Abstract
In the present study, we investigated the roles of PDCD5 (programmed cell death 5) in multidrug re-sistance (MDR) of osteosarcoma cells and the possible lurking mechanisms. An adenovirus expression vector of PDCD5 was constructed and transfected into human adriamycin-resistant osteosarcoma cell line Saos-2/ADM. We found that up-regulation of PDCD5 could significantly enhance the sensitivity of Saos-2/ADM cells towards vincristine, methotrexate, cisplatin and arsenic trioxide (As2O3), and could decrease the capacity of cells to efflux adriamycin. PDCD5 could significantly down regulate the expression of P-glycoprotein (Pgp), but not affect the expression of multidrug resistance associated protein (MRP) or the glutathione S-transferase (GST). PDCD5 was also able to significantly increase the apoptotic activity of modified osteosarcoma cells. Further study of the biological functions of PDCD5 might be helpful in the understanding of the mechanisms of multidrug resistance (MDR) in osteosarcoma and exploring PDCD5 based adjuvant genetic therapy.
Keywords: Osteosarcoma, MDR, PDCD5, apoptosis
Introduction
Osteosarcoma is the most common primary malignant bone tumor, mainly occurring in children and adolescents. Despite current treatment strategies, including a combination of limb salvage surgery and neo-adjuvant chemotherapy, long-term disease-free survival rates are between 60% and 76% in patients with localized disease. The patients whose tumors respond poorly to chemotherapy are at a higher risk of relapse and adverse outcome [1,2]. The most challenging problem that orthopedics oncologists must deal with is multidrug resistance (MDR) induced by the classic chemotherapeutic agents, such as vincristine, methotrexate, adriamycin and cisplatin [1-4]. Previous reports revealed a diverse range of possible mechanisms of MDR, such as extrusion of drug by cell membrane pumps, increase of drug detoxification, DNA damage repair, redistribution of intracellular drug accumulation, modification of drug target molecules, suppression of drug-induced apoptosis, up-regulation of lipids and other biochemical changes [5-9]. However, the precise mechanisms of MDR have not been fully elucidated to this day.
PDCD5 (programmed cell death 5), earlier named TF-1 apoptosis-related gene 19 (TFAR19), is a novel apoptosis-related gene cloned as an increased expression gene during the apoptotic process of TF-1 cells induced by cytokine withdrawal using a cDNA-RDA method. The human PDCD5 gene encodes a protein expressed in tumor cells, which is translocated rapidly from the cytoplasm into the nuclei of cells during apoptosis. The appearance of PDCD5 in the nuclei of apoptotic cells precedes the externalization of phosphatidylserine and fragmentation of chromosomal DNA. And the nuclear translocation of PDCD5 is a universal early event of the apoptotic process, and may be a novel early marker for apoptosis [10]. Several pieces of evidence have suggested that the expression of PDCD5 protein is down-regulated in some human tumors, such as breast cancer [11], hepatocellular carcinoma [12] and gastric cancer [13].
In the previous study, we have firstly found that PDCD5 would facilitate the sensitivity of K562 cells to idarubicin in vitro and in vivo, suggesting that the PDCD5 might transfer high sensitivity to other anticancer drugs through apoptosis [14]. Here the potential roles of PDCD5 in Adriamycin resistant osteosarcoma cell line and the possible underlying mechanisms were further investigated. The results showed that PDCD5 might mediate Adriamycin resistance of osteosarcoma through regulation of Pgp and apoptosis.
Methods and materials
Cell lines and culture
The human osteosarcoma cell line Saos-2, was obtained fromMemorial Sloan-Kettering Cancer Center. Human Adriamycin-resistant osteosarcoma cell line Saos-2/ADM was selected in vitro by growing them in progressively increasing drug concentrations in the medium as described previously [15]. All the cells were routinely maintained in high-glucose Dulbecco’s Modified Eagle’s Minimum Essential Medium (DMEM) (Invitrogen Co., Carlsbad, CA) supplemented with 10% heat-inactivated fetal calf serum (Gibco) in 37°C humidified incubator with a mixture of 95% air and 5% CO2, fed every 3 days with complete medium, and subcultrued when confluence was reached.
Adenovirus infection
The construction and purification of Ad-null and Ad-PDCD5 (E1, E3 deleted, CMV promoter) were performed by the AGTC Gene Technology Company Ltd. (Beijing, China). For infections, cells were plated in DMEM supplemented with 10% heat-inactivated fetal calf serum and cultured until they achieved 70-80% confluence. Culture medium was then replaced with low-serum media (containing 0.5% FBS), and then infected 24 h later with 400 multiplicities of infection (MOI). After 36 h, cells were washed with PBS, and fresh standard medium was added for subsequently study.
