune rejection. DCs are present during both pregnancy and decidualization, which indicates that DCs may play an important role in immune regulation at the maternal-fetal interface [15,16].
This study sought to uncover a contribution of DCs in the pathogenesis of preeclampsia. DCs differentiated from mononuclear cells in the peripheral blood were co-cultured with CD4+ T lymphocytes from preeclampsia patients and healthy pregnant women. The influence of DCs on the production of cytokines by CD4+ T lymphocytes was measured.
Participants and methods
Participants
This prospective study recruited 73 preeclampsia patients from the Department of Obstetrics of Nantong Women and Children Health Care Hospital (Nantong, Jiangsu Province, China), who received treatment between June 2013 and December 2013. Their mean age was 27.11 ± 2.49 years and mean gestational age was 33.95 ± 1.94 weeks. As a control group, 80 healthy pregnant women were selected from the department during the same period. Their mean age was 27.98 ± 3.74 years and mean gestational age was 34.15 ± 1.48 weeks. The diagnostic criteria for preeclampsia were used according to the definition published in 2006 by the Royal College of Obstetricians and Gynecologists [17]. The differences in mean age and gestational age between groups were not statistically significant (P > 0.05), and both groups were free of complications in internal medicine, history of autoimmune diseases, and other obstetric complications. This study was approved by the Hospital Ethics Committee, and informed consent was obtained from the patients.
Reagents and apparatus
rhGM-CSF was purchased from United Pharmaceutical Co (Beijing Medical University, China). LPS, rhIL-4, and RPMI1640 culture medium were purchased from GIBCO (under Applied Biosystems; Grand Island, New York, USA). FITC-labeled anti-human CD80 and CD86 antibodies, PE-labeled anti-human IFN-γ antibody, and Percp-labeled anti-human CD8 antibody were purchased from eBioscience (San Diego, California, USA). FITC-labeled anti-human CD14, CD83, and IL-17 antibodies were purchased from Caltag Laboratories (California, USA). Recombinant human interleukin (IL)-2 was purchased from Kingsley Pharmaceutical (Jiangsu, China). The IL-23 assay kit was purchased from R&D Systems (Minneapolis, Minnesota, USA). FACSCalibur flow cytometer and immunomagnetic beads coupled with an anti-human CD4 were from BD (New Jersey, USA).
Culture of dendritic cells
Peripheral blood (10 mL) was collected from each participant into heparin-coated tubes. Peripheral blood mononuclear cells (PBMCs) were separated by the density-gradient centrifugation method, washed 3 times using Complete RPMI 1640 medium, and placed in a 6-well plate at a density of 5 × 109 cells /L. Cells were cultured at 37°C in 50 mL/L CO2 for 2 hours. Suspended cells were removed, and adherent cells were obtained after the wells were rinsed with RPMI 1640. Medium was added to adherent cells, which were then supplemented with cytokines (100 g/L rhIL-4 and 100 g/L rhGM-CSF) to induce DC differentiation. On the 3rd day of culture, cytokines were added again after half of the medium was renewed. On the 6th day, half of the medium was renewed, and 1 mg/L LPS was added. On the 8th day, suspended cells were collected.
Purified CD4+ T cells
PBMCs were separated by Ficoll gradient centrifugation to produce a cell suspension, and cells were counted. Fifty μL of immunomagnetic beads coupled with an anti-human CD4 antibody were added with mixing. The mixture was incubated at a room temperature for 30 min, then resuspended to 1 mL using BD IMag buffer. The new mixture was incubated at room temperature for 10 min, then the supernatant was removed, and the pellet was resuspended to a final volume of 1 mL using BD IMag buffer. Following a 4-min incubation at room temperature, the supernatant was removed to obtain purified CD4+ T cells.
Cell expansion induced in vitro
The density of purified CD4+ T cells was adjusted to 1 × 109 cells/L. Then, 1 mL/well culture solution containing the adjusted cells were added to a 24-well plate for culture in 4 ways as follows: 1) with 500,000 U/L IL-2 and P-DC (1:100 for CD4+ T); 2) with 500,000 U/L IL-2 and N-DC (1:100 for CD4+ T); 3) with 15 μg/L IL-1β, 20 μg/L IL-6, and P-DC (1:100 for CD4+ T); 4) with 15 μg/L IL-1β as well as 20 μg/L IL-6 and N-DC (1:100 for CD4+ T). On the 3rd day of culture, all cell solutions were supplemented with 500,000 U/L IL-2. After the cells were expanded, on the 2nd day, half of each medium was renewed, and 500,000 U/L IL-2 were again added to maintain cell growth. On the 6th day, expanded cells were collected.
