Abstract
Background: The expression of P21 and TBX2 in laryngeal squamous cell carcinoma (LSCC) and their corresponding adjacent normal laryngeal tissues, as well as their association with clinical pathological features and survival remain unclear. Method: we used the RT-PCR and immunohistochemistry to detect their mRNA and protein levels in 75 LSCC patients. We also use log-rank test and Cox models to compare survival among different groups. Results: The mRNA expression level of TBX2 was up-regulated, while P21 was down-regulated in LSCC compared with their matched adjacent laryngeal tissues (All P < 0.001). The expression of P21 was correlated with tumor stage, lymph node metastasis and smoking; and TBX2 expression was associated with lymph node metastasis, differentiation degree and smoking (All P < 0.05). Patients with high TBX2 and low P21 expression had significantly worse survival than those with low TBX2 and high P21 expression, respectively (All P < 0.05). A significant correlation between expression of TBX2 and P21 (Pearson, P < 0.05) was observed. Furthermore, multivariable analysis showed that patients with low TBX2 and high P21 expression alone had a significantly reduced risk for overall death compared with those with low TBX2 and high P21 expression. The risk for overall death was even lower for patients with both low and high expression of both genes than any other co-expression status of both genes (HR, 0.1; 95% CI, 0.0-0.9). Conclusion: These results suggest that abnormal expression of P21 and TBX2 in tumors may jointly, or individually, predict poor prognosis of LSCC.
Keywords: P21, TBX2, LSCC, survival
Introduction
Head and neck squamous cell carcinomas (HNSCC) rank among the sixth most common type of tumor worldwide, with poor prognosis [1]; and among the HNSCC laryngeal squamous cell carcinoma (LSCC) ranks second [2]. It accounts for approximately half of all HNSCC cases in China [3]. The development and progression of LSCC involve various factors and molecular processes, with an obvious expression anomalism of numerous cellular molecules. P21, also known as CDK-interacting protein 1 (CIP1), senescent cell-derived growth inhibitor 1 (SDI1), wild-type p53-activated fragment 1 (WAF1), melanoma-derived antigen 6 (MDA6) and cyclin-dependent kinase inhibitor 1A (CDKN1A), functions as a cyclin-dependent kinase inhibitor, which is a key cell cycle regulatory protein for cell cycle regulation [4]. It is a well-known mediator of p53-induced cell growth arrest [5] and exerts a determinant role as a regulator of CDK activity [6]. Furthermore, P21 is induced in the context of cellular senescence and affects cell apoptosis. The activation of P21 transcription can activate p53 in senescence and thus leads to cell cycle arrest [7]. Noel et al indicated that p21 is frequently down-regulated in human cancers, and its expression can either inhibit or promote carcinogenesis, depending on the cellular context [4].
The T-box gene family is an archaic gene family as demonstrated by phylogenetic analysis, the members of T-box family are transcription factors that are characterized by a highly conserved region of nearly 200 amino acid residues corresponding to the DNA binding domains known as the T-box [8]. Recent studies suggest that T-box factors may also play a role in controlling cell cycle progression and cancer development [9]. TBX2, short for T-box2, is one of members in T-box family. In humans, it is expressed in a wide variety of tissues including lung, kidney, ovary, prostate, spleen, testis, breast, heart, intestine, thymus, and polymorphonucleocytes [10,11]. Extensive studies have been taken to show its relationship with tumor invasion and metastasis, and the increased expression of TBX2 was related to tumor development and progression [12-15]. However, the expression profiles of p21 and TBX2 and their correlations with clinical parameters and influence on prognosis of LSCC have remained largely unknown. The aim of the study was to investigate the expression of p21 and TBX2 in LSCC and to analyze their relationship with clinicopathologic characters and prognosis in patients with LSCC.