RT-PCR
Total RNA was extracted from cells using TRIzol reagent (Invitrogen, Carlsbad, CA), following the manufacturer’s instructions. Reverse transcription was performed and cDNAs were amplified with the following primer pairs. PDCD5, forward: 5’-GTGATGCGGCCCAACAG-3’; reverse: 5’-ATCCAGAACTTGGGCTAAGATACTG-3’; and beta-actin, forward: 5’-AGCGGGAAATCGTGCGTG-3’; reverse: 5’-CAGGGTACATGGTGGTGCC-3’. All of the PCR products were analyzed on 2.0% agarose gels [16].
Western blotting
Cells were plated at 5 × 105 per well in six well plates. The following day, cells were treated with different anticancer drugs. After treatment, for whole-cell extracts, cells were washed with PBS and lysed in the culture dishes using lysis buffer (150 mM NaCl, 50 mM Tris-HCL, pH 7.4, 2 mM EDTA, 1% NP-40) containing protease inhibitors. Then the total cellular proteins were quantified by Bradford method. Equal amounts of total protein (50-100 μg per lane) were electrophoresed in 12% SDS-PAGE and blotted on a nitrocellulose membrane (0.45 μm, Millipore, USA) in 25 mM Tris-base, 190 mM glycine, and 20% methanol using a semi-dry blotter. Membranes were blocked with 8% fat-free milk powder at room temperature for 2 hours and incubated overnight with primary antibody at 4°C. After three washes for 15 minutes in PBS-Tween 20, the membrane was incubated with the horseradish peroxidase-conjugated goat anti-mouse IgG antibody for 1 hour at room temperature. The membrane was washed again in PBS-Tween 20; enhanced chemiluminescence (Amersham, NJ) was added and monitored for the development of color. The following antibodies were used: anti-Pgp, anti-MRP, anti-GST, anti-Bcl-2, anti-cleaved caspase-3, anti-PDCD5, and anti-beta-actin. All these antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). The density of the bands was assessed using the BioRad Multianalyst software (BioRad, Hercules, CA).
In vitro drug sensitivity assay
Different anticancer drugs were freshly prepared before each experiment. Drug sensitivity was assessed using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay [17]. Briefly, cells were diluted with the standard culture medium to the seeding density (1 × 104/well), suspended in 96-well plates (200 μl/well), and incubated at 37°C for 24 hours. Then, cells were incubated for 72 hours in the absence or presence of various concentrations of the anticancer agents in 200 μl medium. Each assay was performed according to the manufacturer’s instructions. All samples and standards were run in triplicate. After cells being cultured for 72 hours, 50 μl of 2 mg/ml MTT (Sigma) was added into each well, and cells were cultured for another 4 hours. Then, supernatants were discarded and 150 μl DMSO (Sigma) was added into each well to dissolve crystals. Absorbance was determined by spectrophotometry (Biohit, BP800, Finland) using a wavelength of 490 nm. Cell survival rates were calculated according to the formula: survival rate = (mean A490 of treated wells/mean A490 of untreated wells) × 100%. Finally, the IC50s of different anticancer drugs to the osteosarcoma cells were calculated.
Intracellular Adriamycin concentration analysis
Fluorescence intensity of intracellular Adriamycin was assessed by flow cytometry [18,19]. Briefly, osteosarcoma cells in log phase were trypsinized, resuspended and seeded into 6-well plates at a cell density of 1 × 106/well and cultured overnight at 37°C. Adriamycin was added to the medium to the final concentration of 5 μg/ml. The cells were incubated for 1 h, and then were trypsinized and harvested for the determination of Adriamycin accumulation, or alternatively cultured with fresh, drug-free medium for another 45 min for the determination of Adriamycin retention. The harvested cells were washed with ice-cold PBS, and intracellular Adriamycin was detected by flow cytometry using a flow cytometry device (FACS) Vantage flow cytometer (Becton Dickinson, San Diego, CA, USA) with an excitation wavelength of 488 nm and an emission wavelength of 575 nm. All samples and standards were run in triplicate.
