Abstract
Aims: To ascertain the protective role of calcitriol in the development of diabetic nephropathy and unravel the mechanism of the protective effects. Methods: In this prospective study, 69 patients were screened for type 1 diabetes, and 31 patients with type 1 diabetes were enrolled. Among these 31 patients, 24 patients had insufficient or deficient levels of serum vitamin D and 21 patients complied with calcitriol and were followed up. At baseline, these 21 patients who suffered from vitamin D deficiency or insufficiency displayed elevated inflammation markers and urinary albumin excretion in contrast with patients with sufficient vitamin D. Simultaneously, serum 25(OH)D3 level was negatively associated with serum and urinary inflammation markers, such as TNF-α, IL-6, and ICAM-1. Six months later, even though glycol-metabolism was not alleviated, all the serum and urinary inflammation markers decreased significantly. Meanwhile, proteinuria declined with inflammation markers. Results: Calcitriol supplementation alleviated inflammation and proteinuria in patients with type 1 diabetes. Conclusions: Calcitriol might delay the development of diabetic nephropathy through suppressing inflammation.
Keywords: Vitamin D, diabetic nephropathy, inflammation
Introduction
Diabetic nephropathy is one of the most common complications that often lead to end-stage kidney disease [1]. Numerous evidences from in vitro experiments, pathological examinations and epidemiological studies, have shown that inflammation is a cardinal pathogenic mechanism in diabetic nephropathy [2].
Vitamin D is a secosteroid that is generated from 7-dehydrocholesterol in skin under the involvement of ultraviolet light which has been traditionally considered as a key regulator of bone metabolism, calcium and phosphorous homeostasis. However, apart from these traditional calcium-related actions, 1,25-dihydroxyvitamin D3 [1,25(OH)2D3], the physiologically active form of vitamin D, and its synthetic analogs are being increasingly recognized for their potent antiproliferative [3], stimulating cell differentiation [4], immunomodulatory [5], and anti-inflammation activitiesits [6]. Accumulated evidence demonstrated that vitamin D might inhibit inflammatory through inhibition of prostaglandin synthesis and actions, inhibition of stress-activated kinase signaling and resultant production of inflammatory cytokines, and inhibition of nuclear factor κB (NF-κB) signaling and production of pro-angiogenic factors [6].
There were abundant investigations showing that vitamin D deficiency was prevalent in patients with diabetes [7,8]. Since vitamin D plays as an anti-inflammatory factor and inflammation contribute to development of diabetic nephropathy. We, therefore, speculated that vitamin D deficiency played an important role in the development of diabetic nephropathy and supplementation vitamin D might ameliorate the development of diabetic nephropathy by regulating inflammation.
In the present study, we therefore investigated the association between vitamin D and inflammatory factors in Type 1 diabetes, and explored effects of supplementation of calcitriol, active form of vitamin D, on the inflammation and proteinuria due to diabetic nephropathy in patients with type 1 diabetes.
Materials and methods
Participants and investigations
Patients with type 1 diabetes suffered from microalbuminuria recruited from Huai’an First People’s Hospital, Nanjing Medical University were screened (from Dec 2009 to Dec 2011) in this study. Exclusion criteria were hyperparathyroidism, hypercalcemia, nephrolithiasis, chronic kidney disease, malignancy, and taking supplements containing vitamin D or calcium in the past eight weeks and conditions that might affect vitamin D or calcium metabolism (e.g., sarcoidosis). Thirty-one patients in line with the above criteria were enrolled. In addition, thirty healthy people were enrolled as controls. This study was approved by the ethics committee of Huai’an First People’s Hospital (Nanjing Medical University, Jiangsu, China). Written informed consents were obtained from each participant.
Plasma levels of calcium, phosphate, alkaline phosphatase, parathyroid hormone (PTH), and creatinine of participants with and without diabetes were within the normal ranges baseline.