Materials and methods
Patients and biological samples
Seventy-five patients who were diagnosed with LSCC between 2009 and 2010 were included in this study and all samples were acquired with patients’ consent. Immediately after surgical operation, we obtained the tumor samples from the tumor area, and its corresponding peripheral normal laryngeal tissues were acquired from the associated non-cancerous tissue area within 5 cm of the tumor, without involving the assessment of tumor margins. The samples were separated into two parts: one for quantitative analysis and snap-frozen quickly in liquid nitrogen immediately after surgery than stored at -80°C; while the other formalin fixed and paraffin embedded for further use. Histopathological assessment was performed on the paraffin blocks. Medical record review for follow-up status of all patients was performed under direct supervision of the staff head and neck surgeon. Primary tumor subsite, clinical stage, treatment, and vital status were acquired from medical records as assessed between the initial and final patient contact recorded. In addition, patients who had drunk at least one alcoholic beverage per week for at least one year during their lifetime were categorized as “ever drinkers”, while patients who had never had such a manner of drinking were categorized as “never drinkers”. Patients who were categorized as “ever smokers” are related to those who had smoked at least 100 cigarettes in their lifetime, while patients who had smoked fewer than 100 cigarettes in their lifetime were sorted as “never smokers.” This study was approved by the ethics committee of Beijing Tongren Hospital of the Capital Medical University, and all of the patients that including in it had signed the informed consent forms.
Quantitative analysis (qPCR)
Total RNA was extracted for qPCR analysis. The primers and internal reference primers were all synthesized by Beijing Biosynthesis Biotechnology Co., Ltd. (Beijing, China) and the Ct value comparison method was used in this study to evaluate the mRNA expression level. The β-actin was used as the internal reference gene in this study. The relative expression levels of TBX2 and P21 were calculated with the Ct values referring to the number of cycles when the fluorescence signal reached the set threshold in each reaction tube. The primers used in this study are shown in Table 1.
Table 1.
Primers used in this study
| Primers | Sequences |
|---|---|
| β-actin | F: 5’-CACCCTTTCTTGACAAAACCT-3’ |
| R: 5’-AGTGGGGTGGCTTTTAGGA-3’ | |
| TBX2 | F: 5’-ACCGCGTGATAAAACTGGGTT-3’ |
| R: 5’-CAGGGAAGAGGTGGGGAGACT-3’ | |
| P21 | F: 5’-GCGGAACAAGGAGTCAGACAT-3’ |
| R: 5’-CCCAATACTCCAAGTACACTAAGCA-3’ |
Immunohistochemistry
TBX2 and P21 antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), while 4-dimethylaminoazobenzene, streptavidin-peroxidase, and the streptavidin-peroxidase immunohistochemical staining kit were purchased from Beijing Tiangen Biomedical Development Co., Ltd. (Beijing, China). The LSCC tissues and its corresponding adjacent normal laryngeal tissues were preserved. A positive biopsy in the kit was used as the positive control, and the antibody was replaced by phosphate-buffered saline (PBS) as the negative control. Yellow to brownish-yellow granules in cells were calculated as positive cells. Positive cells were counted in a total of 100 cells under high-power microscopy, and scored according to the positive expression rate (0 points for < 10%; 1 point for 11-20%; 3 points for 21-50%; 4 points for > 50%) and staining intensity (0 points for no staining; 2 points for weak; 3 points for strong). The sum of the points for staining intensity and positive expression rate was used as the expression score. A score ≥ 3 was considered to indicate a positive case while a score of 0-2 as a negative case.
Statistical analysis
Data were analyzed via SPSS 17.0 statistical software (SPSS, Inc., Chicago, IL). A paired t-test was used to compare the measurement data, while spearman’s rank correlation analysis was used to analyze the correlation of TBX2 and P21 expression. The differences between TBX2 and P21 expression was evaluated by using the χ2 test. For survival analysis, the primary endpoint is overall death. Overall survival (OS) was defined as the time from first appointment to the date of last follow-up or death from any cause. Patients who were still alive at the end of the study period or lost to follow-up were considered censored. Survival analysis was performed by using Kaplan-Meier analysis, the significance was analyzed with the log-rank test. Multivariable cox proportional hazards regressions were used to evaluate the individual hazard ratio (HR) for OS. The difference was considered significant when the P value was less than 0.05.