DNA fragmentation assay
After incubation with 2 μg/ml of Adriamycin, Adriamycin resistant cells were collected and washed twice with cold PBS [20]. Cell pellets were resuspended in lysis buffer and incubated overnight at 50°C with continuously shaking. The nucleic acids were extracted with phenol-chloroform, precipitated with ethanol-sodium acetate, and redissolved in deionized water containing 100 mg/ml RNase A. After incubation in a water bath at 37°C for 30 minutes, the DNA samples were analyzed on 2.0% agarose gel containing 0.1 μg/ml ethidium bromide.
Annexin V staining
After incubation with 2 μg/ml of Adriamycin, Adriamycin resistant cells were washed twice with cold PBS and resuspended in 100 μl binding buffer at a concentration of 1 × 106/ml. Then, 5 μl Annexin V-FITC (BD Biosciences, USA) and 10 μl of 20 μg/ml propidium iodide (50 μg/mL, Sigma) were added to these cells. After incubation at room temperature for 15 minutes, 400 μl Annexin-binding buffer was added to each sample, and the samples were kept on ice for counting the stained cells by flow cytometry. Annexin V binds to those cells that express phosphatidylserine on the outer layer of the cell membrane, and propidium iodide stains the cellular DNA of those cells with a compromised cell membrane. The fluorescence intensity of individual cells was measured by a flow cytometer (Becton Dickinson, USA), at least 10,000 cells were counted and the data were analyzed using Becton Dickinson Cell Fit Software (BD, USA). Viable cells were negative for both PI and annexin V; apoptotic cells were positive for annexin V and negative for PI, whereas late apoptotic dead cells displayed both high annexin V and PI labeling Non-viable cells which underwent necrosis, were positive for PI and negative for annexin V. The apoptotic percentage of 10,000 cells was determined.
Statistical analysis
The data were expressed as means ± SD. We determined the changes of IC50s and Adriamycin content by One-way analysis of variance (ANOVA). The statistical package for the social sciences (SPSS) software (version 17 for Windows; SPSS Inc., Chicago, IL, US) was used to analyze the data. P < 0.05 was considered significant.
Results
The expression level of PDCD5 in Adriamycin resistant Saos-2 cells
Expression of PDCD5 was down-regulated in Adriamycin resistant osteosarcoma cells. We detected the expression of PDCD5 in Saos-2 and Saos-2/ADM by both RT-PCR and Western blotting, and observed down regulated expression of PDCD5 in Saos-2/ADM cells at both mRNA and protein levels (Figure 1).
Figure 1.
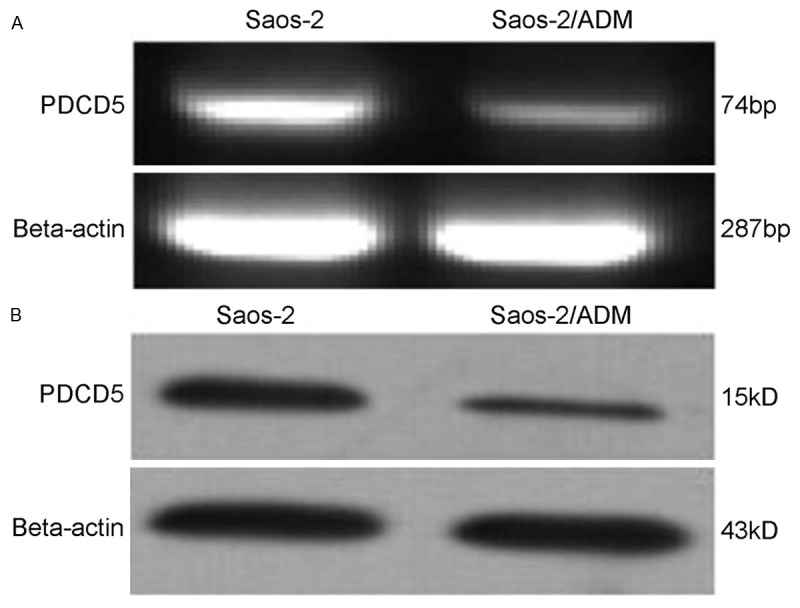
Expression of PDCD5 in Saos-2 osteosarcoma as detected by the RT-PCR (A) and Western blotting (B). PCR products were electrophoresed on a 2.0% agarose gel, with beta-actin as an internal control. For Western blotting, cell lysates were separated on a 12% SDS polyacrylamide gel, and the blot was probed with a specific monoclonal anti-PDCD5 antibody, with beta-actin as an internal standard to normalize loading protein.
