Abstract
Objective: This study aims to investigate the effect of captopril and losartan on the electrophysiology of myocardial cells parameters in ventricular vulnerable period and effective refractory period of myocardial ischemia rats. Methods: 96 wistar rats were enrolled in the study and divided into six groups: Captopril myocardial ischemia group, losartan myocardial ischemia group, myocardial ischemia control group, captopril normal group, losartan normal group and normal control group (n=16). We observed morphological changes of myocardial tissue in each group. The cardiac electrophysiological parameters in effective refractory period of each group were measured. Creatine kinase (CK), alanine aminotransferase (GOT), lactate dehydrogenase (LDH), the expression of Cardiotrophin 1 (CT-1) and malonaldehyde (MDA) were detected. Results: Compared the losartan and captopril group with the control group, (P<0.05). Losartan and captopril can shorten the ventricular vulnerable period of the normal group and ischemic group. There was no interaction effect between losartan and captopril group and the acute myocardial ischemia group. The effect of losartan and captopril on time window in ventricular vulnerable period showed that compared with the control group (P<0.05). Losartan and captopril had a significant effect on prolonged effective refractory period of normal and ischemic rats. There was no interaction effect between losartan and captopril group and the acute myocardial ischemia group. Compared with the myocardial ischemia control group, CK, GOT, LDH and MDA decreased in captopril and losartan myocardial ischemia groups (P<0.05). Conclusion: Losartan and captopril had a significant effect on prolonged effective refractory period and shorten ventricular vulnerable period, they can also effectively prevent arrhythmias.
Keywords: Losartan, captopril, myocardial ischemia, rats
Introduction
Angiotensin-converting enzyme inhibitor (ACEI) captopril is a conventional anti-hypertension and anti-congestive heart failure drug. In addition, data showed captopril can not only effectively protect the heart through own function, but also effectively improve the quality of life and increases survival rate in patients, even effectively through structural reconstruction of the left ventricle in the patient to relieve myocardial fibrosis and improve ventricular hypertrophy and ultimately improve the survival rate in patients with heart failure [1-4]. And it can reduce myocardial ischemia-reperfusion damage, improve ischemic cardiomyopathy fibrinolytic system, diastolic blood vessels and anti-atherosclerosis formation. Angiotensin II receptor antagonists (angiotensin receptor blockade, ARB) losartan is an important inhibition drugs for the renin-angiotensin system (RAS). It can decrease blood pressure and protect organs, which is widely used in the treatment of cardiovascular diseases. Losartan can block the combination of angiotensin II (Ang II) and AT1, which can help blood vessels to dilate, relax vascular smooth muscles, increase renal sodium and water excretion, so as to protect the target organ and antihypertensive [5-7].
Currently captopril and losartan were mainly used as antihypertensive and anti-heart-failure drugs. However, reports on the correlation between these two drugs and arrhythmia are not many. With the development of science and deep research, there is a direct or indirect interactions between captopril and losartan and arrhythmias. Related studies showed ACEI can effectively act on the ion channel of cardiomyocyte, but the report on the interaction between it and myocardial electrophysiological are not many. The purpose of this study is to analyze the effects of captopril and losartan on myocardial ischemia electrophysiological parameters, biochemical parameters and the expression of related genes, which providing experimental and clinical basis for the effective prevention of cardiac arrhythmias.
Material and methods
Experimental animals
A total of 96 specific pathogen-free male wistar rats weighing 230-250 were obtained from the animal experimental center of Peking Union Medical College. These rats were kept in a clean and quiet environment with a room temperature and relative humidity at 40-50%. They had free access to food and drinking water and allowed to acclimate to the environment prior to experimental initiation.
Rats were divided into six groups: normal control group (A), captopril normal group (B), losartan normal group (C), myocardial ischemia control group (D), captopril myocardial ischemia group (E) and losartan myocardial ischemia group (F) (n=16).