Results
mRNA levels of TBX2 and P21
qPCR was applied to uncover the potential changes in the mRNA levels of TBX2 and P21 expression in LSCC as well as its corresponding adjacent normal laryngeal tissues. The qPCR results showed significant differences in both gene expressions between the cancerous and adjacent tissues (P < 0.05). The expression of TBX2 in the cancerous tissue significantly increased (P < 0.05) while the P21 mRNA expression in the cancerous tissue decreased compared with those in the corresponding adjacent normal laryngeal tissues (P < 0.05), respectively, as shown in Table 2.
Table 2.
mRNA expression of P21 and TBX2 in LSCC and adjacent normal tissues
| Tissue type | N | P21 | P | TBX2 | P |
|---|---|---|---|---|---|
| Adjacent tissue | 75 | 0.572±0.217 | 2.128±0.779 | ||
| Cancer tissue | 75 | 1.074±0.394 | 0.000 | 1.077±0.292 | 0.000 |
Protein expression of TBX2 and P21
Immunohistochemical analyses of TBX2 and P21 expression in LSCC and their corresponding adjacent normal laryngeal tissues were performed to detect the expression of both proteins (Table 3). Both TBX2 and P21 proteins were expressed in the cell nuclear (Figure 1). The positive expression rates and scores of TBX2 significantly increased in the cancerous tissue compared with those in the adjacent tissue (P < 0.05). For P21, however, its protein expression level significantly decreased in LSCC compared with those in the adjacent tissues (P < 0.05). These results indicate that the increased TBX2 and reduced p21 protein expressions in LSCC compared with those in adjacent normal tissues are consistent with their mRNA expressions in those tissues, respectively.
Table 3.
MMP11 and P14ARF protein expression level in LSCC and adjacent tissues
| Tissue type | n | TBX2-Positive N (%) | P | P21-Positive N (%) | P |
|---|---|---|---|---|---|
| Adjacent tissue | 75 | 18 (24.0%) | 58 (77.3%) | ||
| Cancer tissue | 75 | 55 (73.3%) | 0.000 | 31 (41.3%) | 0.000 |
Figure 1.
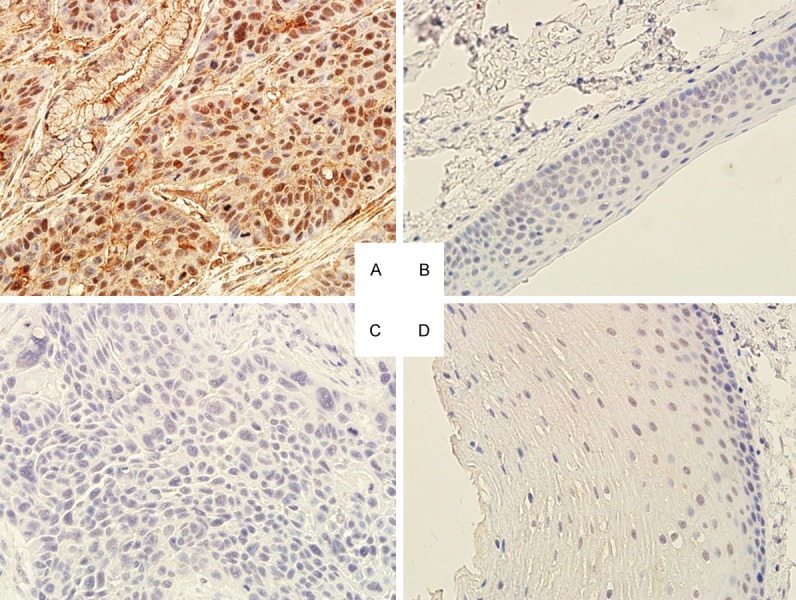
Immunostaining of TBX2 and P21 in LSCC tissues. A: LSCC tissues with high TBX2 expression in the cytoplasm and nuclear; B: TBX2 weakly expressed in the adjacent tissue; C: P21 weekly expressed in LSCC tissue; and D: P21 positively expressed in the adjacent tissue.
