Abstract
The typical balance between Th17 and Treg T cell subsets is altered in various autoimmune diseases. Here, inflammatory markers in patients with acute exacerbation of chronic obstructive pulmonary disease (COPD) (n=32) and stable COPD (n=36) were compared with smokers with normal lung function (n=40, control group). Flow cytometry was used to detect proportions of Th17 and Treg cells in the peripheral blood. ELISA of induced sputum samples was used to detect IL-17 (secreted by Th17 cells) and TGF-β1 (secreted by Treg cells) concentrations. The proportion of Th17 cells in peripheral blood and secreted IL-17 and TGF-β1 levels in sputum were significantly higher in acute exacerbation of COPD patients than in stable COPD and control groups (P < 0.05). Additionally, the proportion of Treg cells was lower than in stable COPD but higher than in controls. Th17 proportions were negatively correlated with Treg proportions in both acute exacerbation and stable COPD patients, and positively correlated with IL-17 levels (P < 0.05). Further, there was a positive correlation between Treg proportion and TGF-β1 levels (P < 0.05). Thus, COPD patients have shifts in the balance of Th17/Treg cells. Acute exacerbation of COPD is shifted toward a pro-inflammatory response, while stable COPD is shifted toward an anti-inflammatory response. This finding may provide a new direction for future clinical treatment of COPD by seeking to repair the disrupted balance in T cells.
Keywords: Chronic obstructive pulmonary disease, COPD, Th17 cells, Treg cells
Introduction
Chronic obstructive pulmonary disease (COPD) produces chronic bronchitis and/or emphysema due to airflow obstruction that results from abnormal inflammatory responses following exposure to harmful gases and particles. Importantly, COPD can develop into pulmonary heart disease and respiratory failure. Indeed, COPD is associated with high mortality and disability rates; globally, its incidence rate is as high as 9-10% in people over 40. The most significant risk factor for COPD is cigarette smoking [1].
Recent studies have uncovered important roles for CD4+T cells [including Th1 cells, Th17 cells, and regulatory T cells (Treg) and their cytokines in the development of COPD [2]. In particular, the roles of Th17 cells (pro-inflammatory) and regulatory T cells (anti-inflammatory) are increasingly investigated [3]. Th17 and Treg cells are normally in a state of balance; if this balance is shifted toward Th17 cells, inflammatory responses are triggered throughout the body [4]. A mouse model of COPD, established using chronic cigarette smoke exposure, exhibited increased Th17 cell populations and decreased Treg populations, accompanied by changes in their respective cytokines, in the disease state [5]. However, information on such inflammatory changes in human COPD remains limited.
This study sought to provide a new direction for the clinical treatment of COPD by studying the ratios of Th17 and Treg cells and expression of their cytokines in peripheral blood at different stages of COPD.
Participants and methods
Participants
The study recruited participants from the Department of thoracic surgery, the First Affiliated Hospital of Zhengzhou University (Zhengzhou, Henan Province, P. R. China), who were hospitalized between October 2012 and May 2014. Participants were divided into groups by lung function, as follows: group A had acute exacerbation of COPD (AECOPD; n=32); group B had stable COPD (SCOPD; n=36); group C (n=40) comprised a smoking population with normal lung function as confirmed by the Health Examination Center of the hospital. Inclusion criteria for group A were: 1) ages 40-80 years; 2) either gender; 3) meeting the diagnostic criteria in Diagnosis and Treatment Guideline for Chronic Obstructive Pulmonary Disease [4]; 4) smoking index > 300; 5) clinical manifestations of acute exacerbation, such as rapid and worse expiration, increased sputum expectoration volume and purulent sputum. Inclusion criteria for group B comprised items 1-4 as in group A; additionally, acute exacerbation, other concurrent lung infections, serious disorders involving the immune system and other systems, and malignant tumors were ruled out. Group C served as a control group; the inclusion criteria were: ages 40-80 years, genders, normal cardiopulmonary function, and smoking index > 300. SP-1M Handheld Spirometer (SCHILLER) was used strictly according to the guidelines for Detection of Lung Function [4]. This study followed the principles of informed consent, and was approved by the Ethics Committee of the hospital.