Detection of IL-23 with ELISA method
The supernatant of DCs that were cultured to day 8 was obtained, centrifuged at 2000-3000 x g for 20 min, and detected according to the kit instructions.
Detection of dendritic cell phenotypes
RMPI was used to adjust the density of monocyte-derived DCs to 1 × 109 cells/L. FITC-labelled anti-human CD14, CD83, CD80, and CD86 antibodies as well as FITC-labeled IgG (as an isotype control) were added to each tube. Cells were incubated away from light for 30 min and washed with PBS 3 times. Cells were fixed with 300 μL of 20 g/L paraformaldehyde, and flow cytometry was used to detect the mature phenotypes of DCs. Cellquest Software (BD Biosceinces, Shanghai, China) was used to analyze the data.
Detection of intracellular cytokines
PBMCs were cultured in a 96-well plate (200 μL and 2 × 106 cells/mL per well) and treated with ionomycin and phorbol 12-myristate 13-acetate (PMA). Next, cells were incubated at 37°C in 50 mL/L CO2 for 3 hours, then treated with monensin (eBioscience), and cultured again for 2 hours. The supernatant was removed by centrifugation, and 50 μL of the remaining liquid was aliquoted into four test tubes. CD8-Percp was added to each tube and cells were incubated away from light at 4°C for 30 minutes. Each sample was washed 2 times using 1 mL of staining buffer; the cells in each tube were then fixed in 500 μL of 40 g/L paraformaldehyde at 4°C for 30 minutes. After washing 2 times using 1 mL of staining buffer, each tube received 500 μL of 1 g/L saponin-PBS, and after cell membrane permeation at 4°C for 15 minutes, the supernatant was removed by centrifugation. Cells were sealed with 20 μL of 100 mL/L bovine serum albumin (Sigma); IFN-γ-PE and IL-17-FITC were added to the tubes to perform intracellular cytokine staining. Meanwhile, isotype control tubes were prepared. After washing with PBS, samples were fixed with 300 μL of 40 g/L paraformaldehyde, and detected with flow cytometry. Cellquest Software was used to analyze the data. Region 1 (R1) for lymphocytes was set in the bitmap of side scattered light (SSC) versus forward light scatter (FSC). Gate 1(G1) = R1 was set to select CD4 + T cell zone, thereby analyzing the percentages of IL-17 and IFN-γ + in CD4 + T cells.
Statistical analysis
SAS9.2 was used to analyze data by Student’s t-test. P < 0.05 was considered to indicate a statistically significant difference.
Results
Phenotypes of dendritic cells derived from preeclampsia patients and healthy pregnant women
To identify potential differences in dendritic cells, PBMCs from healthy pregnant women and women with preeclampsia were induced to form DC by stimulation with LPS, GM-CSF, and IL-4. Flow cytometry detected cells from both groups that weakly expressed CD14. Cells with strong expression of CD83, CD86, and CD80 marked mature DCs. DCs derived from preeclampsia patients had significantly higher expression of CD83, CD80, and CD86 than DCs derived from healthy pregnant women (each P value < 0.05; Figure 1).
Figure 1.
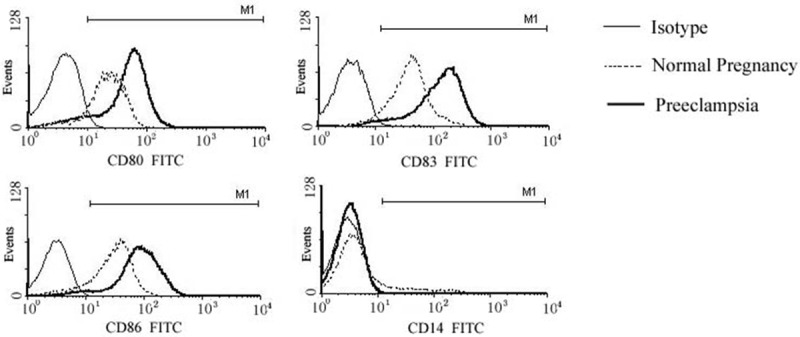
Monocyte-derived dendritic cell cytokine expression in healthy pregnant women (n = 80) and pregnant women with preeclampsia (n = 73) (x̅ ± s). Note: #P < 0.05 vs healthy pregnancy.
Secretion of IL-23 by derived DCs
Activated DCs and macrophages can produce the cytokine IL-23 [18]. IL-23 acts on CD4 + T lymphocytes to promote differentiation of Th17 cells, which produce IL-17 and induce the production of IFN-γ and the differentiation of Th1 cells [19]. Thus, secretion of IL-23 by cultured DCs was measured in the culture medium. The concentration of IL-23 in the medium of DCs derived from preeclampsia patients was significantly higher than the concentration secreted by DCs derived from healthy pregnant women (P < 0.001; Table 1).