In vitro drug sensitivity assay
To investigate whether PDCD5 is involved in the reversal of MDR in osteosarcoma cell line Saos-2, we attempted to upregulate PDCD5 by adenovirus transfection and determine the in vitro anticancer drug sensitivities of these cell lines. Western blotting showed that PDCD5 expression was increased in cells transfected with Ad-PDCD5 (Figure 2). Therefore, we used these transfected cells for the subsequent experiments. We compared the in vitro sensitivity of the transfectants and their respective control cells to four commonly used anticancer drugs. The results showed that Ad-PDCD5 transfected Saos-2 cells in which PDCD5 was over expressed had significantly lower IC50 for all the five drugs than Saos-2 cells transfected with Ad-null as a control (Table 1).
Figure 2.
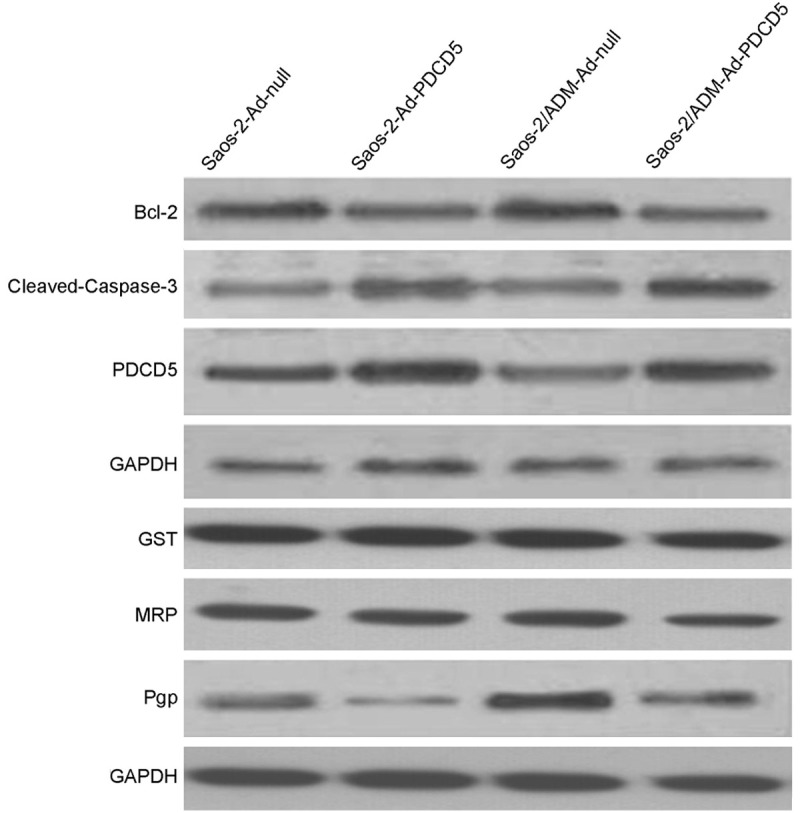
Western blotting analysis of Pgp, MRP, GST, Bcl-2, cleaved-caspase-3 and PDCD5 in osteosarcoma cells. GAPDH was used as an internal control.
Table 1.
IC50s (μg/L) of anticancer drugs for different osteosarcoma cells
| ADM | VCR | MTX | CDDP | As2O3 | |
|---|---|---|---|---|---|
| Saos-2 | 126.31 ± 21.67 | 7.45 ± 1.29 | 24.24 ± 4.58 | 121.46 ± 17.34 | 869.25 ± 196.45 |
| Saos-2-Ad-null | 131.58 ± 19.19 | 6.98 ± 1.36 | 25.65 ± 3.98 | 98.83 ± 15.98 | 850.39 ± 162.98 |
| Saos-2-Ad-PDCD5 | 85.74 ± 17.45a | 5.63 ± 0.95a | 16.27 ± 2.73a | 67.56 ± 12.72a | 571.18 ± 10.65a |
| Saos-2/ADM | 6946.47 ± 1189.58 | 373.96 ± 27.67 | 42.45 ± 13.01 | 176.17 ± 37.73 | 1128.42 ± 276.68 |
| Saos-2/ADM-Ad-null | 6754.76 ± 957.37 | 384.93 ± 41.84 | 41.28 ± 10.47 | 186.12 ± 12.98 | 1098.34 ± 194.34 |
| Saos-2/ADM-Ad-PDCD5 | 4638.14 ± 914.69b | 218.45 ± 46.87b | 26.22 ± 3.97b | 127.76 ± 14.61b | 673.98 ± 15.66b |
Note: survival rate of osteosarcoma cell to anticancer agents were evaluated by MTT assay as described in materials and methods. The IC50 values were determined by the dose-effect curves of different anticancer agents. Data are means ± SD of different independent experiment.