Pathological characteristics
The mRNA expression levels were analyzed to explore the potential relationship between both protein and mRNA expression levels of TBX2 and P21 and their relationships with clinical pathological parameters (Table 4). We found that TBX2 and P21 were negatively correlated (correlation coefficient, -0.352, P = 0.001). In the 75 cases of LSCC tissues, TBX2 was correlated with the differentiation degree and lymph node metastasis; and the patients with lymph node metastasis and lower differentiation had shown a much stronger TBX2 expression compared with those with negative lymph node metastasis and higher differentiation (P < 0.05). In contrast, P21 expression was also associated with lymph node metastasis and the tumor stage; and the patients in the later stage and with lymph node metastasis had shown a much lower expression level (P < 0.05). Furthermore, both TBX2 and P21 had a significant correlation with tobacco smoking. These results suggest that the increased expression level of TBX2 may be associated with the malignancy of tumors while P21 expression may be negatively correlated with the degree of malignancy.
Table 4.
Correlations between the protein/mRNA expressions of MMP11 and P14ARF with clinical pathological characteristics in 75 LSCC cases
| Factors | Cases (%) | TBX2 | P21 | ||
|---|---|---|---|---|---|
|
| |||||
| Protein-positive N (%) | mRNA-upregulated N (%) | Protein-positive N (%) | mRNA-upregulated N (%) | ||
| Age (years) | |||||
| ≥ 60 | 32 (42.7%) | 24 (75.0%) | 27 (84.4%) | 11 (34.4%) | 9 (28.1%) |
| < 60 | 43 (57.3%) | 31 (72.1%) | 31 (72.1%) | 20 (46.5%) | 14 (32.6%) |
| Gender | |||||
| Male | 67 (89.2%) | 49 (73.1%) | 52 (77.6%) | 27 (40.3%) | 20 (29.9%) |
| Female | 8 (10.8%) | 6 (75.0%) | 6 (75.0%) | 4 (50.0%) | 3 (37.5%) |
| Tumor stage | |||||
| I-II | 24 (32.0%) | 17 (70.8%) | 19 (79.2%) | 16 (66.7%) | 17 (70.8 %) |
| III-IV | 51 (68.0%) | 38 (74.5%) | 39 (76.5%) | 15 (29.4%)a | 6 (11.8%)a |
| Lymph node metastasis | |||||
| Absent | 30 (40.0%) | 15 (50.0%) | 17 (56.7%) | 7 (23.3%) | 5 (16.7%) |
| Present | 45 (60.0%) | 40 (88.9%)a | 41 (91.1%)a | 24 (53.3%)a | 18 (40.0%a |
| Differentiation degree | |||||
| High | 27 (36.0%) | 15 (55.6%) | 16 (59.3%) | 10 (37.0%) | 7 (25.9%) |
| M.-low | 48 (64.0%) | 40 (83.3%)a | 42 (87.5%)a | 21 (43.8%) | 16 (33.3%) |
| Smoking | |||||
| Ever | 57 (76.0%) | 46 (80.7%) | 48 (84.2%) | 17 (29.8%) | 11 (19.3%) |
| Never | 18 (24.0%) | 9 (50.0%)a | 10 (55.6%)a | 14 (77.8%)a | 12 (70.6%)a |
| Alcohol | |||||
| Ever | 58 (77.3%) | 42 (72.4%) | 46 (79.3%) | 25 (43.1%) | 16 (27.6%) |
| Never | 17 (22.7%) | 13 (76.5%) | 12 (70.6%) | 6 (35.3%) | 7 (41.2%) |
| Treatment | |||||
| Surgery only | 34 (45.7) | 25 (73.5) | 24 (70.6) | 23 (67.6) | 26 (76.5) |
| Combined | 41 (54.3) | 30 (73.2) | 31 (75.6) | 32 (78.0) | 29 (70.7) |
P < 0.05;
M., moderate.