Table 1.
Concentration of IL-23 secreted by dendritic cells derived from healthy pregnant women and women with preeclampsia (x̅ ± s)
| Group | IL-23 (ng/L) | t | P |
|---|---|---|---|
| Healthy pregnancy (n = 80) | 96.13 ± 33.21 | -7.57 | < 0.001 |
| Preeclampsia (n = 73) | 134.18 ± 28.53 |
Detection of the percentages of CD4+IL-17+Th17 and CD4+IFN-γ+Th1 cells by flow cytometry
To determine whether the differences in DC marker expression and secretion of IL-23 could alter the profile of Th17/Th1 cells in preeclampsia, CD4 + T cells were separated by immunomagnetic beads. Before separation, the purity of CD4 + T cells was 48.05 ± 11.31%; after separation, the purity improved significantly, to 96.07 ± 3.69% (P < 0.05).
Flow cytometry was used to identify IL-17 + (Th17) and IFN-γ + (Th1) subsets of CD4 + T cells (Figure 2). Four different culture supplements were tested (Figure 3). CD4 + cells that secreted IFN-γ+ were treated with IL-2 and N-DC; their purity reached 19.98 ± 4.51%. Following supplementation with IL-2 and P-DC, their purity reached 34.04 ± 3.97%; this difference was statistically significant (P < 0.01). In comparison, addition of IL-6, P-DC, and IL-1β resulted in a purity of 11.19 ± 2.70%, while addition of IL-6, N-DC, and IL-1β resulted in a purity of 10.99 ± 2.79%. However, for CD4 + cells that secreted IL-17, supplementation with IL-6, P-DC, and IL-1β or IL-6, N-DC, and IL-1β resulted in purities of 12.01 ± 2.69% and 26.03 ± 6.51%, respectively; this difference was statistically significant (P < 0.01). In contrast, supplementation with L-2 and N-DC resulted in a purity of 1.99 ± 0.78%, and supplementation with IL-2 and P-DC resulted in a purity of 3.01 ± 0.98%.
Figure 2.
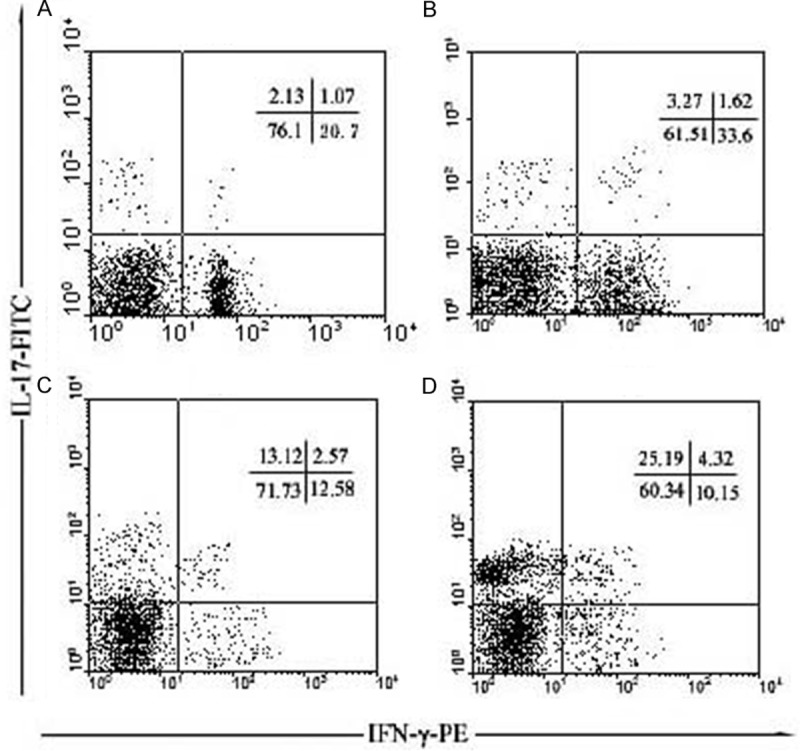
Typical results for CD4+IFN-γ+Th1 and CD4+IL-17+Th17 cell subsets detected by flow cytometry. Note: A: Purified CD4+ T + N-DC + IL-2, B: Purified CD4+ T + P-DC + IL-2, C: Purified CD4+ T + N-DC + IL-1β + IL-6, D: Purified CD4+ T + P-DC + IL-1β + IL-6.
Figure 3.