Patients with type 1 diabetes were divided into two groups according to serum 25-hydroxyvitamin D3 [25(OH)D3] level. Patients had deficient or insufficient 25(OH)D3 were supplied with calcitriol (the active form of vitamin D, Roche Group) 0.25 μg/daily; patients with sufficient 25(OH)D3 were served as controls. All the patients therefore were followed up for 6 months (Figure 1). Fasting C-peptide, glycosylated hemoglobin (HbA1C), serum calcium, serum phosphorus, parathyroid hormone and 25(OH)D3 levels, fasting serum C-reactive protein (CRP), Tumor necrosis factor alpha (TNF-α and Interleukin 6 (IL-6), urinary albumin concentration, urinary TGF-1 and MCP-1 in 24 h urine were assessed twice at baseline and endpoint respectively. Stimulated C-peptide meal test was performed at baseline and at 6 months with blood samples drawn at 0 and 30, 60, and 120 min. C-peptide was evaluated by chemiluminescence on an ROCHE E170 and serum 25(OH)D3 were detected by radioimmunoassay on StatFax-2100.
Figure 1.
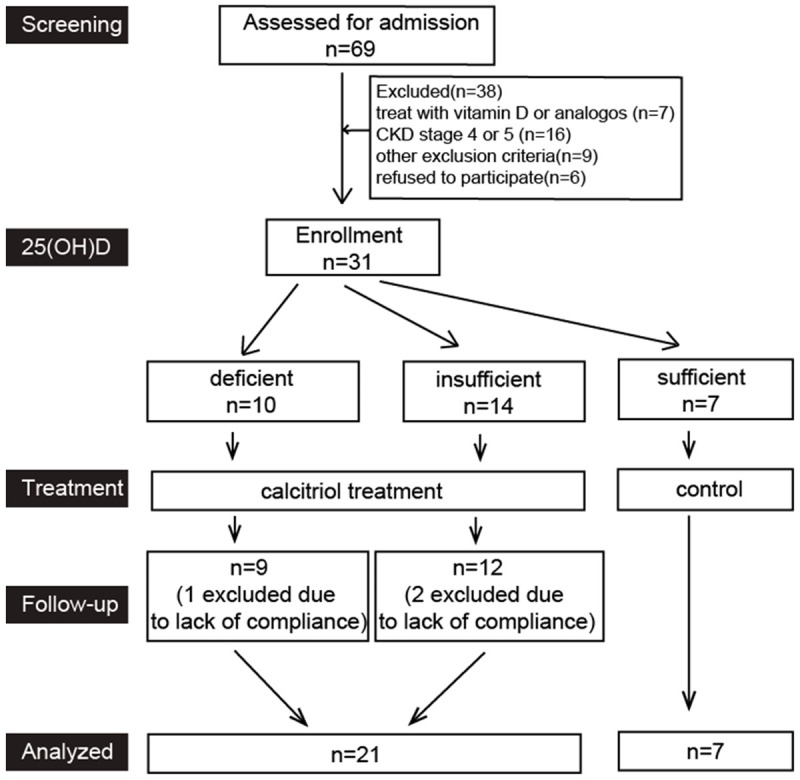
Flow chat of this study.
In this study, patients continued their intensive insulin therapy. Prescriptions of irbesartan or enalapril were given to all the patients with proteinuria. Serum 25(OH)D3 was defined as follow: deficiency (< 20 ng/dl), insufficiency (20-30 ng/dl) and sufficiency (> 30 ng/dl).
Enzyme-linked immunosorbent assay
Serum levels of IL-6, Interleukin-10 (IL-10) and TNF-α were measured using enzyme-linked immunosorbent assay (ELISA) kits following the manufacturer’s instructions (Invitrogen, US). All samples were measured in duplicates.
Statistical analysis
Data are presented as the mean ± SD in clinical data and mean ± SEM in experimental animal data. Statistical significance was assessed using an unpaired t-test, correlation and one-way ANOVA. Differences with P < 0.05 were regarded as significant. All statistical analyses were performed using Stata 11.0 software.
Results
25(OH)D3 and inflammatory cytokines in type 1 diabetes
Patients with type 1 diabetes showed a lower 25(OH)D3 level compared with healthy controls. Accompany with lower 25(OH)D3 level, type 1 diabetes patients displayed a higher serum CRP, TNF-α and IL-6 levels. And, there were no differences of serum calcium and phosphorous concentration between healthy controls and patients with type 1 diabetes (Table 1). Additionally, serum vitamin D level was negatively related to serum (high sensitive) hsCRP, TNF-α and IL-6 levels (Figure 2A). Urinary MCP-1, TGF-1, as well as urinary albumin excretion, were slightly higher in patients with insufficient or deficient serum levels of 25(OH)D3 than in patients with sufficient 25(OH)D3 (Table 2).
Table 1.