Housing and procedures involving experimental animals were in accordance with the Guide for the Care and Use of Laboratory Animals. All experimental procedures were approved by the Care of Experimental Animals Committee of our hospital.
Acute myocardial ischemia modeling
The rats were anesthetized with an intraperitoneal injection of urethane (4 ml/kg, 25%), isolated in each layer after anesthesia to exposure organs. Tube was leading into the cartilage at 4-5 tracheal rings. Thoracic contour obvious fluctuate with cartilage frequency is the successful intubation. Ventilator respiratory rate is 60 beats/min, respiration ratio is 2:1, tidal volume 3 ml/mg. A longitudinal incision was made on the left side of the sternum, the incision should be parallel to the sternum. The muscle of rats was isolated layer by layer, the corresponding ribs were fixed and cut. Outer membrane of left ventricle and heart were gradually exposed. Found the left coronary artery, ligated the left anterior descending coronary artery with No. 7/0 thread. The infarcted area was pale intraoperative, recorded ECG before and after surgery. ST elevated and fused with T-wave into single-phase curve represents the successful ligation.
The treatment of animal models
Captopril group: After anesthesia, captopril 4 mg/h/mg and isoproterenol 0.32 mg/h/mg were administrated by intravenous infusion via micro pump, began stimulation after 30 min of the treatment. losartan group: After anesthesia, losartan 4 mg/h/mg and isoproterenol 0.32 mg/h/mg were administrated by intravenous infusion via micro pump ,began stimulation after 30 min of the treatment .
Electrical stimulation treatment
After the rats were anesthetized, the left thoracotomy was performed. Outer membrane of left ventricle and heart were gradually exposed. One end of the bipolar electrodes which used as the pacing and stimulation electrodes was fixed to the left ventricle apex. Another end of the electrode was fixed to the stimulator. The stimulation started after 5 ml of ischemia.
The detection of physiological indicators
Time window determination in ventricular vulnerable period of fixation strength
S1-S2 programmed electrical stimulation, stimulus intensities were S1: 2V and S2: 8V, pulse width were 2 ms, every time triggered eight S1 and one S2, S1-S1 interval time was 150 ms. Scanning order is from end-diastolic in reverse. S1-S2 decremented 2 ms each time. When S2 cause ventricular tachycardia and ventricular fibrillation, S2 is the outer margin of time-window, while when S2 is at cardiac cycle, S2 is the inner margin of time-window. The difference of the inner margin and outer margin of S1-S2 is the time-window of ventricular vulnerable period.
The effective refractory period measurement
S1-S2 programmed electrical stimulation, the effective refractory period is defined as the longest S1-S2 interval QRS wave which S2 can not induce. In which S2 stimulus intensity was 2 times of the diastolic threshold, the pulse width was 2 ms. Scanning order is from end-diastolic in reverse.
Observation of the myocardial tissue morphology
The 1 mm×1 mm×1 mm of myocardial tissues in left apex ischemia region were taken and fixed with 4% formalin, then they were stained with routine HE staining and observed under light microscope.
Determination of serum myocardial enzyme and MDA
Serum myocardial enzyme CK, GOT and LDH were determined with automatic biochemical analyzer. MDA was determined with Thio barbituric acid (TBA) method.
Detection of the expression level of CT-1 in the myocardium
The total RNA of transplanted tumor specimens was extracted with an RNA Isolation Kit (Promega (Beijing) Biotech Co., Ltd., Beijing, China) according to the manual. The process of reverse transcription was conducted using PrimeScript 1st Strand cDNA Synthesis Kit (TaKaRa Biotech (Dalian) Co., Ltd., Dalian, China). The primers for PCR were as follows: CT-1 F: 5’-GCGTCTTCTCAGCTAAGG-3’, CT-1 R: 5’-GAACACACATGGATGACATAG-3’; β-actin F: 5’-TCAGGTCATCACTATCGGCAAT-3’, R: 5’-AAAGAAAGGGTGTAAAACGCA-3’.