Blood samples
Peripheral venous blood (5 mL) was collected from each fasting participant in the morning. Samples were anticoagulated (placed in 2 heparin anticoagulant tubes), diluted in PBS, centrifuged, and washed. Peripheral blood mononuclear cell (PBMC) suspension was prepared by placing 100 μL of the cell suspension into two tubes and adjusting the cell concentration to 2×106 cells/mL. Cells were sorted (see following methods) using a flow cytometer (FACS Calibur, Beckman Coulter).
Flow cytometry
For Th17 cell detection, one tube of PBMCs was treated with ionomycin (1 μg/mL), bacterin (0.7 μg/mL), phorbol ester (50 μg/mL), and brefeldin (1 μg/mL). The tube was placed into a 5% CO2 incubator at 37°C and was incubated for 4 h. A PBS solution was used for washing and centrifugation. Next, 10 μL of APC-conjugated anti-CD3 monoclonal antibody (R&D Systems) and 20 μL of FITC-conjugated anti-CD4 antibody (R&D Systems) were added to the tube. Following mixing, samples were incubated at 4°C away from light for 30 min and washed with PBS. Next, permeabilization buffer was added, and samples were incubated away from light for 60 min and subsequently washed with PBS. 5 μL of PE-conjugated anti-IL-17 (R&D Systems) was added, and samples were incubated away from light for 30 min. After washing with PBS, samples were stored at 4°C until detection. Cellquest Software (BD Biosciences, California, USA) was used for data analysis.
For detection of Treg cell detection, 10 μL of APC-conjugated anti-CD3 monoclonal antibody, 10 μL of FITC-conjugated anti-CD4, and 10 μL of PE-conjugated anti-CD25 (R&D Systems) were added. Samples were incubated away from light for 30 min and washed with PBS. Permeabilization buffer was added, and samples were incubated away from light for 60 min. Samples were washed again, and PECy5-conjugated anti-FOXP3 (R&D Systems) was added. Samples were incubated away from light for 60 min, washed, and stored at 4°C until detection. FCS Express Version 3 developed (De novo Software) was used for data analysis.
Enzyme-linked immunosorbent assay (ELISA)
A double-antibody sandwich ELISA kit was used to detect IL-17 and TGF-β1 according to manufacturer instructions (Wuhan Boster Biological Technology, China). All participants was recruited for being induced sputum with hypertonic saline in the morning. At 10 minutes before being induced, each individual inhale salbutamol (400 μg), and 30 g/L hypertonic saline by using ultrasonic atomization for 15 min. Then, sputum was expectorated with force to reach the culture plates. Forceps were used to take out sticky mucus with a great density so as to be separated from the saliva and be collected. The mucus was weighed and added with 0.1% dithiothreitol ( 4 times the mucus in volume ) to mix completely; the mixture was treated in a water bath at 37°C for 10 min, filtered through mesh sieve and centrifuged at 2000 r/min (r=8 cm) for 10 min to precipitate cells; and then, the supernatant was stored at -80°C. The frozen supernatant was taken to undergo ELISA detection of IL-17 and TGF-β1, in which, the detailed operation was done according to the instructions in the kit.
Statistical treatment
SPSS17.0 was used for statistical analysis. Measurement data are expressed as mean ± standard deviation; the means in multiple groups were compared using univariate analysis of variance and pairwise comparison between groups (SNK method). If heterogeneity of variance was found in multiple groups, a Kruskal-Wallis test was used for analysis, and pairwise comparison was performed with Wilcoxon rank-sum test. For bivariate correlation analysis, Spearman correlation was used to analyze the correlation between Th17 and Treg cells. P < 0.05 was considered to indicate a statistically significant difference.
Results
Comparison of characteristics between participant groups
Participant characteristics are shown in Table 1. All participants were male. No significant difference was detected between groups in the mean age (F=0.418, P=0.829). However, significant differences were observed between the groups in the smoking index (F=12.742, P < 0.001). Lung function was measured using forced expiratory volume in one second (FEV1) and forced vital capacity (FVC). Analysis of variance identified significant differences in FEV1, FEV1/FVC, and FEV1/predicted value, with group C having significantly higher values for each than groups A and B, and group B had significantly higher FEV1/FVC and FEV1/pred than group A.
Table 1.