P < 0.05, compared with Saos-2-Ad-null;
P < 0.05, compared with Saos-2/ADM-Ad-null.
PDCD5 down regulated MDR1 expression
Adriamycin was fluorescent and this characteristic provided easy monitoring of its intracellular accumulation and retention by flow cytometric (FCM). As shown in Table 2, decreased accumulation of Adriamycin in PDCD5-overexpressed cells was observed as compared with that of the controls (P < 0.05). To study whether the alteration in ADM accumulation and retention is mediated by MDR-1 (Pgp), MRP1 or GST, the expression of these two molecules in the transfectants and their parental cell lines were detected by Western blotting. We observed that down expression of PDCD5 was accompanied by increased expression of MDR-1. Meanwhile, all these cell lines exhibited no obvious difference of MRP and GST expression as shown in Figure 2.
Table 2.
Adriamycin accumulation and retention in various cells
| Cell lines | Accumulations | Retention |
|---|---|---|
| Saos-2 | 61.5 ± 5.20 | 29.6 ± 3.17 |
| Saos-2-Ad-null | 59.6 ± 6.09 | 31.5 ± 2.96 |
| Saos-2-Ad-PDCD5 | 74.5 ± 6.92a | 36.8 ± 3.12a |
| Saos-2/ADM | 38.3 ± 3.51 | 20.4 ± 1.98 |
| Saos-2/ADM-Ad-null | 41.9 ± 5.52 | 21.6 ± 2.05 |
| Saos-2/ADM-Ad-PDCD5 | 67.4 ± 6.41b | 32.2 ± 3.86b |
Note: Adriamycin accumulation and retention were measured by flow cytometry, and intracellular Adriamycin concentration was represented by average fluorescence intensity. Values were represented as mean ± SD.
P < 0.05, compared with Saos-2-ad-null cells;
P < 0.05, compared with Saos-2/ADR-ad-null cells.
Effect of PDCD5 on apoptosis
Induction of apoptosis is an important mechanism of anticancer drugs. We investigated the capacity of Saos-2 cells to undergo PDCD5-induced apoptosis by DNA fragment and Annexin V staining. Treatment of Saos-2/ADM-Ad-null and Saos-2/ADM-Ad-PDCD5 cells with 2 μg/ml of ADM resulted in internucleosomal DNA fragmentation, evidenced by the formation of DNA ladders on agarose gels, a hallmark of cells undergoing apoptosis. No DNA ladders were detected in the sample from control cells. Similarly, results of Annexin V staining suggested that Adriamycin could induce Saos-2/ADM-Ad-PDCD5 cells apoptosis and the apoptotic rate of Saos-2/ADM-Ad-PDCD5 cells was significantly higher than that of Saos-2/ADM-Ad-null cells.
Effect of PDCD5 on proteins regulating apoptosis
To gain insight into the molecular mechanisms involved in PDCD5-mediated apoptosis, the expressions of Bcl-2 and Caspase-3 were assessed in Saos-2 cells treated with Adriamycin. As shown in Figure 2, the expression of Bcl-2 protein was decreased in response to Adriamycin. However, the level of cleaved Caspase-3 was increased after Adriamycin treatment.
Discussion
The programmed cell death5 (PDCD5) gene was first cloned from TF-1 cells in Laboratory of Medical Immunology of Peking University in 1999. It has been reported that PDCD5 is obviously upregulated in the process of apoptosis. In the process of cells undergoing apoptosis, PDCD5 protein translocates to the nucleus and accumulates in the nucleus. Then, it induces chromosome DNA fragmentation and phosphatidylserine (PS) externalization without other stimuli. In this process of apoptosis, some holds that the PDCD5 might bind to the DNA double strand and induce its break [10]. However, the biological function of PDCD5 is far from being completely clear.
Increased exclusion of intracellular drugs is one of the common and important mechanisms of MDR. Our study demonstrated that PDCD5 increased Adriamycin accumulation and retention in osteosarcoma cells. It has been demonstrated that Pgp, MRP-1 and GST are the most important drug transporters in mammalian cells [21-23]. In the present study, we showed that the expression of Pgp was downregulated by PDCD5, suggesting that downregulation of Pgp is likely responsible for the increase in drug accumulation and retention, and thus is one of the mechanisms by which PDCD5 reverses osteosarcoma MDR. However, it is noticed that in this study, PDCD5 reversed the resistance of Saos-2 cells not only against drugs that are specific substrates of Pgp such as ADM and VCR [24], but also against the anticancer drugs that are not adequate substrates of Pgp such as CDDP, MTX and As2O3 [25], suggesting that mechanisms other than the Pgp pathway exists.