Statistical analysis
All data was analyzed with SPSS 17.0 software. Data were expressed as mean SD values. Mean comparison were compared with t test. P<0.05 has statistical significance.
Results
The effect of captopril on ventricular vulnerable period time window
Compared the captopril group with the control group, the effect of captopril on ventricular vulnerable period time window result showed P<0.05. Captopril can shorten the ventricular vulnerable period of the normal group and ischemic group. There was no interaction effect between captopril group and the acute myocardial ischemia group (F=0.521, P=0.502). The effect of captopril on time window in ventricular vulnerable period showed that captopril had a significant effect on prolonged effective refractory period of rats in both groups, and there was no interaction effect between captopril group and the acute myocardial ischemia group (F=2.731. P=0.124). Compared the losartan group with the control group, the effect of losartan on ventricular vulnerable period time window result showed P<0.05. Losartan can shorten the ventricular vulnerable period of the normal group and ischemic group. There was no interaction effect between losartan group and the acute myocardial ischemia group (F=0.864, P=0.356). The effect of captopril on time window in ventricular vulnerable period showed that losartan had a significant effect on prolonged effective refractory period of rats in both groups,and there was no interaction effect between losartan group and the acute myocardial ischemia group (F=1.526, P=0.178) (Table 1).
Table 1.
The comparison of the effect of captopril and losartan on ventricular vulnerable period time window and effective refractory period of ischemic rats and normal rats (n=16)
| Group | item | A | B | D | E |
|---|---|---|---|---|---|
| Captopril | time window (ms) | 24.03±8.96 | 7.14±4.54 | 35.41±7.89 | 20.63±6.15 |
| effective refractory period (ms) | 39.24±11.35 | 34.63±8.21 | 59.32±8.42 | 45.26±6.74 | |
| Losartan | time window (ms) | 24.03±8.96 | 35.41±7.89 | 11.25±5.67 | 22.75±6.63 |
| effective refractory period (ms) | 39.24±11.35 | 34.63±8.21 | 51.35±12.53 | 44.32±6.84 |
A: Normal control group, B: Captopril normal group, C: Losartan normal group, D: Myocardial ischemia control group, E: Captopril myocardial ischemia group, F: Losartan myocardial ischemia group.
Pathological changes of myocardial tissue
No abnormal myocardial tissues were observed in the normal control group. There was various degree of necrosis with inflammatory cell infiltration in ischemia group. The area of myocardial necrosis reduced and myocardial fibers arranged neatly in group E and F when compared with group D (Figure 1).
Figure 1.
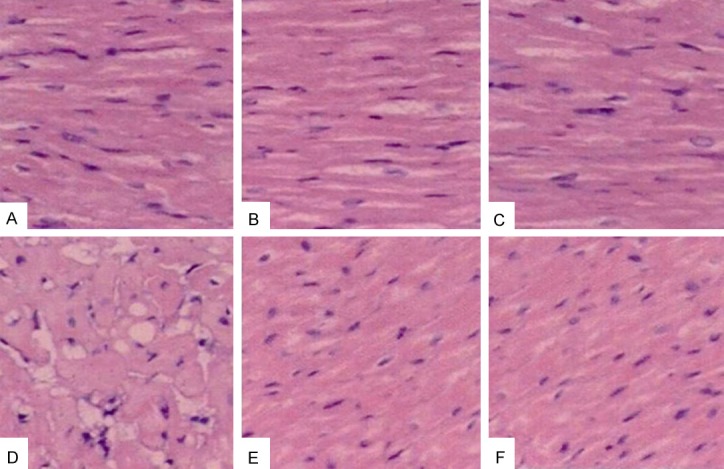
HE staining of myocardial tissue in different groups. A: Normal control group, B: Captopril normal group, C: Losartan normal group, D: Myocardial ischemia control group, E: Captopril myocardial ischemia group, F: Losartan myocardial ischemia group.