The proportion of CD4+IFN-γ+Th1, CD4+IL-17+Th17 cell subsets by four different culture methods. Note: A: Purified CD4+ T + N-DC + IL-2, B: Purified CD4+ T + P-DC + IL-2, C: Purified CD4+ T + N-DC + IL-1β + IL-6, D: Purified CD4+ T + P-DC + IL-1β + IL-6. Note: #P < 0.01, B vs A; ##P < 0.01, D vs C.
Discussion
Pregnancy is a successful natural semiallograft, and healthy pregnancy depends on the dynamic balance between the maternal immune system and the fetal immune system. In particular, the dynamic balance between various subtypes of CD4+ T lymphocytes and their cytokines plays an important role in healthy pregnancy.
In this study, we induced differentiation of mononuclear cells from peripheral blood of women with preeclampsia and healthy pregnant women to study DCs. We found that the phenotypes of DCs derived from preeclampsia patients differed from those derived from healthy pregnant women. The expression of CD80, CD83, and CD86 costimulatory molecules on the cell surface of DCs from preeclampsia patients were higher than those from healthy pregnant women. The actions of various cytokines stimulated the reproductive activity of allogeneic lymphocytes, indicating that the DCs derived from healthy pregnant women were kept at an immunosuppressive state, while those of preeclampsia patients were kept at an active state of inflammation. In preeclampsia patients, the DC-produced IL-12 could be embryotoxic, and also can induce T lymphocytes to differentiate into Th1 cells. IL-12 has been demonstrated to be toxic to trophocytes [20].
The concentration of IL-23 secreted by derived DCs into the culture medium was higher in the preeclampsia group than in the control group. Further, the DC-stimulated reproductive activity of allogeneic lymphocytes was assessed. When DCs, IL-1β, and IL-6 jointly acted on CD4 + T lymphocytes, more IL-17 + cells were produced, which shows that IL-23 alone cannot induce T cells to differentiate into Th17 cells, consistent with the findings of Duhen et al. [21]. In this study, under the same culture conditions, the capacity of DCs derived from preeclampsia patients to produce inflammatory cytokines was higher than that of DCs derived from healthy pregnant women. Furthermore, the DCs in the preeclampsia patients were differentiated into Th1 and Th17 cells. CD4 + T lymphocytes are closely related with pregnancy outcomes [22,23]. In patients with preeclampsia, the dynamic balance of T-cell subgroups is disturbed, and there is an increase in the Th1- and Th17-derived inflammatory cytokines. Thus, this process can promote pregnancy pathology with the potential for miscarriage. In healthy pregnant women, the T-cell subgroups are maintained at a state of balance. Therefore, the key to preventing or treating preeclampsia may be in understanding the biological changes of DCs in preeclampsia and use the characteristics of DCs to maintain the immune balance in pregnant women.
In summary, the pathogenesis of preeclampsia is complex. Immune regulation functions throughout pregnancy, and the manifestations of Th1/Th17 cells may be only a part of immune imbalance. Further investigation into the changes and roles of Th1/Th17 cells in preeclampsia is needed to open new prospects for the prevention, diagnosis, and treatment of preeclampsia.
Acknowledgements
This work was supported by Jiangsu Provincial Health Department (Grant No. F 201213).
Disclosure of conflict of interest
None.