Serum level of 25(OH)D3 and inflammatory cytokines in patients with type 1 diabetes
| Patients with diabetes | Healthy controls | |
|---|---|---|
| Age | 24.03 ± 5.53 | 25 ± 2.21 |
| Sex (M/F) | 15/16 | 14/16 |
| HbA1C (%) | 7.98 ± 0.57* | 5.11 ± 0.27 |
| 25(OH)D3 (ng/ml) | 25.08 ± 8.94* | 32.7 ± 4.94 |
| PTH (pg/ml) | 43.81 ± 6.0* | 41.3 ± 6.25 |
| Calcium (mmol/l) | 2.16 ± 0.88 | 2.29 ± 0.06 |
| Phosphorus (mmol/l) | 1.64 ± 0.25 | 1.63 ± 0.26 |
| CRP (mg/L) | 1.37 ± 0.32* | 0.46 ± 0.16 |
| TNF-α (pg/ml) | 58.4 ± 10.27* | 30.45 ± 4.76 |
| IL-6 (pg/ml) | 45.16 ± 7.3* | 27.73 ± 4.55 |
P < 0.05 compared to healthy controls.
Figure 2.
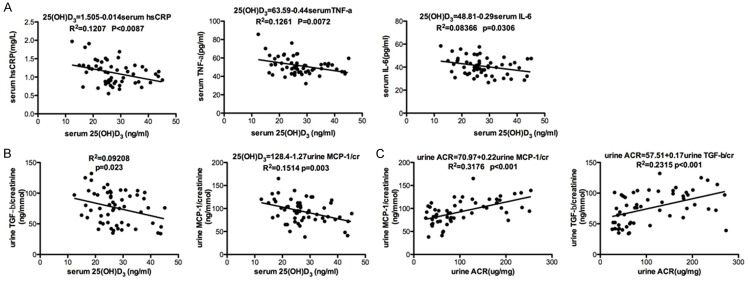
Relations of serum 25(OH)D3 level with serum hsCRP, serum TNF-α, serum IL-6 (A), unrin TGF-b and unrin MCP-1 (B), unrine ACR with MCP-1 and TGF-b (C).
Table 2.
Effects of calcitriol on patients with type 1 diabetes
| Calcitriol group | Control group | |||
|---|---|---|---|---|
|
|
|
|||
| Baseline | Month 6 | Baseline | Month 6 | |
| Age (y) | 23.95 ± 5.21 | 24.86 ± 7.01 | ||
| Sex (male/female) | 11/10 | 4/3 | ||
| Duration (y) | 9.81 ± 3.25 | 7.86 ± 4.26 | ||
| ACEI/ARB | 6/15 | 2/5 | ||
| SBP (mmHg) | 107.1 ± 10.63 | 104.71 ± 9.83* | 105.71 ± 10.23 | 104.57 ± 6.8 |
| DBP (mmHg) | 68.9 ± 6.07 | 67.14 ± 5.85* | 67.14 ± 5.61 | 66.86 ± 2.97 |
| HbA1C (%) | 7.88 ± 0.58 | 7.1 ± 0.5 | 7.71 ± 0.69 | 7.31 ± 0.4 |
| AUCC-peptide (nmol/L × 120 min) | 35.94 ± 12.3 | 33.2 ± 9.6 | 34.67 ± 7.27 | 31.05 ± 6.6 |
| Daily insulin dose (u) | 24.19 ± 5.53 | 24.57 ± 6.32 | 26.57 ± 3.6 | 26.86 ± 4.74 |
| 25(OH)D3 (ng/ml) | 20.61 ± 4.29 | 27.16 ± 2.9* | 38.48 ± 4.4* | 37.16 ± 5.88 |
| PTH (pg/ml) | 42.82 ± 1.3 | 41.68 ± 0.98 | 39.21 ± 1.67 | 40.8 ± 1.17 |
| Calcium (mmol/l) | 2.17 ± 0.1 | 2.24 ± 0.11* | 2.28 ± 0.08* | 2.25 ± 0.08 |
| Phosphorus (mmol/l) | 1.22 ± 0.2 | 1.21 ± 0.18 | 1.28 ± 0.27 | 1.23 ± 0.11 |
| hsCRP (mg/L) | 1.34 ± 0.34 | 0.94 ± 0.22* | 1.03 ± 0.19* | 1.05 ± 0.18 |
| TNF-a (pg/ml) | 57.7 ± 10.7 | 47.09 ± 7.73* | 48.84 ± 5.03* | 50.07 ± 4.8 |
| IL-6 (pg/ml) | 44.04 ± 7.29 | 39.88 ± 7.86* | 37.3 ± 6.99* | 38.14 ± 8.01 |
| Urine MCP-1/creatinine (ng/mmol) | 99.38 ± 25.29 | 89.57 ± 21.46* | 89.43 ± 30.21 | 87.43 ± 22.59 |
| Urine TGF-b/creatinine (ng/mmol) | 79 ± 28.89 | 72.33 ± 25.61* | 74.43 ± 27.23 | 76.14 ± 25.47 |
| Urinary albumin excretion (μg/mg) | 127.05 ± 84.79 | 104.81 ± 74.05* | 101.96 ± 59.38 | 102.7 ± 59.8 |
P < 0.05 compared to baseline data of patients with calcitriol.