Association of TBX2 and P21 expression with survival in LSCC
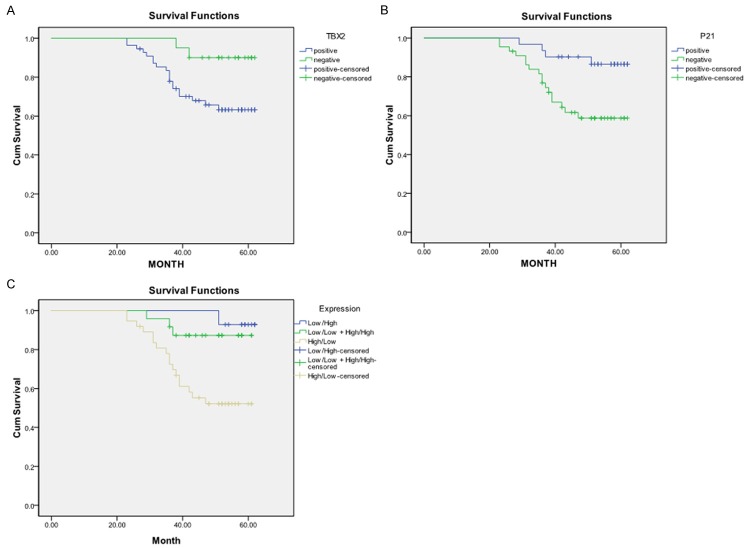
Figure 2 shows the univariate Kaplan-Meier analyses of survival with respect to the death from all causes. At a median follow-up time of 67 months (range, 4-67 months), 21 deaths occurred from any causes. The patients with high TBX2 and low P21 expression had a notably worse overall survival than patients with low TBX2 and high P21 expression (Log-rank: P = 0.031 for TBX2 and P = 0.010 for P21, respectively, Figure 2A, 2B). Patients with high and low expression of both TBX2 and P21 had an even worse survival than patients with low and high expression of either of genes or any other co-expression status of both genes (P = 0.002, Figure 2C). Furthermore, the results of multivariable Cox proportional hazards regression analysis regarding the association between expression of both genes and risk of overall death are shown in Table 5. Estimates of association were adjusted for potential confounders including age, gender, tumor differentiation, TNM stage, postoperative treatment, smoking and alcohol use, and Lymph node metastasis. Compared with patients having low p21 and high TBX2 expression of either of genes, the patients with high p21 and low TBX2 expression had significantly reduced risk of overall death (HR, 0.3, 95% CI, 0.1-0.9 for p21 and HR, 0.3, 95% CI, 0.1-0.7 for TBX2). The risk for overall death was even lower for patients with high p21 and low TBX2 expression of both genes (HR, 0.1; 95% CI, 0.0-0.9) compared with any other co-expression status of both genes (Table 5).
Figure 2.
Survival analysis by expression of TBX2 (A), P21 (B), and their combination (C) among 75 LSCC patients.
Table 5.
Multivariable survival analysis by expression of p21 and TBX2 alone or in combination in 75 LSCC patients
| Gene expression | Total (75) | Events | Survival (OS) |
|---|---|---|---|
|
|
|
||
| (Overall deaths) | aHR, 95% CI | ||
|
|
|
||
| N (%) | N (%) | ||
| P21 | |||
| Low | 44 (58.7) | 17 (80.1) | 1.0 |
| High | 31 (41.3) | 4 (19.9) | 0.3 (0.1-0.9) |
| TBX2 | |||
| High | 55 (73.3) | 19 (90.5) | 1.0 |
| Low | 20 (26.7) | 2 (9.5) | 0.3 (0.1-0.7) |
| Combined p21 and TBX2 | |||
| Low/High | 37 (49.3) | 17 (81.0) | 1.0 |
| Low/Low + High/High | 24 (32.0) | 3 (14.3) | 0.2 (0.1-0.6) |
| High/Low | 14 (18.7) | 1 (4.7) | 0.1 (0.0-0.9) |
Adjusted for age, sex, smoking, alcohol, overall stage, differentiation, node metastasis, and treatment in Cox’s models.