References
- 1.Powe CE, Levine RJ, Karumanchi SA. Preeclampsia, a disease of the maternal endothelium the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation. 2011;123:2856–2869. doi: 10.1161/CIRCULATIONAHA.109.853127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berg CJ, Mackay AP, Qin C, Callaghan WM. Overview of maternal morbidity during hospitalization for labor and delivery in the United States: 1993-1997 and 2001-2005. Obstet Gynecol. 2009;113:1075–1081. doi: 10.1097/AOG.0b013e3181a09fc0. [DOI] [PubMed] [Google Scholar]
- 3.MacKay AP, Berg CJ, Atrash HK. Pregnancy-related mortality from preeclampsia and eclampsia. Obstet Gynecol. 2001;97:533–538. doi: 10.1016/s0029-7844(00)01223-0. [DOI] [PubMed] [Google Scholar]
- 4.Dekker GA, Robillard PY, Hulsey TC. Immune maladaptation in the etiology of preeclampsia: a review of corroborative epidemiologic studies. Obstet Gynecol Surv. 1998;53:377–82. doi: 10.1097/00006254-199806000-00023. [DOI] [PubMed] [Google Scholar]
- 5.Sakai M, Tsuda H, Tanebe K, Sasaki Y, Saito S. Interleukin-12 secretion by peripheral blood mononuclear cells is decreased in normal pregnant subjects and increased in preeclamptic patients. Am J Repro Immunol. 2002;47:91–97. doi: 10.1034/j.1600-0897.2002.1o020.x. [DOI] [PubMed] [Google Scholar]
- 6.Flier J, Boorsma DM, van Beek PJ, Nieboer C, Stoof TJ, Willemze R, Tensen CP. Differential expression of CXCR3 targeting chemokines CXCL10, CXCL9, and CXCL11 in different types of skin inflammation. J Pathol. 2001;194:398–405. doi: 10.1002/1096-9896(200108)194:4<397::aid-path899>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 7.Patel DD, Zachariah JP, Whichard LP. CXCR3 and CCR5 ligands in rheumatoid arthritis synovium. Clinical Immunology. 2001;98:39–45. doi: 10.1006/clim.2000.4957. [DOI] [PubMed] [Google Scholar]
- 8.Saito S, Sakai M. Th1/Th2 balance in preeclampsia. J Repro Immunol. 2003;59:161–173. doi: 10.1016/s0165-0378(03)00045-7. [DOI] [PubMed] [Google Scholar]
- 9.Szarka A, Rigó J, Lázár L, Beko G, Molvarec A. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunol. 2010;11:59. doi: 10.1186/1471-2172-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li X, Yang A, Huang H, Zhang X, Town J, Davis B, Cockcroft DW, Gordon JR. Induction of type 2 T helper cell allergen tolerance by IL-10 -differentiated regulatory dendritic cells. Am J Respir Cell Mol Biol. 2010;42:190–199. doi: 10.1165/rcmb.2009-0023OC. [DOI] [PubMed] [Google Scholar]
- 11.Panda B, Panda A, Ueda I, Abrahams VM, Norwitz ER, Stanic AK, Young BC, Ecker JL, Altfeld M, Shaw AC, Rueda BR. Dendritic cells in the circulation of women with preeclampsia demonstrate a pro-inflammatory bias secondary to dysregulation of TLR receptors. J Reprod Immunol. 2012;94:210–215. doi: 10.1016/j.jri.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Sallusto F, Lanzavecchia A. Heterogeneity of CD4+ memory T cells: functional modules for tailored immunity. Eur J Immunol. 2009;39:2076–2082. doi: 10.1002/eji.200939722. [DOI] [PubMed] [Google Scholar]
- 13.Lee YK, Mukasa R, Hatton RD, Weaver CT. Developmental plasticity of Th17 and Treg cells. Curr Opin Immunol. 2009;21:274–280. doi: 10.1016/j.coi.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 14.Joffre O, Nolte MA, Spörri R. Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol Rev. 2009;227:234–247. doi: 10.1111/j.1600-065X.2008.00718.x. [DOI] [PubMed] [Google Scholar]
- 15.Blois SM, Alba Soto CD, Tometten M, Klapp BF, Margni RA, Arck PC. Lineage, maturity, and phenotype of uterine murine dendritic cells throughout gestation indicate a protective role in maintaining pregnancy. Biol Reprod. 2004;70:1018–1023. doi: 10.1095/biolreprod.103.022640. [DOI] [PubMed] [Google Scholar]
- 16.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tierney JP, Welsh J, Owen P Effective Gynaecology in Glasgow Group. Management of early pregnancy loss--a complete audit cycle. J Obstet Gynaecol. 2006;26:229–232. doi: 10.1080/01443610500537898. [DOI] [PubMed] [Google Scholar]
- 18.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from γδ T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nature Rev Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 20.Saito S, Nakashima A, Shima T, Ito M. Th1/Th2/Th17 and Regulatory T-Cell Paradigm in Pregnancy. Am J Repro Immunol. 2010;63:601–610. doi: 10.1111/j.1600-0897.2010.00852.x. [DOI] [PubMed] [Google Scholar]
- 21.Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nature Immunol. 2009;10:857–863. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- 22.Toldi G, Molvarec A, Stenczer B, Müller V, Eszes N, Bohács A, Bikov A, Rigó J Jr, Vásárhelyi B, Losonczy G, Tamási L. Peripheral T(h)1/T(h)2/T(h)17/regulatory T-cell balance in asthmatic pregnancy. Int Immunol. 2011;23:669–677. doi: 10.1093/intimm/dxr074. [DOI] [PubMed] [Google Scholar]
- 23.Lissauer D, Goodyear O, Khanum R, Moss PA, Kilby MD. Profile of maternal CD4 T-cell effector function during normal pregnancy and in women with a history of recurrent miscarriage. Clin Science (Lod) 2014;126:347–354. doi: 10.1042/CS20130247. [DOI] [PubMed] [Google Scholar]