Effects of calcitriol on patients with type 1 diabetes
Patients with vitamin D insufficiency and deficiency exhibited higher inflammatory factors, such as serum hsCRP, TNF-α and IL-6, compared with patients with sufficient vitamin D. Six months later, serum calcium and 25(OH)D3 level were alleviated significantly with calcitriol supplement in patients originally with vitamin D insufficiency and deficiency, however the serum PTH and serum phosphorous did not change. Simultaneously, calcitriol supplement did not change fasting blood glucose, HbA1c, AUCC-peptide, daily insulin consumption. Interestingly, calcitriol treatment did not alter glucose metabolism, however calcitriol reduced blood pressure and rescued serum calcium. Along with decreased inflammation markers, proteinuria level in patients with low vitamin D declined significantly six months later (Table 2).
Discussion
Vitamin D3 (cholecalciferol), well accepted as pleiotropic steroid hormone, acts principal physiological role of regulation of calcium and phosphorus homeostasis and bone mineralization [9]. In humans, Vitamin D3 is mainly generated in the skin in response to sun exposure [10]; then, vitamin D3 is transported to liver, where it is converted to circulating form of vitamin D, 25(OH)D3, by 25-hydroxylases; finally, 25(OH)D3 is transported to the kidney and converted to the most active form, 1,25(OH)2D3, by the 1-hydroxylase [11].
Numerous evidences show that vitamin D insufficiency is common in diabetes patients and associated with diabetes [7,8]. In this study, patients with diabetes exhibited a lower serum 25(OH)D3 level by contrast with the healthy controls, which is accordance with what Evaraj et al. reported [12] in 2011. Individuals with vitamin D insufficiency are prone to develop into diabetes because vitamin D insufficiency worsen insulitis [8], impairing insulin secretion and inducing insulin resistance [13]. On the other hand, supplementation of 1,25(OH)2D3 or its analogues reduced incidence of diabetes and ameliorated symptoms of diabetes in both human and experimental animals [14,15], even though supplement of calcitriol is ineffective in preserving residual b-cell functions in recent-onset type 1 diabetes [16,17].
Apart from protecting against the pathogenesis of diabetes, vitamin D is well considered as an anti-inflammation factor [18-21]. Vitamin D deficiency is related with inflammation-linked vascular endothelial dysfunction [22]. Furthermore, vitamin D supplementation is considered to alleviate vascular endothelial cells by acting anti-inflammation role [18]. Additionally, vitamin D can reduce the susceptibility to gingival inflammation through its anti-inflammatory effects [23]. In the present study, in accordance with previous reports, vitamin D level was negatively related with serum CRP, TNF-α and IL-6 levels and urinal inflammation factors in patients with type 1 diabetes. This indicated that vitamin D deficiency might aggravate inflammatory state in patients with type 1 diabetes.