Determination of serum myocardial enzyme
The results were shown in Table 2. Compared with normal control group, the levels of serum CK, GOT and LDH increased in ischemia group (P<0.05), while they decreased in group E and F when compared with group D (P<0.05).
Table 2.
The comparison of serum myocardial enzyme in different group (n=16)
| group | CK (U/L) | GOT (U/L) | LDH (U/L) |
|---|---|---|---|
| A | 435±38 | 86±9 | 208±42 |
| B | 419±56 | 90±3 | 180±45 |
| C | 421±28 | 78±10 | 179±58 |
| D | 1583±1251 | 155±331 | 520±221 |
| E | 840±781,2 | 110±251,2 | 405±221,2 |
| F | 902±371,2 | 108±191,2 | 375±241,2 |
Compared with control group, P<0.05;
Compared with ischemic group, P<0.05.
A: Normal control group, B: Captopril normal group, C: Losartan normal group, D: Myocardial ischemia control group, E: captopril myocardial ischemia group, F: Losartan myocardial ischemia group.
The expression level of CT-1 in the myocardium
Compared with normal control group, the expression levels of CT-1 increased in ischemia groups, it also increased in group E and F when compared with group D. The results of RT-PCR were shown in Figure 2.
Figure 2.
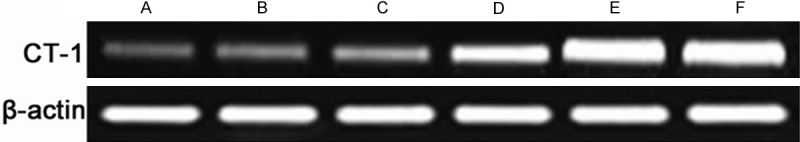
RT-PCR results of CT-1 expression levels in myocardial tissue in different groups. A: Normal control group, B: Captopril normal group, C: Losartan normal group, D: Myocardial ischemia control group, E: Captopril myocardial ischemia group, F: Losartan myocardial ischemia group.
Determination of MDA
As shown in Figure 3, the concentration of MDA in ischemia groups were higher than that of control group (P<0.05), while the concentration of MDA in group E and F were lower than that of group D (P<0.05).
Figure 3.
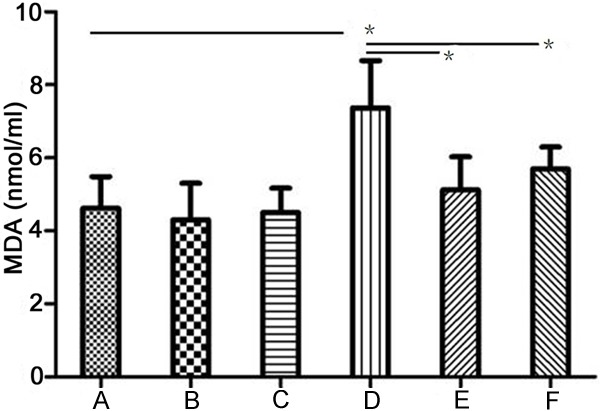
MDA concentration in different groups. A: Normal control group, B: Captopril normal group, C: Losartan normal group, D: Myocardial ischemia control group, E: Captopril myocardial ischemia group, F: Losartan myocardial ischemia group. *P<0.05.
Discussion
Captopril and losartan were mainly used as antihypertensive and anti-heart-failure drugs, reports on the correlation between these two drugs and arrhythmia are not many [8]. With the development of science and deep research, there is a direct or indirect interactions between captopril and losartan and arrhythmias. Related studies showed ACEI can effectively act on the ion channel of cardiomyocyte, but the report on the interaction between it and myocardial electrophysiological are not many. The main effect of ACEI is to reducing angiotensin II (Ang II) content by effective suppression of the renin-angiotensin system (RAS) and angiotensin-converting enzyme activity. Data in this study showed that captopril can effectively prolong the effective refractory period and shorten the vulnerable period in rats, so as to effectively prevent the occurrence of arrhythmias. The reason is that captopril can reduce L-type calcium channel current, improve cardiac arrhythmia which caused by the Ca ion overload [9,10].