Demographic and clinical characteristics in participants with and without COPD
| Group | Age (years) | Smoking index | FEV1 (L) | FEV1/FVC (%) | FEV1/pred (%) |
|---|---|---|---|---|---|
| A (n=32) | 65±8 | 812±192☆ | 1.14±0.08☆ | 41±5.12☆,★ | 35±3.22☆,★ |
| B (n=36) | 67±5 | 794±387☆ | 1.25±0.15☆ | 59±8.43☆ | 43±7.14☆ |
| C (n=40) | 62±7 | 592±284 | 3.10±0.39 | 89±4.25 | 110±15.09 |
| F | 0.418 | 12.742 | 542.31 | 774.59 | 982.24 |
| P | 0.829 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
P < 0.05, vs. group C;
P < 0.05, vs. group B.
Group A: acute exacerbation of COPD; Group B: stable COPD; Group C: smokers without COPD. FEV1: forced expiratory volume in one second; FVC: forced vital capacity; pred: predicted FEV1.
Inflammatory markers in COPD
The ratios of Th17 and Treg cells were analyzed in PBMCs of participants with COPD and without COPD to determine whether the ratio serves as a marker for COPD progression. Because the data set showed heterogeneity of variance between groups, Wilcoxon and Kruskal-Wallis rank sum tests were used to compare data; pairwise comparisons were also used. The proportions of Th17 and Treg cells in CD3+/CD4+T cells were significantly different between gr-oups A and C (P < 0.05); Groups B and C differed significantly in proportions of Treg cells (P < 0.05; Table 2). T cells express cytokines that promote or fight inflammation. Specifically, Th17 cells secrete the pro-inflammatory cytokine interleukin-17 (IL-17); Treg cells secrete the anti-inflammatory cytokine TGF-β1.The levels of IL-17 and TGF-β1 were determined by ELISA of induced sputum, and differed significantly between groups, with both markers detected at higher levels in group A than group C (P < 0.05); only TGF-β1 differed significantly between groups B and C (P < 0.05; Table 2). Further, pairwise comparisons revealed significantly higher levels of both cytokines in group A compared to group B (P < 0.05).
Table 2.
Comparison of Th17 and Treg cells as well as IL-17 and TGF-β1 as inflammatory cytokines in three groups
| Groups | Th17 (%) | Treg (%) | Th17/Treg (%) | IL-17 (ng/L) | TGF-β1 (ng/L) |
|---|---|---|---|---|---|
| A (n=32) | 2.92±0.14☆,★ | 8.84±0.62☆,★ | 33.28±3.81☆,★ | 81.00±10.32☆,★ | 1363.05±139.90☆,★ |
| B (n=36) | 1.21±0.07 | 11.94±0.38☆ | 10.20±0.90☆ | 12.41±1.54 | 802.23±44.36☆ |
| C (n=40) | 1.35±0.07 | 5.33±0.83 | 26.01±4.58 | 4.84±0.98 | 58.10±13.46 |
| H | 87.867 | 94.853 | 85.08 | 97.834 | 94.830 |
| P | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
P < 0.05, vs. group C;
P < 0.05, vs. group B.
Because the data were not normally distributed, Spearman’s method was used to determine correlation. The proportions of Th17 and Treg cells were negatively correlated with one another in groups A and B (Figure 1). Further, for both groups, the proportion of Th17 cells was positively correlated with the level of IL-17 expression (Figure 2), and the proportion of Treg cells was positively correlated with the level of TGF-1β expression (Figure 3). In group C, these indexes were not correlated.
Figure 1.

Correlation of the proportion of Th17 and Treg cells in the peripheral blood of patients with COPD. Note: A. group A; B. group B; C. group C.
Figure 2.
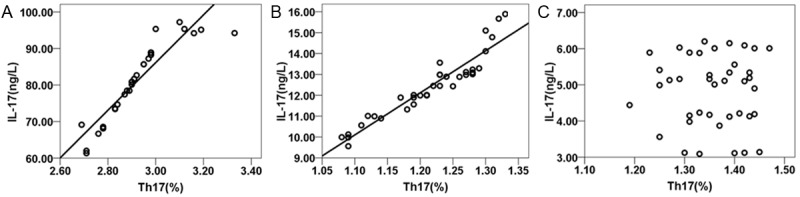
Correlation of the proportion of Th17 cells in the peripheral blood and level of IL-17 expression in the induced sputum of patients with COPD. Note: A. group A; B. group B; C. group C.
