Abstract
Objectives: The newly identified adipokine chemerin has been shown to be associated with the components of MetS, inflammation and insulin resistance. In this study, the relationship between serum chemerin levels and the presence and severity of coronary artery disease (CAD) was evaluated in patients with MetS. Methods: The study population consisted of 84 MetS patients (43 patients with CAD and 41 without CAD), who had coronary angiography for suspected coronary artery disease, and 46 healthy individuals as a control group. Angiographic CAD was defined as ≥ 50% luminal diameter stenosis of at least one major epicardial coronary artery. The severity of CAD was determined by the Gensini score. Serum chemerin levels were measured with enzyme linked immunosorbent assay (ELISA). Results: Serum chemerin levels were significantly higher in patients with MetS (n=84) than those in the control group (120.47±25.32 vs. 90.4±11.4 ng/ml P < 0.001). In addition, MetS patients with CAD had higher chemerin levels than MetS patients without CAD (128.7±26.6 vs. 115.7±15.2 ng/ml, P < 0.001). Serum chemerin levels had a significant positive correlation with the Gensini score (r=0.58, P < 0.001). Multivariate logistic regression demonstrated that serum high-density lipoprotein cholesterol (HDL-C) and chemerin levels were significant and independent predictors for determining the presence of angiographic CAD (OR=1.009, 95% CI: 0.972-1.057; P=0.003 and OR=0.925, 95% CI: 0.896-0.922; P < 0.001, respectively). Conclusion: This study demonstrated that in patients with MetS, chemerin levels were higher in patients with CAD than patients without CAD and also showed a significant positive correlation with CAD severity.
Keywords: Chemerin, metabolic syndrome, coronary artery disease, gensini score
Introduction
Metabolic syndrome (MetS) is characterized by multiple risk factors, which are related to cardiovascular diseases. Factors include abdominal obesity, insulin resistance, high triglyceride (TG) levels, high blood pressure, impaired glucose tolerance and low levels of high density lipoprotein (HDL-C). Patients with MetS are at risk for CAD and related morbidity and mortality [1,2]. For this reason, they must be carefully evaluated with regard to CAD.
Adipose tissue is not merely a lipid store but also an endocrine organ that secretes cytokines and adipokines, such as leptin, adiponectin, tumor necrosis factor-alpha (TNF-α) and resistin [3-5]. Adipokines have several systemic effects on brain, liver, muscle, lymphoid organs and vasculature [6). Adipokines affect the functions of endothelial cells, arterial smooth muscle cells and macrophages in the atherosclerotic process related to obesity [7-9]. Previous studies have demonstrated that adipokines released from adipose tissue play a significant role in the development of CAD in patients with MetS [10-13].
Chemerin is a newly discovered adipokine, which is released from liver and white fat tissue [14]. Chemerin also helps to regulate expression of adipocyte genes, such as glucose transporter-4, adiponectin and leptin, which play a role in the differentiation of adipocytes, and also lipid and glucose metabolism [15]. Previous studies have demonstrated the relationship between chemerin and MetS components, insulin resistance and inflammation [16-18]. The relationship between chemerin levels and the development of coronary atherosclerosis in MetS patients has not yet been investigated.
The purpose of this study was to assess the relationship between chemerin levels and the presence and extent of CAD in patients with MetS.
Methods
Study population
Randomly selected patients, who underwent diagnostic coronary angiography (CAG) for suspected coronary artery disease (CAD) at Ondokuz Mayıs University Hospital between February 1, 2012 and March 1, 2014, were enrolled in this prospective study. Among the subjects, 84 had MetS [43 with CAD (19 men and 24 women; mean age 53.1±9.5 years) and 41 without CAD (20 men and 21 women; mean age 53.7±9.2 years)], and 46 were healthy (21 men and 25 women; mean age 52.6±9.1 years).