Discussion
Few studies have explored the roles of TBX2 and P21 in LSCC. Our current study evidently indicates that both TBX2 and P21 individually, or in combination, influence survival and might be served as prognostic biomarkers of LSCC patients. In human TBX2 exists in variety tissues [10,11], and TBX2 is over-expressed in several human cancers such as breast cancer [12], melanomas [14], liver cancer [16]. Moreover, the increased level of TBX2 may contribute to carcinogenesis through bypassing checkpoints which enable tetraploid cells to re-enter the cell cycle in a P21- and P53- dependent manner [17,18]. P21 is the first identified inhibitor of cyclin-dependent kinase (CDK) complexes and has various significant functions in cell biology, and is well known as a mediator of p53-induced cell growth arrest [5]. It can interact with the CDK1 and CDK2 via an additional CDK-binding site in the N-terminus and thus exerts its inhibitory control over the cell cycle [19]. To date, the expression level of P21 during tumor progression is up-regulated or down-regulated is still controversial. Hu et al indicated that the expression of P21 was repressed by focally amplified lncRNA on chromosome 1 (FAL1), and associates with the epigenetic repressor of BMI1 [20]. TBX2 also can directly repress the expression of P21, as previously reported [14]. P21 expression level was down-regulated in LSCC (22) and SCC of hypopharynx (23). On the contrary, others found that P21 was up-regulated in several cancers including esophageal SCC and oral SCC [24,25].
Our study demonstrated that the expression level of P21 was down-expressed while TBX2 was over-expressed in LSCC in both mRNA and protein levels. TBX2 was correlated with the differentiation degree, lymph node metastasis of LSCC patients and P21 was associated with the tumor stage and lymph node metastasis. For TBX2, its expression increased in patients with higher differentiation and lymph node metastasis, suggesting that TBX2 was correlated with tumor malignance. In contrast, the p21 expression was much lower and negatively correlated with the tumor stage and lymph node metastasis, which is in agreement with the investigation in HNSCC [24]. The possible mechanisms that cause altered expression could be due to its regulators such as FAL1 [20], TBX2 [21]. In addition, both TBX2 and P21 had a significant correlation with tobacco smoking, patients with ever tobacco smoking show much higher TBX2 expression as well as lower P21 expression than those who were never smokers, indicating that tobacco smoking is another influence factor which affect the anomalous expression of TBX2 and P21 in LSCC.
Our data also showed that patients with high-level TBX2 expression had obviously lower survival compared with those with low levels of TBX2. Several studies have demonstrated that over-expression of TBX2 is related to a lower survival among patients with non-small cell lung cancer [26] and colorectal cancer [27]. In contrast, patients with low-level of P21 had obviously shorter survival compared with those with high expression levels. These results thus might indicate that both TBX2 and P21 might individually or in combination affect the prognosis of LSCC. Intriguingly, in this study we found that there exists a negative correlation between the expression levels of TBX2 and P21. The potential mechanism may due to the fact that TBX2 can directly repress the P21 expression as Sharon et al suggested in their investigation [28]. They show that TBX2 can bind and repress the p21 promoter both in vitro and in vivo by using an integration of in vitro DNA-binding, transfection and chromatin immunoprecipitation assays. They also illustrated that small interfering RNA-mediated down-regulation of TBX2 expression resulted in a strong activation of p21 expression. Timo et al found similar results that TBX2 can repress the expression of P21 in lung cancer [29]. Therefore, more studies are needed to further investigations to understand the relationship between these two genes.
In conclusion, the expression level of TBX2 was over-expressed while P21 was down-expression in LSCC in both mRNA and protein levels; and a negative correlation between the expression levels of TBX2 and P21 was observed. Moreover, both expressions were related to the malignancy, several clinicopathological features, and prognosis of LSCC. Thus, our current study may provide evidence that both TBX2 and P21 may contribute to the development, progression and prognosis of LSCC. However, further larger studies are still necessary for validation of our findings and an investigation of the molecular mechanisms underlying the observed correlations.