It is well accepted that diabetes mellitus is a proinflammatory state characterized by in-creased circulating biomarkers of inflammation, such as hsCRP, SVCAM and SICAM [24,25]. Furthermore, accumulated evidence indicated that inflammation has a crucial role in micro- and macro-vascular complications in diabetes [24], including diabetic nephropathy. Accumulated evidences indicated that diabetic nephropathy, contributing to excess morbidity and mortality in patients with diabetes, was thought prominently be triggered by inflammation in the pathogenesis of diabetic kidney disease [26,27]. Furthermore Sugimoto et al. demonstrated that synthesis of proinflammatory cytokines increased in several types of renal cells (glomerular, endothelial, tubular and mesangial cells), monocytes, macrophages and T cells [28], and these cytokines are important in the pathogenesis of diabetic nephropathy [29,30]. In this study, patients with type 1 diabetes displayed an increased circulating CRP, TNF-α and IL-6 level and urinary MCP-1 and TGF-β; Since proinflammatory cytokines, especially IL-6 and TNF-α are considered to have key roles in the pathogenesis of diabetic nephropathy [29,30], we therefore determined the proteinuria, a marker of diabetic nephropathy, in patients with type 1 diabetes. With increased inflammatory factors levels, patients with type 1 diabetes exhibited enhanced urinary micro-albumin levels compared to the healthy controls. We also found that microalbuminuria level was compatible with secretion of urine TGF- and MCP-1. These data implicated that diabetes was associated with inflammatory state in kidney; moreover the inflammatory state was related with the early stage of diabetic nephropathy.
We supplied calcitriol to patients with type 1 diabetes who had vitamin D insufficiency or deficiency. Serum level of vitamin D was negatively associated with inflammation, and meanwhile microalbuminuria was aggravated by inflammation. We evaluated whether calcitriol supplement rescue microalbuminuria in these patients. In the present study, proteinuria was alleviated with calcitriol supplementation in patients with Type 1 diabetes. Furthermore, in the present study, patients with calcitriol supplementation showed an apparent decrease of inflammatory factors, such as CRP, TNF-α and IL-6 in serum, and TGF-, MCP-1 in urine, although the level of inflammatory factors in these patients did not rescued to the normal level. It is indicated that calcitriol played a role of anti-inflammatory in type 1 diabetes as well in cancers patients [31] and in human monocytes [32]. Interestingly, in the present study, proteinuria level of type 1 diabetes patients supplemented with calcitriol, accompany with serum cytokines and urine cytokines, declined notably compared with those with sufficient vitamin D. This implicated that vitamin D alleviated proteinuria might through suppressing inflammation. In addition, calcitriol supplementation did not alleviate hyperglycemia and β-cell functions. These indicated that calcitriol might delay the progress of diabetic nephropathy through anti-inflammation effects rather than ameliorating glycol-metabolism.
In the present study, patients with vitamin D insufficiency or deficiency exhibited lower serum calcium compared with patients with sufficient vitamin D, although the serum calcium level was still in normal range. With calcitriol supplement, the serum calcium was rescued to the similar concentration to patients with sufficient vitamin D. As we known, besides the direct effects of vitamin D on glycol-metabolism, vitamin D regulates glucose metabolism indirectly through mediating calcium. Here we did not find difference in HbA1c and insulin doze between patients with vitamin D insufficiency or deficiency and patients with vitamin D sufficiency. Even when the serum calcium of those patients was rescued to the level of patients with vitamin D sufficiency, the HbA1c of the patients with deficient or insufficient vitamin D was not altered. There was no significant alleviation in glycol-metabolism might due to inadequate sample volume.
There were some shortcomings in the present study. We did not explore which signaling pathway calcitriol played the role of anti-inflammation. One more disadvantage was that we only supplemented calcitriol to patients with scarcity of vitamin D, therefore we could not know whether there is protective effect of calcitriol on patients with sufficient vitamin D.
Even through there are some shortcomings in the present study, we still could conclude that vitamin D attenuates proteinuria resulted from diabetic nephropathy might through suppressing the secretion of inflammation cytokines. The present study suggests that it is necessary and vital for supplementation vitamin D in patients with type 1 diabetes for retarding the development of diabetic nephropathy, because vitamin D supplementation plays a protective role in development of diabetic nephropathy despite there are no benefit to islet β-cells.
Acknowledgements
This study was supported by a grant for innovation person from the Huai’an First Hospital to Li Mao.
Disclosure of conflict of interest
None.