All subjects were classified according to the modified National Cholesterol Education Program (NCEP) criteria for MetS (19) and were diagnosed as MetS patients if three or more of the following criteria were met: (1) waist circumference (WC) over 90 cm in men and over 80 cm in women, (2) systolic blood pressure ≥ 130 mmHg or diastolic pressure ≥ 85 mmHg, (3) triglyceride ≥ 150 mg/dl, (4) HDL-C < 40 mg/dl for men and < 50 mg/dl for women, and (5) fasting glucose ≥ 110 mg/dl.
Patients with acute coronary syndrome (ACS), diabetes mellitus, previously documented CAD, suspected myocarditis or pericarditis, advanced renal and liver disease, known malignant disease, systemic inflammatory disease or autoimmune disease were excluded. This study was approved by the institutional ethics committee, and informed consents were obtained from all the participants.
Coronary angiography analysis
All of the patients had coronary angiography using the Judkins technique with a femoral approach. Images were stored in a digital angiographic system (ACOM.PC; Siemens AG, Germany) and collected at a rate of 15 frame/s. Iopromide was used as a contrast agent (Ultravist 370, Schering AG, Berlin Germany). Recordings were analyzed by two independent cardiologists, and angiographic CAD was defined as ≥ 50% luminal diameter stenosis of at least one major epicardial coronary artery. The severity of CAD was determined using the Gensini score, which is a measure of the extent of coronary stenosis according to degree and location. In the Gensini scoring system, larger segments are more heavily weighted with scores ranging from 0.5 to 5.0. The narrowing of the coronary artery lumen is rated 2 for 0% to 25% stenosis, 4 for 26% to 50%, 8 for 51% to 75%, 16 for 76% to 90%, 32 for 91% to 99%, and 64 for 100%. The Gensini index is the sum of the total weights for each segment [20].
Standard echocardiography
Echocardiographic examinations were done while patients were lying in the left lateral decubitus position using a GE Vingmed Vivid 7 (GE Vingmed Ultrasound, Horten, Norway) by an experienced cardiologist. Parasternal long axis, short axis, apical four chamber and two chamber views were obtained and measured with M-Mode, 2-D and continuous wave Doppler and pulsed wave Doppler. Measurements were performed according to recommendations by the American Society of Echocar-diography [21]. Values were measured on three separate beats and then averaged for all parameters.
Biochemical measurements
Anthropometric (height, weight and blood pressures), clinical and laboratory analyses were performed. Peripheral venous blood samples were collected, after a 12-h fasting period, from all the subjects. Biochemical parameters were measured using an Abbott ARCHITECT c8000 (Abbott Laboratories, USA) auto-analyzer with commercial kits. Hematologic parameters were obtained using an Abbott CellDyn 3700 (Abbott Laboratories, USA) device with laser and impedance methods. Serum chemerin levels were measured using a Human Chemerin (CHEMERIN) ELISA kit (Hangzhou Eastbiopharm Co. Hangzou, CHINA), a Multiwash (Tri-Continent Scientific, USA) microplate washer and Synergy 4 Microplate Reader (Biotek, USA), according to standard procedures. Standard curves were generated using a four-parameter curve fitting equation, and chemerin levels were calculated according to this curve, with values given as ng/ml.
Statistical analysis
Statistical analyses were based on the SPSS 15.0 (Statistical Package for Social Sciences) program. The Kolmogorov-Smirnov test was used to check normal distribution of all parameters. Categorical variables were expressed in percentage, whereas numerical variables were presented as mean values ± standard deviation (SD). For statistical analysis, the background data were analyzed by the Kruskal-Wallis Test and the chi-square test to examine the overall balance among the three groups. If the Kruskal-Wallis test was statistically significant, the Wilcoxon rank-sum test was performed to assess which differences were significant. Multivariate logistic-regression analysis was also performed and the model included potential confounders (age, sex, body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), TG, total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), HDL-C, fasting glucose, and chemerin) for CAD. Inclusion of variables into final models was based on both clinical and statistical considerations. A value of P < 0.05 was considered significant. We employed a Bonferroni approach for assessing the significance of group differences and declared a significant difference between control and MetS groups only when the associated P-value was less 0.017.