Acknowledgements
The authors thank all clinical staff from the department for their help with subject recruitment for this study and laboratory personnel for laboratory management. This work was supported by the grants from the National Natural Science Foundation of China (grant number: 81302374 and 81241084), Beijing Natural Science Foundation (grant numbers: 7121005 and 5122016), and Beijing Nova Program (grant number: xx2013043), Capital Development Fund for medical research (grant numbers: 2011-2005-06).
Abbreviations
- LSCC
laryngeal squamous cell carcinoma
- OS
overall survival
- HR
hazard ratio
- CI
confidence interval
- HNSCC
head and neck squamous cell carcinoma.
References
- 1.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J. Clin. Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E. Cancer statistics, 2009. CA: a cancer journal for clinicians, Cancer Stat. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.Chen K, Song F, He M. Trends in head and neck cancer incidence in Tianjin, China, between 1981 and 2002. Head Neck. 2009;31:175–182. doi: 10.1002/hed.20946. [DOI] [PubMed] [Google Scholar]
- 4.Warfel NA, El-Deiry WS. P21WAF1 and tumourigenesis: 20 years after. Curr Opin Oncol. 2013;25:52–58. doi: 10.1097/CCO.0b013e32835b639e. [DOI] [PubMed] [Google Scholar]
- 5.el Deiry WS, Tokino T, Velculescu VE. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 6.Harper JW, Adami GR, Wei N. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 7.Alcorta DA, Xiong Y, Phelps D. Involvement of the cyclin-dependent kinase inhibitor p16 (INK4a) in replicative senescence of normal human fibroblasts. Proc Natl Acad Sci U S A. 1996;93:13742–13747. doi: 10.1073/pnas.93.24.13742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson V, Conlon FL. The T-box family. Genome Biol. 2002;3:REVIEWS3008. doi: 10.1186/gb-2002-3-6-reviews3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abrahams A, Parker MI, Prince S. The T-box Transcription Factor Tbx2: Its role in Development and Possible Implication in Cancer. IUBMB Life. 2010;62:92–102. doi: 10.1002/iub.275. [DOI] [PubMed] [Google Scholar]
- 10.Campbell C, Goodrich K, Casey G. Cloning and mapping of a human gene (TBX2) sharing a highly conserved protein motif with the drosophila omb gene. Genomics. 1995;28:255–260. doi: 10.1006/geno.1995.1139. [DOI] [PubMed] [Google Scholar]
- 11.Law DJ, Gebuhr T, Garvey N. Identification, characterization, and localization to chromosome 17q21-22 of the human TBX2 homolog, member of a conserved developmental gene family. Mamm Genome. 1995;6:793–797. doi: 10.1007/BF00539006. [DOI] [PubMed] [Google Scholar]
- 12.Sinclair CS, Adem C, Naderi A. TBX2 is preferentially amplified in BRCA1- and BRCA2-related breast tumors. Cancer Res. 2002;62:3587–3591. [PubMed] [Google Scholar]
- 13.Mahlamaki EH, Barlund M, Tanner M. Frequent amplification of 8q24, 11q, 17q, and 20q-specific genes in pancreatic cancer. Genes Chromosomes Cancer. 2002;35:353–358. doi: 10.1002/gcc.10122. [DOI] [PubMed] [Google Scholar]
- 14.Vance KW, Carreira S, Brosch G. Tbx2 is overexpressed and plays an important role in maintaining proliferation and suppression of senescence in melanomas. Cancer Res. 2005;65:2260–2268. doi: 10.1158/0008-5472.CAN-04-3045. [DOI] [PubMed] [Google Scholar]