References
- 1.Ritz E, Rychlik I, Locatelli F, Halimi S. End-stage renal failure in type 2 diabetes: A medical catastrophe of worldwide dimensions. Am J Kidney Dis. 1999;34:795–808. doi: 10.1016/S0272-6386(99)70035-1. [DOI] [PubMed] [Google Scholar]
- 2.Navarro-Gonzalez JF, Mora-Fernandez C, Muros de Fuentes M, Garcia-Perez J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nature reviews. Nephrology. 2011;7:327–340. doi: 10.1038/nrneph.2011.51. [DOI] [PubMed] [Google Scholar]
- 3.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7:684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- 4.Bikle D. Nonclassic actions of vitamin D. J Clin Endocrinol Metab. 2009;94:26–34. doi: 10.1210/jc.2008-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Correale J, Ysrraelit MC, Gaitan MI. Immunomodulatory effects of Vitamin D in multiple sclerosis. Brain. 2009;132:1146–1160. doi: 10.1093/brain/awp033. [DOI] [PubMed] [Google Scholar]
- 6.Krishnan AV, Feldman D. Mechanisms of the anti-cancer and anti-inflammatory actions of vitamin D. Annu Rev Pharmacol Toxicol. 2011;51:311–336. doi: 10.1146/annurev-pharmtox-010510-100611. [DOI] [PubMed] [Google Scholar]
- 7.Karvonen M, Jantti V, Muntoni S, Stabilini M, Stabilini L, Tuomilehto J. Comparison of the seasonal pattern in the clinical onset of IDDM in Finland and Sardinia. Diabetes Care. 1998;21:1101–1109. doi: 10.2337/diacare.21.7.1101. [DOI] [PubMed] [Google Scholar]
- 8.Mathieu C, Gysemans C, Giulietti A, Bouillon R. Vitamin D and diabetes. Diabetologia. 2005;48:1247–1257. doi: 10.1007/s00125-005-1802-7. [DOI] [PubMed] [Google Scholar]
- 9.Holick MF. Vitamin D and bone health. J Nutr. 1996;126:1159S–1164S. doi: 10.1093/jn/126.suppl_4.1159S. [DOI] [PubMed] [Google Scholar]
- 10.MacLaughlin JA, Anderson RR, Holick MF. Spectral character of sunlight modulates photosynthesis of previtamin D3 and its photoisomers in human skin. Science. 1982;216:1001–1003. doi: 10.1126/science.6281884. [DOI] [PubMed] [Google Scholar]
- 11.Haddad JG, Matsuoka LY, Hollis BW, Hu YZ, Wortsman J. Human plasma transport of vitamin D after its endogenous synthesis. J Clin Invest. 1993;91:2552–2555. doi: 10.1172/JCI116492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devaraj S, Yun JM, Duncan-Staley CR, Jialal I. Low vitamin D levels correlate with the proinflammatory state in type 1 diabetic subjects with and without microvascular complications. Am J Clin Pathol. 2011;135:429–433. doi: 10.1309/AJCPJGZQX42BIAXL. [DOI] [PubMed] [Google Scholar]
- 13.Tai K, Need AG, Horowitz M, Chapman IM. Vitamin D, glucose, insulin, and insulin sensitivity. Nutrition. 2008;24:279–285. doi: 10.1016/j.nut.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein NS, Hart J. Histologic features associated with lymph node metastasis in stage T1 and superficial T2 rectal adenocarcinomas in abdominoperineal resection specimens. Identifying a subset of patients for whom treatment with adjuvant therapy or completion abdominoperineal resection should be considered after local excision. Am J Clin Pathol. 1999;111:51–58. doi: 10.1093/ajcp/111.1.51. [DOI] [PubMed] [Google Scholar]
- 15.Hypponen E, Laara E, Reunanen A, Jarvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358:1500–1503. doi: 10.1016/S0140-6736(01)06580-1. [DOI] [PubMed] [Google Scholar]
- 16.Walter M, Kaupper T, Adler K, Foersch J, Bonifacio E, Ziegler AG. No effect of the 1alpha, 25-dihydroxyvitamin D3 on beta-cell residual function and insulin requirement in adults with new-onset type 1 diabetes. Diabetes Care. 2010;33:1443–1448. doi: 10.2337/dc09-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bizzarri C, Pitocco D, Napoli N, Di Stasio E, Maggi D, Manfrini S, Suraci C, Cavallo MG, Cappa M, Ghirlanda G, Pozzilli P. No protective effect of calcitriol on beta-cell function in recent-onset type 1 diabetes: the IMDIAB XIII trial. Diabetes Care. 2010;33:1962–1963. doi: 10.2337/dc10-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Querfeld U. Vitamin D and inflammation. Pediatr Nephrol. 2013;28:605–610. doi: 10.1007/s00467-012-2377-4. [DOI] [PubMed] [Google Scholar]