Results
Baseline clinical characteristics
Basal and laboratory findings of the patients are shown in Table 1. Patients with MetS had significantly higher waist circumference, BMI, SBP, DBP and fasting blood glucose, levels than those of the control group (P < 0.017). At the same time, HDL-C levels were significantly lower in MetS patients who had CAD than those of the control group and MetS patients who did not have CAD (P < 0.017). No significant differences were found regarding statin, angiotensin converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB) usage in MetS patients, regardless of their CAD status.
Table 1.
Baseline clinical characteristics
| MetS patients | ||||
|---|---|---|---|---|
|
|
||||
| Variables | Controls (n=46) | Without CAD (n=41) | With CAD (n=43) | P value |
| Age (years) | 52.6±9.1 | 53.7±9.2 | 53.1±9.5 | 0.792 |
| Male sex n (%) | 21 (46) | 20 (49) | 19 (45) | 0.452 |
| Smoking n (%) | 25 (55) | 22 (54) | 24 (56) | 0.243 |
| Waist circumference (cm) | 84.7±8.4 | 89.8±9.8a | 90.1±10.5a | 0.009 |
| BMI (kg/m2) | 27.6±3.7 | 29.5±1.8a | 30.9±1.5a | 0.032 |
| SBP (mmHg) | 118.8±12.9 | 125.8±20.8a | 133.2±19.4a | 0.048 |
| DBP (mmHg) | 70.5±11.1 | 78.3±13.9a | 82.7±12.5a | 0.044 |
| Fasting glucose (mg/dL) | 91.12±10.4 | 107.54±12.43a | 111.67±13.11a | 0.009 |
| TC (mg/dL) | 172.1±28.1 | 174.2±39.2 | 181.6±38.4 | 0.377 |
| TG (mg/dL) | 150.9±56.1 | 166.2±72.3 | 178.1±137.7 | 0.527 |
| LDL-C (mg/dL) | 95.7±23.7 | 102.3±37.1 | 107.3±26.9 | 0.370 |
| HDL-C (mg/dL) | 38.1±10.2 | 37.8±7.1 | 35.1±10.2a,b | 0.007 |
| Chemerin (ng/ml) | 90.4±11.4 | 115.7±15.2a | 128.7±26.6a,b | < 0.001 |
| Gensini score | 34.41±11.9 | |||
| Cardiovacular medication | ||||
| Statins n (%) | 12 (29) | 14 (32) | 0.342 | |
| ACEI/ARB n (%) | 14 (34) | 15 (35) | 0.568 | |
Abbreviations: CAD, coronary artery disease; MetS, metabolic syndrome; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; T, total cholesterol; TG, triglycerides; LDL-C, low-density cholesterol; HDL-C, high-density lipoprotein cholesterol; ACEI, anjiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker. Data are presented as means ± SD.
P < 0.017 compared with control subjects.
P < 0.017 compared with MetS patients without CAD.
Serum chemerin levels
Serum chemerin levels were significantly higher in MetS patients (n=84) than those in the control group (120.47±25.32 vs. 90.4±11.4 ng/ml, P < 0.001, Figure 1A). In addition, MetS patients with CAD had higher chemerin levels compared to patients without CAD (128.7±26.6 vs. 115.7±15.2 ng/ml, P < 0.001, Figure 1B).
Figure 1.