It can effectively improve the density of sodium current. Data showed individual guinea captopril irrigation cardiomyocytes can significantly reduce the current density of Na, and the inhibition of captopril on Na ions is closely related with voltages and time [10]. Na current plays a crucial role in depolarizing current. So captopril can effectively reduce the excitability of myocardial cells and prolong effective refractory period of the ventricle. In addition, studies also show that captopril can change the fast sodium inward current and slow inward calcium current, so as to improve the effect of the inward current within the myocardium. Captopril can also control the proliferation of myocardial cells and vascular smooth muscle cell, which play a protective role in myocardial cells and smooth muscle cells, and thus to improve cardiovascular function in rats and effectively remodel myocardial tissue [11].
Angiotensin system is an enzyme-linked reaction which can transform the angiotensinogen (AGT) into angiotensin. Angiotensin exists in various systems of the body, especially in the cardiovascular system. Losartan is the AT1 subtype receptor l blocker of angiotensin II. Angioten\sin II receptor antagonist losartan can relax vascular smooth muscle and dilate blood vessels [12-15]. This study showed that losartan can significantly shorten the ventricular vulnerable period and extend the effective refractory period in rats, effectively preventing arrhythmias. The possible reason is that it can suppress the generation of Ang II voltage and enhance L2 calcium current density. The key component of the cardiac action potential is the L-type calcium currents [16]. Losartan can extend EPR by inhibiting L-type calcium channel current abnormal increase which induced by Ang II. Ang II itself can also promote inward rectifier potassium channel current and result in a reduction time of action potential, so as to shorten EPR. It can reduce the ATP activity to inhibit Na-K pump, which lead to the overload of intracellular calcium and result in a further decrease of myocardial blood flow, eventually leading to myocardial ischemia [17]. These factors ultimately result in the increased incidence of arrhythmias, while losartan can effectively prevent arrhythmia through the inhibition of Ang II.
CT-1 could promote the survival and growth of immature myocardial cells, it has myocardial protective effect. In this study we found that captopril and losartan could promote the expression of CT-1, to some extent it could reduce myocardial injury. MDA increased after ischemia while captopril and losartan could decreased it.
In summary, the results of this study showed that losartan and captopril have a significantly prevention and treatment effect on arrhythmias.
Acknowledgements
This project was supported by the Capital Medical Development Foundation (ZD201125).
Disclosure of conflict of interest
None.
References
- 1.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau J, Shi VC, Solomon SD, Swedberg K, Zile MR PARADIGM-HF Committees and Investigators. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF) Eur J Heart Fail. 2013;15:1062–73. doi: 10.1093/eurjhf/hft052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qian Q, Manning DM, Ou N, Klarich MJ, Leutink DJ, Loth AR, Lopez-Jimenez F. ACEi/ARB for systolic heart failure: closing the quality gap with a sustainable intervention at an academic medical center. J Hosp Med. 2011;6:156–160. doi: 10.1002/jhm.803. [DOI] [PubMed] [Google Scholar]
- 3.Warden BA, Freels JP, Furuno JP, Mackay J. Pharmacy-managed program for providing education and discharge instructions for patients with heart failure. Am J Health Syst Pharm. 2014;71:134–139. doi: 10.2146/ajhp130103. [DOI] [PubMed] [Google Scholar]