- 19.Guillot X, Semerano L, Saidenberg-Kermanac’h N, Falgarone G, Boissier MC. Vitamin D and inflammation. Joint Bone Spine. 2010;77:552–557. doi: 10.1016/j.jbspin.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 20.Boxer RS, Dauser DA, Walsh SJ, Hager WD, Kenny AM. The association between vitamin D and inflammation with the 6-minute walk and frailty in patients with heart failure. J Am Geriatr Soc. 2008;56:454–461. doi: 10.1111/j.1532-5415.2007.01601.x. [DOI] [PubMed] [Google Scholar]
- 21.Shea MK, Booth SL, Massaro JM, Jacques PF, D’Agostino RB Jr, Dawson-Hughes B, Ordovas JM, O’Donnell CJ, Kathiresan S, Keaney JF Jr, Vasan RS, Benjamin EJ. Vitamin K and vitamin D status: associations with inflammatory markers in the Framingham Offspring Study. Am J Epidemiol. 2008;167:313–320. doi: 10.1093/aje/kwm306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jablonski KL, Chonchol M, Pierce GL, Walker AE, Seals DR. 25-Hydroxyvitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension. 2011;57:63–69. doi: 10.1161/HYPERTENSIONAHA.110.160929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dietrich T, Nunn M, Dawson-Hughes B, Bischoff-Ferrari HA. Association between serum concentrations of 25-hydroxyvitamin D and gingival inflammation. Am J Clin Nutr. 2005;82:575–580. doi: 10.1093/ajcn.82.3.575. [DOI] [PubMed] [Google Scholar]
- 24.Devaraj S, Cheung AT, Jialal I, Griffen SC, Nguyen D, Glaser N, Aoki T. Evidence of increased inflammation and microcirculatory abnormalities in patients with type 1 diabetes and their role in microvascular complications. Diabetes. 2007;56:2790–2796. doi: 10.2337/db07-0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Devaraj S, Dasu MR, Rockwood J, Winter W, Griffen SC, Jialal I. Increased toll-like receptor (TLR) 2 and TLR4 expression in monocytes from patients with type 1 diabetes: further evidence of a proinflammatory state. J Clin Endocrinol Metab. 2008;93:578–583. doi: 10.1210/jc.2007-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim AK, Tesch GH. Inflammation in diabetic nephropathy. Mediators Inflamm. 2012;2012:146154. doi: 10.1155/2012/146154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Navarro JF, Mora C, Maca M, Garca J. Inflammatory parameters are independently associated with urinary albumin in type 2 diabetes mellitus. Am J Kidney Dis. 2003;42:53–61. doi: 10.1016/s0272-6386(03)00408-6. [DOI] [PubMed] [Google Scholar]
- 28.Sugimoto H, Shikata K, Wada J, Horiuchi S, Makino H. Advanced glycation end products-cytokine-nitric oxide sequence pathway in the development of diabetic nephropathy: aminoguanidine ameliorates the overexpression of tumour necrosis factor-alpha and inducible nitric oxide synthase in diabetic rat glomeruli. Diabetologia. 1999;42:878–886. doi: 10.1007/s001250051241. [DOI] [PubMed] [Google Scholar]
- 29.Alexandraki K, Piperi C, Kalofoutis C, Singh J, Alaveras A, Kalofoutis A. Inflammatory process in type 2 diabetes: The role of cytokines. Ann N Y Acad Sci. 2006;1084:89–117. doi: 10.1196/annals.1372.039. [DOI] [PubMed] [Google Scholar]
- 30.Navarro-Gonzalez JF, Mora-Fernandez C. The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol. 2008;19:433–442. doi: 10.1681/ASN.2007091048. [DOI] [PubMed] [Google Scholar]
- 31.Hawk ET, Viner JL, Dannenberg A, DuBois RN. COX-2 in cancer--a player that’s defining the rules. J Natl Cancer Inst. 2002;94:545–546. doi: 10.1093/jnci/94.8.545. [DOI] [PubMed] [Google Scholar]
- 32.Dickie LJ, Church LD, Coulthard LR, Mathews RJ, Emery P, McDermott MF. Vitamin D3 down-regulates intracellular Toll-like receptor 9 expression and Toll-like receptor 9-induced IL-6 production in human monocytes. Rheumatology. 2010;49:1466–1471. doi: 10.1093/rheumatology/keq124. [DOI] [PubMed] [Google Scholar]