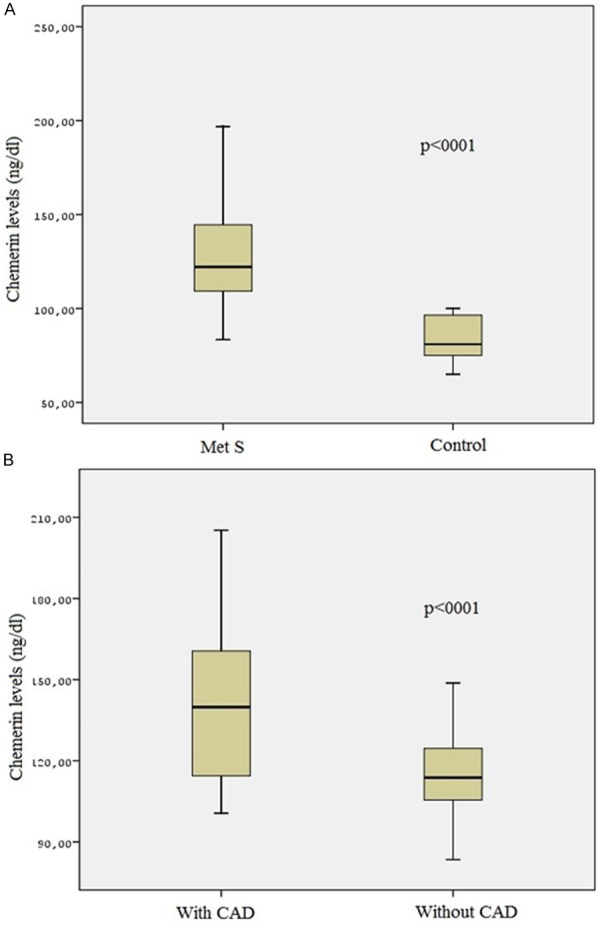
A: The levels of chemerin in the metabolic syndrome and the control group. B: The levels of chemerin in the metabolic syndrome patients with CAD and without CAD.
Association of serum chemerin levels with clinical characteristics and angiographic risk score
Chemerin levels in MetS patients showed a significant positive correlation with BMI, SBP, serum TG levels, fasting blood glucose and showed a significant negative correlation with HDL-C levels (Table 2). Moreover, chemerin levels had a significant positive correlation with the Gensini score (r=0.58, P < 0.001, Figure 2).
Table 2.
Correlation analyses between serum chemerin levels and various parameters in metabolic syndrome patients with coronary artery disease
| Variables | r value | P value |
|---|---|---|
| Age | 0.05 | 0.634 |
| Sex (Male) | 0.11 | 0.108 |
| BMI | 0.32 | < 0.001 |
| SBP | 0.29 | 0.015 |
| DBP | 0.08 | 0.322 |
| Fasting glucose | 0.22 | 0.012 |
| TG | 0.21 | 0.042 |
| TC | 0.05 | 0.522 |
| HDL | -0.35 | < 0.001 |
| LDL | 0.07 | 0.436 |
| Gensini score | 0.58 | < 0.001 |
Abbreviations: BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, triglycerides; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density cholesterol.
Figure 2.
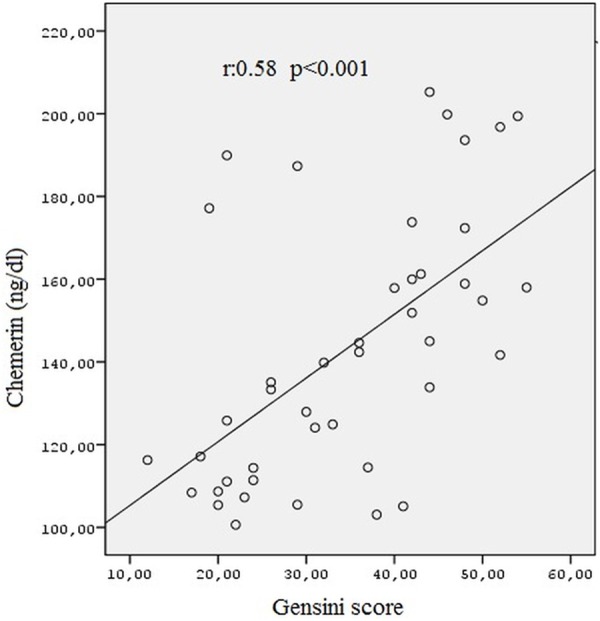
The correlation analysis of chemerin levels and Gensini score.