- 4.Banka G, Heidenreich PA, Fonarow GC. Incremental cost-effectiveness of guideline-directed medical therapies for heart failure. Am Coll Cardiol. 2013;61:1440–1446. doi: 10.1016/j.jacc.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 5.Honjo T, Yamaoka-Tojo M, Inoue N. Pleiotropic effects of ARB in vascular metabolism--focusing on atherosclerosis-based cardiovascular disease. Curr Vasc Pharmacol. 2011;9:145–152. doi: 10.2174/157016111794519273. [DOI] [PubMed] [Google Scholar]
- 6.Yamada S. Pleiotropic effects of ARB in metabolic syndrome. Curr Vasc Pharmacol. 2011;9:158–161. doi: 10.2174/157016111794519318. [DOI] [PubMed] [Google Scholar]
- 7.Zoja C, Corna D, Gagliardini E, Conti S, Arnaboldi L, Benigni A, Remuzzi G. Adding a statin to a combination of ACE inhibitor and ARB normalizes proteinuria in experimental diabetes, which translates into full renoprotection. Am J Physiol Renal Physiol. 2010;299:F1203–211. doi: 10.1152/ajprenal.00045.2010. [DOI] [PubMed] [Google Scholar]
- 8.Kim DH, Choi WG, Kwan J, Park KS, Lee WH. Effects of early losartan therapy on ventricular late potentials in acute myocardial infarction. Ann Noninvasive Electrocardiol. 2008;13:371–377. doi: 10.1111/j.1542-474X.2008.00253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alvin Z, Laurence GG, Coleman BR, Zhao A, Hajj-Moussa M, Haddad GE. Regulation of L-type inward calcium channel activity by captopril and angiotensin II via the phosphatidyl inositol 3-kinase pathway in cardiomyocytes from volume-overload hypertrophied rat hearts. Can J Physiol Pharmacol. 2011;89:206–215. doi: 10.1139/Y11-011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Picciotto G, Sargiotto A, Petrarulo M, Rabbia C, De Filippi PG, Roccatello D. Reliability of captopril renography in patients under chronic therapy with angiotensin II (AT1) receptor antagonists. J Nucl Med. 2003;44:1574–1581. [PubMed] [Google Scholar]
- 11.Vasiuk IuA. [Assessment of natriuretic peptides levels in the study of efficacy of perindopril and captopril in myocardial infarction with systolic dysfunction] . Ter Arkh. 2005;77:89–93. [PubMed] [Google Scholar]
- 12.Zhang N, Ji Z. Effects of caveolin-1 and P-ERK1/2 on Ang II-induced glomerular mesangial cell proliferation. Ren Fail. 2013;35:971–977. doi: 10.3109/0886022X.2013.808956. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen MT, Lee DH, Delpire E, McDonough AA. Differential regulation of Na+ transporters along nephron during ANG II-dependent hypertension: distal stimulation counteracted by proximal inhibition. Am J Physiol Renal Physiol. 2013;305:F510–519. doi: 10.1152/ajprenal.00183.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barauna VG, Magalhaes FC, Campos LC, Reis RI, Kunapuli SP, Costa-Neto CM, Miyakawa AA, Krieger JE. Shear stress-induced Ang II AT1 receptor activation: G-protein dependent and independent mechanisms. Biochem Biophys Res Commun. 2013;434:647–652. doi: 10.1016/j.bbrc.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Shibayama Y, Hitomi H, Nakano D, Kobori H, Mori H, Deguchi K, Masaki T, Ichihara A, Nishiyama A. Role of (pro) renin receptor in Ang II-mediated EGF receptor transactivation. Front Biosci (Elite Ed) 2013;5:697–705. doi: 10.2741/e650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H, Ding J, Fan Q, Liu S. TRPC6 up-regulation in Ang II-induced podocyte apoptosis might result from ERK activation and NF-kappaB translocation. Exp Biol Med (Maywood) 2009;234:1029–1036. doi: 10.3181/0901-RM-11. [DOI] [PubMed] [Google Scholar]
- 17.Chen JX, Stinnett A. Disruption of Ang-1/Tie-2 signaling contributes to the impaired myocardial vascular maturation and angiogenesis in type II diabetic mice. Arterioscler Thromb Vasc Biol. 2008;28:1606–1613. doi: 10.1161/ATVBAHA.108.169235. [DOI] [PMC free article] [PubMed] [Google Scholar]