One of the primary causes of chemotherapy failure in osteosarcoma is resistance to chemotherapeutic drugs. Attempts to improve chemotherapy outcomes by increasing drug concentration or by changing to another agent have only been partly successful, and often result in unacceptably severe side effects. Adriamycin, an anthracycline anticancer agent, is the primary chemotherapeutic agent to osteosarcoma. Adriamycin is an inhibitor of DNA topoisomerase II and is used as an anticancer drug that generates DNA double strand breaks resulting in DNA damage. It has been reported that MDR-1 might mediate the Adriamycin-related MDR osteosarcoma [26]. The Pgp, encoded by MDR1 gene, functions as an ATP-dependent drug efflux pump, in effect to remove Adriamycin out of the cell membrane to decrease the intracellular concentrations [24]. But the precise mechanism of resistance of Adriamycin is not completely elucidated and is the subject of future experimental work.
Attenuated apoptotic capability is an important potential mechanism of MDR. It is reported that the Adriamycin resistant lung cancer cells would processed a large number of changes in the proteomic changes contributed to a more apoptosis resistant state [26]. In this study, the difference of PDCD5 gene between the wild and Adriamycin resistant osteosarcoma cell line was demonstrated for the first time from the transcription and translation of mRNA to proteins. This difference may partially explain the biological behavior of the Adriamycin resistant osteosarcoma cells. The result suggested that reduced PDCD5 may contribute to the malignancy of the refractory osteosarcoma in clinical practice.
As is known to us, the PDCD5 is a novel cloned apoptosis related gene. And the exact mechanism by which PDCD5 regulates apoptosis remains to be elucidated. Our previous study has uncovered that the adenovirus-PDCD5 would facilitate the sensitivity of K562 cells to idarubicin in vitro and in vivo, suggesting that the PDCD5 might transfer high sensitivity to other anticancer drugs through apoptosis [14]. In this present study, we detected that the PDCD5 gene could partly reverse the Adriamycin resistance of osteosarcoma cells. The investigated bcl-2 was found to be down-regulated by PDCD5, and the cleaved caspase-3 was up-regulated by PDCD5. We proposed that the PDCD5 might reverse the Adriamycin resistance by exerting its apoptosis-accelerating effects through the mitochondrial activation pathway. Whether other apoptosis related molecules was involved in the reversal of osteosarcoma’s Adriamycin resistance by PDCD5 is left for further investigation [27,28].
It must be emphasized that this study was performed using only Saos-2 cells, its relevance to osteosarcoma cells and its clinical implications need to be further investigated.
In conclusion, PDCD5 is involved in the reversal of osteosarcoma MDR. Modulation of Pgp expression is most likely one of the underlying mechanisms. How PDCD5 regulates MDR-1 and other possible mechanism and molecules that are involved in PDCD5 reversed MDR remain to be further elucidated.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 81202124 and 81301727).
Disclosure of conflict of interest
None.