Association of serum chemerin levels with CAD in MetS patients
In MetS patients, simple logistic regression analysis revealed that HDL-C (OR=1.114, 95% CI: 1.031-1.203, P=0.006), BMI (OR=0.750, 95% CI: 0.554-1.015, P=0.034) and serum chemerin levels (OR=0.941, 95% CI: 0.906-0.976; P < 0.001) were associated with the presence of angiographic CAD (P < 0.05, Table 3). These variables were then entered into a backward, stepwise, multivariate logistic regression model. Multivariate logistic regression demonstrated that serum HDL-C and chemerin levels were significant and independent predictors for determining the presence of angiographic CAD (OR=1.009, 95% CI: 0.972-1.057, P=0.003 and OR=0.925, 95% CI: 0.896-0.922; P < 0.001, respectively, Table 3).
Table 3.
Logistic regression analysis for the presence of CAD in patients with MetS (n=84)
| Univariate Logistic Regression | Multivarite Logistic Regression Analysis | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Odds Ratio | 95% CI | P Value | Odds Ratio | 95% CI | P Value | |
| Age (years) | 0.979 | 0.915-1.048 | 0.538 | |||
| Sex (Male) | 5.260 | 0.812-34.077 | 0.482 | |||
| BMI (kg/m2) | 0.750 | 0.554-1.015 | 0.034 | 0.718 | 0.512-1.003 | 0.056 |
| SBP (mm Hg) | 1.095 | 1.011-1.186 | 0.266 | |||
| DBP (mm Hg) | 0.866 | 0.788-0.996 | 0.431 | |||
| TG (mg/dL) | 1.002 | 0.994-1.011 | 0.594 | |||
| TC (mg/dL) | 0.985 | 0.946-1.025 | 0.465 | |||
| LDL-C (mg/dL) | 1.009 | 0.969-1.051 | 0.594 | |||
| HDL-C (mg/dL) | 1.114 | 1.031-1.203 | 0.006 | 1.009 | 0.972-1.057 | 0.003 |
| Fasting glucose (mg/dL) | 0.991 | 0.982-1.000 | 0.057 | |||
| Chemerin (ng/ml) | 0.941 | 0.906-0.976 | < 0.001 | 0.925 | 0.896-0.922 | < 0.001 |
Abbreviations: BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, triglycerides; TC, total cholesterol; LDL-C, low-density cholesterol; HDL-C, high-density lipoprotein cholesterol.
Discussion
Results from our study demonstrated that in MetS patients, chemerin levels were higher in patients with CAD than those in patients without CAD and showed a significant positive correlation with CAD severity.
Metabolic syndrome patients have a higher risk for the development of cardiovascular diseases and related mortality than that of the general population [2]. Adipokines released from adipose tissue and related to inflammation in obesity have been previously demonstrated to play a role in the pathogenesis of CAD in metabolic syndrome patients [10-13].
Previous studies have demonstrated that chemerin, a newly identified adipokine, is related to glucose and lipid metabolism, insulin resistance, and inflammation [15-18,22]. Studies also reported that serum chemerin levels are increased in MetS patients and correlate with components of MetS [23,24]. The correlation of chemerin with inflammation and MetS components, which are also CAD risk factors, led us to determine the relationship between chemerin levels and development of coronary atherosclerosis.
Studies that have attempted to determine the relationship between chemerin levels and development of coronary atherosclerosis have provided conflicting results. Two studies have demonstrated that chemerin levels were significantly higher in Chinese individuals with CAD than those in a Chinese control group [25,26]. In addition, Dong et al. [24] reported that chemerin levels were higher in MetS patients who had CAD than those in MetS patients who did not have CAD. In another study, which enrolled 131 Korean patients, serum chemerin levels were found to have a significant correlation with cardiometabolic parameters and percentage of coronary artery narrowing [27]. In our study, we also found that serum chemerin levels were higher in MetS patients who had CAD than those in MetS patients who did not have CAD. On the other hand, Lehrke et al. [17] demonstrated no relationship between serum chemerin levels and coronary atherosclerosis development when comparing the extent of coronary atherosclerosis using multi-slice CT angiography in 303 Caucasian patients and adjusting for other cardiovascular risk factors. Several reasons can explain the conflicting results, such as the use of multi-slice CT angiography to evaluate coronary atherosclerosis index and enrollment of patient populations with different ethical backgrounds and basal characteristics. Variability could also arise when measuring total chemerin levels, because chemerin ELISA kits can detect pro-chemerin as well as some proteolytically processed short forms of chemerin in their study. In addition, the adipokine chemerin may play a role in the pathogenesis of CAD in MetS patients.