Figure 3.

Correlation of the proportion of Treg cells in the peripheral blood and level of TGF-β expression in the induced sputum of patients with COPD. Note: A. group A; B. group B; C. group C.
Discussion
T cell subsets play varying but important roles in inflammatory responses. Th17 and Treg cells in the peripheral blood are closely related with the differentiation of CD4+CD25+ Treg cells, where they are respectively involved in inflammatory responses and immune inhibition [6]. A Th17/Treg balance, separate from the Th1/Th2 balance, is proposed to be important in immunity [7]. An imbalance in Th17/Treg is detected in various autoimmune diseases and is demonstrated to aggravate inflammatory responses in the diseases [8]. Therefore, the Th17/Treg ratio may also reflect the inflammatory responses in COPD patients. A negative correlation between Th17 cells and Treg cells was identified in this study in the peripheral blood of patients with acute exacerbated COPD (A) and stable COPD (B). This finding indicates that pro-inflammatory Th17 cells and anti-inflammatory Treg cells inhibit each other in terms of differentiation and functions. Interestingly, the Th17/Treg ratio in group A was higher than in the control group, while that of group B was lower than in the control group; thus, patients with acute exacerbation of COPD have a balance shifted toward the promotion of inflammatory responses, while patients with stable COPD have a balance shifted toward the inhibition of inflammatory responses.
Th17 cells produce the pro-inflammatory cytokine IL-17 and can promote the activation of bronchial fibroblasts, epithelial cells, and smooth muscle cells. Those cells are, in turn, induced to express cytokines like IL-6, IL-8, and granulocyte colony-stimulating factor (G-CSF) to promote the local infiltration and proliferation of neutrophil granulocytes in the airway, and aggravate inflammatory responses involving the airway and lungs [9]. Therefore, the proportion of Th17 cells in the peripheral blood can serve as an index that reflects the inflammatory responses of COPD patients. Here, the proportion of Th17 cells in the acute exacerbated COPD group (A) was higher than that in the stable COPD group (B). This finding is consistent with the findings of Miyahara et al. [10]. The results suggest that the proportion of Th17 cells increases in the peripheral blood of patients with acute exacerbation of COPD, secreting additional IL-17 to promote the inflammatory responses in the lung. Individuals with stable COPD, however, did not exhibit a significantly higher proportion of Th17 cells or increased secretion of IL-17 compared to controls, and thus were not correlated with lung function. This is consistent with the findings of Doe et al. [11] biopsy of the bronchial mucosa did not uncover differences in IL-17+ cells in individuals with COPD compared with smokers with normal lung function. In contrast, Zhang et al. [12] found markedly increased levels of IL-17 in the peripheral blood, as well as a negative correlation of this level with the degree of limitation of air flow, in patients with stable COPD compared with smokers with normal lung function. However, these differences could be related to other factors including sex; thus, to avoid potential bias, all participants in this study were male.
In contrast to Th17 cells, Treg cells can be immunosuppressive, secreting the anti-inflammatory cytokines IL-10 and TGF-β1 to strengthen immune tolerance and inhibit inflammatory responses; Treg cells are also subject to feedback regulation by TGF-p [13]. Here, proportions of Treg cells and levels of secreted TGF-β1 in group B were significantly different from those in group A and group C, and Treg cells and TGF-β1 had a positive correlation. Thus, patients with stable COPD appear to have a balance shifted toward anti-inflammatory responses. This finding is consistent with the findings of Lee et al. [14], in that there was a marked increase in Treg cells in the lung tissue and peripheral blood in patients with acute exacerbation of COPD and patients with emphysema compared with healthy individuals. Further, these results corroborate a report for a mouse model of airway diseases, in which transfusions of CD4+CD25+ Treg cells could inhibit the occurrence of allergic inflammation [15].
In summary, the normal balance between Th17 and Treg cells is disturbed in patients with COPD. Patients with acute exacerbation of COPD exhibit pro- inflammatory responses, while patients with stable COPD exhibit anti-inflammatory responses. These findings provide a basis for the hypothesis of autoimmunity of COPD patients, and repairing this disrupted balance may offer a new therapeutic target for COPD patients.