References
- 1.Meyers PA, Heller G, Healey J, Huvos A, Lane J, Marcove R, Applewhite A, Vlamis V, Rosen G. Chemotherapy for nonmetastatic osteogenic sarcoma: the Memorial Sloan-Kettering experience. J. Clin. Oncol. 1992;10:5–15. doi: 10.1200/JCO.1992.10.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Meyers PA, Gorlick R, Heller G, Casper E, Lane J, Huvos AG, Healey JH. Intensification of preoperative chemotherapy for osteogenic sarcoma: results of the Memorial Sloan-Kettering (T12) protocol. J. Clin. Oncol. 1998;16:2452–2458. doi: 10.1200/JCO.1998.16.7.2452. [DOI] [PubMed] [Google Scholar]
- 3.Jaffe N, Frei E 3rd, Watts H, Traggis D. High-dose methotrexate in osteogenic sarcoma: a 5-year experience. Cancer Treat Rep. 1978;62:259–264. [PubMed] [Google Scholar]
- 4.Saeter G, Alvegard TA, Elomaa I, Stenwig AE, Holmstrom T, Solheim OP. Treatment of osteosarcoma of the extremities with the T-10 protocol, with emphasis on the effects of preoperative chemotherapy with single-agent high-dose methotrexate: a Scandinavian Sarcoma Group study. J. Clin. Oncol. 1991;9:1766–1775. doi: 10.1200/JCO.1991.9.10.1766. [DOI] [PubMed] [Google Scholar]
- 5.Lage H, Perlitz C, Abele R, Tampe R, Dietel M, Schadendorf D, Sinha P. Enhanced expression of human ABC-transporter tap is associated with cellular resistance to mitoxantrone. FEBS Lett. 2001;503:179–184. doi: 10.1016/s0014-5793(01)02722-3. [DOI] [PubMed] [Google Scholar]
- 6.Litman T, Druley TE, Stein WD, Bates SE. From MDR to MXR: new understanding of multidrug resistance systems, their properties and clinical significance. Cell Mol Life Sci. 2001;58:931–959. doi: 10.1007/PL00000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu RH, Pelicano H, Zhou Y, Carew JS, Feng L, Bhalla KN, Keating MJ, Huang P. Inhibition of glycolysis in cancer cells: a novel strategy to overcome drug resistance associated with mitochondrial respiratory defect and hypoxia. Cancer Res. 2005;65:613–621. [PubMed] [Google Scholar]
- 8.Zhang M, Latham DE, Delaney MA, Chakravarti A. Survivin mediates resistance to antiandrogen therapy in prostate cancer. Oncogene. 2005;24:2474–2482. doi: 10.1038/sj.onc.1208490. [DOI] [PubMed] [Google Scholar]
- 9.Wang LC, Okitsu CY, Zandi E. Tumor necrosis factor alpha-dependent drug resistance to purine and pyrimidine analogues in human colon tumor cells mediated through IKK. J Biol Chem. 2005;280:7634–7644. doi: 10.1074/jbc.M413384200. [DOI] [PubMed] [Google Scholar]
- 10.Liu H, Wang Y, Zhang Y, Song Q, Di C, Chen G, Tang J, Ma D. TFAR19, a novel apoptosis-related gene cloned from human leukemia cell line TF-1, could enhance apoptosis of some tumor cells induced by growth factor withdrawal. Biochem Biophys Res Commun. 1999;254:203–210. doi: 10.1006/bbrc.1998.9893. [DOI] [PubMed] [Google Scholar]
- 11.Hedenfalk I, Duggan D, Chen Y, Radmacher M, Bittner M, Simon R, Meltzer P, Gusterson B, Esteller M, Kallioniemi OP, Wilfond B, Borg A, Trent J, Raffeld M, Yakhini Z, Ben-Dor A, Dougherty E, Kononen J, Bubendorf L, Fehrle W, Pittaluga S, Gruvberger S, Loman N, Johannsson O, Olsson H, Sauter G. Gene-expression profiles in hereditary breast cancer. N Engl J Med. 2001;344:539–548. doi: 10.1056/NEJM200102223440801. [DOI] [PubMed] [Google Scholar]
- 12.Xu XR, Huang J, Xu ZG, Qian BZ, Zhu ZD, Yan Q, Cai T, Zhang X, Xiao HS, Qu J, Liu F, Huang QH, Cheng ZH, Li NG, Du JJ, Hu W, Shen KT, Lu G, Fu G, Zhong M, Xu SH, Gu WY, Huang W, Zhao XT, Hu GX, Gu JR, Chen Z, Han ZG. Insight into hepatocellular carcinogenesis at transcriptome level by comparing gene expression profiles of hepatocellular carcinoma with those of corresponding noncancerous liver. Proc Natl Acad Sci U S A. 2001;98:15089–15094. doi: 10.1073/pnas.241522398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang YH, Zhao M, Li WM, Lu YY, Chen YY, Kang B, Lu YY. Expression of programmed cell death 5 gene involves in regulation of apoptosis in gastric tumor cells. Apoptosis. 2006;11:993–1001. doi: 10.1007/s10495-006-6714-6. [DOI] [PubMed] [Google Scholar]