- 15.Davis E, Teng H, Bilican B. Ectopic Tbx2 expression results in polyploidy and cisplatin resistance. Oncogene. 2008;27:976–984. doi: 10.1038/sj.onc.1210701. [DOI] [PubMed] [Google Scholar]
- 16.Renard CA, Labalette C, Armengol C. Tbx3 is a downstream target of the Wnt/b-catenin pathway and a critical mediator of b-catenin survival functions in liver cancer. Cancer Res. 2007;67:901–910. doi: 10.1158/0008-5472.CAN-06-2344. [DOI] [PubMed] [Google Scholar]
- 17.Bunz F, Dutriaux C, Lengauer T. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- 18.Andreassen PR, Lacroix FB, Lohez OD. Neither p21WAF1 nor 14-3-3r prevents G2 progression to mitotic catastrophe in human colon carcinoma cells after DNA damage, but p21WAF1 induces stable G1 arrest in resulting tetraploid cells. Cancer Res. 2001;61:7660–7668. [PubMed] [Google Scholar]
- 19.Chen J, Saha P, Kornbluth S. Cyclin-binding motifs are essential for the function of p21cip1. Mol Cell Biol. 1996;16:4673–4682. doi: 10.1128/mcb.16.9.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu X, Feng Y, Zhang D. A Functional Genomic Approach Identifies FAL1 as an Oncogenic Long Noncoding RNA that Associates with BMI1 and Represses p21 Expression in Cancer. Cancer Cell. 2014;26:344–357. doi: 10.1016/j.ccr.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prince S, Carreira S, Vance KW. Tbx2 directly represses the expression of the p21(WAF1) cyclin-dependent kinase inhibitor. Cancer Res. 2004;64:1669–1674. doi: 10.1158/0008-5472.can-03-3286. [DOI] [PubMed] [Google Scholar]
- 22.Hirvikoski P, Kellokoski JK, Kumpulainen EJ. Downregulation of p21/WAF1 is related to advanced and dedifferentiated laryngeal squamous cell carcinoma. J Clin Pathol. 1999;52:440–444. doi: 10.1136/jcp.52.6.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chien CY, Huang CC, Cheng JT. The clinicopathological significance of p53 and p21 expression in squamous cell carcinoma of hypopharyngeal cancer. Cancer Lett. 2003;201:217–223. doi: 10.1016/s0304-3835(03)00484-1. [DOI] [PubMed] [Google Scholar]
- 24.Zhang M, Li J, Wang L. Prognostic significance of p21, p27 and survivin protein expression in patients with oral squamous cell carcinoma. Oncol Lett. 2013;6:381–386. doi: 10.3892/ol.2013.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taghavi N, Biramijamal F, Sotoudeh M. Association of p53/p21 expression with cigarette smoking and prognosis in esophageal squamous cell carcinoma patients. World J Gastroenterol. 2010;16:4958–4967. doi: 10.3748/wjg.v16.i39.4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Z, Guo Y. High TBX2 expression predicts poor prognosis in non-small cell lung cancer. Neoplasma. 2014;61:476–480. [PubMed] [Google Scholar]
- 27.Han Y, Tu WW, Wen YG. Increased expression of TBX2 is a novel independent prognostic biomarker of a worse outcome in colorectal cancer patients after curative surgery and a potential therapeutic target. Med Oncol. 2013;30:688. doi: 10.1007/s12032-013-0688-3. [DOI] [PubMed] [Google Scholar]
- 28.Prince S, Carreira S, Vance KW. Tbx2 Directly Represses the Expression of the p21WAF1 Cyclin-Dependent Kinase Inhibitor. Cancer Res. 2004;64:1669–1674. doi: 10.1158/0008-5472.can-03-3286. [DOI] [PubMed] [Google Scholar]
- 29.Lüdtke TH, Farin HF, Rudat C. Tbx2 Controls Lung Growth by Direct Repression of the Cell Cycle Inhibitor Genes Cdkn1a and Cdkn1b. PLoS Genet. 2013;9:e1003189. doi: 10.1371/journal.pgen.1003189. [DOI] [PMC free article] [PubMed] [Google Scholar]