Disclosure of conflict of interest
None.
References
- 1.Rabe K, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, Zielinski J. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 2.Afshar R, Medoff BD, Luster AD. Allergic asthma: a tale of many T cells. Clin Exp Allergy. 2008;38:1847–1857. doi: 10.1111/j.1365-2222.2008.03119.x. [DOI] [PubMed] [Google Scholar]
- 3.Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunol Rev. 2008;223:87–113. doi: 10.1111/j.1600-065X.2008.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fromer L, Cooper CB. A review of the GOLD guidelines for the diagnosis and treatment of patients with COPD. Int J Clin Pract. 2008;62:1219–1236. doi: 10.1111/j.1742-1241.2008.01807.x. [DOI] [PubMed] [Google Scholar]
- 5.Wang H, Peng W, Weng Y, Ying H, Li H, Xia D, Yu W. Imbalance of Th17/Treg cells in mice with chronic cigarette smoke exposure. Int Immunopharmacol. 2012;14:504–512. doi: 10.1016/j.intimp.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Turner MD, Nedjai B, Hurst T, Pennington DJ. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim Biophys Acta. 2014;1843:2563–2582. doi: 10.1016/j.bbamcr.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 7.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal development al pathways for the generation of pathogenic effectors Th17 and regulatory T cells. Nature. 2006;411:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 8.Eisenstein EM, Williams CB. The Treg/Th17 Cell Balance: A New Paradigm for Autoimmunity. Ped Res. 2009;65:26R–31R. doi: 10.1203/PDR.0b013e31819e76c7. [DOI] [PubMed] [Google Scholar]
- 9.Oboki K, Ohno T, Saito H, Nakae S. Th17 and allergy. Allergol Int. 2008;57:121–134. doi: 10.2332/allergolint.R-07-160. [DOI] [PubMed] [Google Scholar]
- 10.Miyahara Y, Odunsi K, Chen W, Peng G, Matsuzaki J, Wang RF. Generation and regulation of human CD4+ IL-17-producing T cells in ovarian cancer. Proc Natl Acad Sci U S A. 2008;105:15505–15510. doi: 10.1073/pnas.0710686105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doe C, Bafadhel M, Siddiqui S, Desai D, Mistry V, Rugman P, McCormick M, Woods J, May R, Sleeman MA, Anderson IK, Brightling CE. Expression of the T helper 17-associated cytokines IL-17A and IL-17F in asthma and COPD. Chest. 2010;38:1140–1147. doi: 10.1378/chest.09-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J, Chu S, Zhong X, Lao Q, He Z, Liang Y. Increased expression of CD4+IL-17 cells in the lung tissue of patients with stable chronic obstructive pulmonary disease (COPD) and smokers. Int Immunopharmacol. 2013;15:58–66. doi: 10.1016/j.intimp.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 13.Hussein AA, Saliba WI, Martin DO, Shadman M, Kanj M, Bhargava M, Dresing T, Chung M, Callahan T, Baranowski B, Tchou P, Lindsay BD, Natale A, Wazni OM. Plasma B-type natriuretic peptide levers and recurrent arrhythmia after successful ablation of lone atria fibrillation. Circulation. 2011;123:2077–2082. doi: 10.1161/CIRCULATIONAHA.110.007252. [DOI] [PubMed] [Google Scholar]
- 14.Lee SH, Goswami S, Grudo A, Song LZ, Bandi V, Goodnight-White S, Green L, Hacken-Bitar J, Huh J, Bakaeen F, Coxson HO, Cogswell S, Storness-Bliss C, Corry DB, Kheradmand F. Anticlastic auto immunity in tobacco smoking-induced emphysema. Nat Med. 2007;13:567–569. doi: 10.1038/nm1583. [DOI] [PubMed] [Google Scholar]
- 15.Wozakowska-Kaplon B, Bartkowiak R, Grabowska U, Janiszewsak G. B-type natriuretic peptide level after sinus rhythm restoration in patients with persistent atrial fibrillation-clinical significance. Kardiol Pol. 2010;68:781–786. [PubMed] [Google Scholar]