- 14.Ruan GR, Chen SS, Chang Y, Li JL, Qin YZ, Li LD, Hao L, Fu JY, Liu YR, Huang XJ. [Adenovirus-mediated PDCD5 gene transfer sensitizes apoptosis of K562 cells induced by etoposide] . Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2007;15:936–940. [PubMed] [Google Scholar]
- 15.Zhao H, Guo W, Peng C, Ji T, Lu X. Arsenic trioxide inhibits the growth of adriamycin resistant osteosarcoma cells through inducing apoptosis. Mol Biol Rep. 2010;37:2509–2515. doi: 10.1007/s11033-009-9765-2. [DOI] [PubMed] [Google Scholar]
- 16.Wang N, Lu HS, Guan ZP, Sun TZ, Chen YY, Ruan GR, Chen ZK, Jiang J, Bai CJ. Involvement of PDCD5 in the regulation of apoptosis in fibroblast-like synoviocytes of rheumatoid arthritis. Apoptosis. 2007;12:1433–1441. doi: 10.1007/s10495-007-0070-z. [DOI] [PubMed] [Google Scholar]
- 17.Li X, Guo W, Shen DH, Yang RL, Liu J, Zhao H. [Expressions of ERCC2 and ERCC4 genes in osteosarcoma and peripheral blood lymphocytes and their clinical significance] . Beijing Da Xue Xue Bao. 2007;39:467–471. [PubMed] [Google Scholar]
- 18.Durand RE, Olive PL. Flow cytometry studies of intracellular adriamycin in single cells in vitro. Cancer Res. 1981;41:3489–3494. [PubMed] [Google Scholar]
- 19.Zhang K, Wang X, Wang H. Effect and mechanism of Src tyrosine kinase inhibitor sunitinib on the drug-resistance reversal of human A549/DDP cisplatin-resistant lung cancer cell line. Mol Med Rep. 2014;10:2065–2072. doi: 10.3892/mmr.2014.2440. [DOI] [PubMed] [Google Scholar]
- 20.Raile K, Hille R, Laue S, Schulz A, Pfeifer G, Horn F, Kiess W. Insulin-like growth factor I (IGF-I) stimulates proliferation but also increases caspase-3 activity, Annexin-V binding, and DNA-fragmentation in human MG63 osteosarcoma cells: co-activation of pro- and anti-apoptotic pathways by IGF-I. Horm Metab Res. 2003;35:786–793. doi: 10.1055/s-2004-814140. [DOI] [PubMed] [Google Scholar]
- 21.Pakos EE, Ioannidis JP. The association of P-glycoprotein with response to chemotherapy and clinical outcome in patients with osteosarcoma. A meta-analysis. Cancer. 2003;98:581–589. doi: 10.1002/cncr.11546. [DOI] [PubMed] [Google Scholar]
- 22.van Triest B, Pinedo HM, Telleman F, van der Wilt CL, Jansen G, Peters GJ. Cross-resistance to antifolates in multidrug resistant cell lines with P-glycoprotein or multidrug resistance protein expression. Biochem Pharmacol. 1997;53:1855–1866. doi: 10.1016/s0006-2952(97)82448-3. [DOI] [PubMed] [Google Scholar]
- 23.Yan F, Jiang Y, Li YM, Zhen X, Cen J, Fang WR. Reversal of P-glycoprotein and multidrug resistance-associated protein 1 mediated multidrug resistance in cancer cells by HZ08 Isomers, tetrataisohydroquinolin derivatives. Biol Pharm Bull. 2008;31:1258–1264. doi: 10.1248/bpb.31.1258. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Xia Q, Cui J, Diao Y, Li J. Reversion of P-glycoprotein-mediated multidrug resistance by diallyl trisulfide in a human osteosarcoma cell line. Oncol Rep. 2014;31:2720–2726. doi: 10.3892/or.2014.3154. [DOI] [PubMed] [Google Scholar]
- 25.Tsai HC, Huang CY, Su HL, Tang CH. CCN2 enhances resistance to cisplatin-mediating cell apoptosis in human osteosarcoma. PLoS One. 2014;9:e90159. doi: 10.1371/journal.pone.0090159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang LH, Yang JY, Yang SN, Li Y, Ping GF, Hou Y, Cui W, Wang ZZ, Xiao W, Wu CF. Suppression of NF-kappaB signaling and P-glycoprotein function by gambogic acid synergistically potentiates adriamycin -induced apoptosis in lung cancer. Curr Cancer Drug Targets. 2014;14:91–103. doi: 10.2174/1568009613666131113100634. [DOI] [PubMed] [Google Scholar]
- 27.Igney FH, Krammer PH. Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer. 2002;2:277–288. doi: 10.1038/nrc776. [DOI] [PubMed] [Google Scholar]
- 28.Weber K, Harper N, Schwabe J, Cohen GM. BIM-mediated membrane insertion of the BAK pore domain is an essential requirement for apoptosis. Cell Rep. 2013;5:409–420. doi: 10.1016/j.celrep.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]