Chemerin is accepted as a chemokine that plays a role in the inflammatory process, induces leukocyte migration and modulates chemotaxis and activation of macrophages and dendritic cells [22]. Strong correlations between atherosclerosis and inflammation are well known and led to the evaluation of the contribution of chemerin to the atherosclerotic process. In a study done by Kostopoulos et al. [28] in which the correlation between chemerin and the development of atherosclerotic lesion was evaluated, chemerin levels were found to be high in atherosclerotic lesions, periadventitial adipose tissue, foam cells and vascular smooth muscle cells (VSMC). Moreover, a significant correlation was found between the severity of the atherosclerotic lesion and chemerin levels released from cells within the lesions. Likewise, Spiroglou et al. [29] confirmed the correlation between epicardial chemerin levels and coronary atherosclerosis. In a study done by Hart et al. [30], chemerin was shown to stimulate the adhesion of macrophages to extracellular matrix protein fibronectin and vascular cell adhesion molecule 1 (VCAM-1). This process was proposed to contribute to the progression of atherosclerosis. In addition, previous studies have demonstrated that chemerin activates MMP-2 and MMP-9 that belong to the matrix metalloproteinase (MMP) class which plays a key role in plaque instability [31] and induce the expression of adhesion molecules such as intercellular adhesion molecule 1 (ICAM-1) and E-selectin [32]. Also it was hypothesized that chemerin could be a new biomarker for coronary atherosclerosis [25]. In light of this information, a significant correlation can be assu-med between chemerin, which plays a role in the inflammatory response during the atherosclerotic process, and the Gensini score. This may be related to intensive plaque burden, possibly reflecting the intensive inflammatory process. Accordingly, the increased Gensini score in our study may be related to an intensive inflammatory state. To our knowledge, our study is the first one that demonstrates the correlation between chemerin levels and extent of CAD.
Bozaoğlu et al. [18,33] have demonstrated that serum chemerin levels are higher in MetS patients than those in the control group and showed a significant correlation between chemerin levels and BMI, blood pressure, fasting blood glucose, and TG and HDL-C levels in different patient populations. Similarly, in our study, chemerin levels were higher in MetS patients than those in the control group and showed a significant positive correlation with BMI, SBP, serum TG levels, fasting blood glucose and significant negative correlation with HDL-C levels. It can be proposed that chemerin, which has a significant correlation with MetS components that contribute to the atherosclerotic process, may play a role MetS-related atherosclerosis.
Study limitations
The main limitation of our study is the low number of the patients. It is also incapable, up to certain extent, to explain the effect of the chemerin on clinical events and plaque stability. Further studies are needed in order to explicate the role of chemerin in the atherosclerotic process.
Conclusion
Results from our study have demonstrated that in MetS patients, chemerin levels were higher in the subjects with CAD than those in without CAD and showed a significant positive correlation with CAD severity. Chemerin could be used as a biomarker to identify high risk patients, such as MetS or diabetic patients, who could develop atherosclerotic heart disease. Because serum chemerin levels also correlate with the extent of the disease, it may also be helpful in guiding the treatment strategy.
Disclosure of conflict of interest
None.
References
- 1.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 2.Solymoss BC, Bourassa MG, Marcil M, Levesque S, Varga S, Campeau L. Long-term rates of cardiovascular events in patients with the metabolic syndrome according to severity of coronary angiographic alterations. Coron Artery Dis. 2009;20:1–8. doi: 10.1097/MCA.0b013e32831624a5. [DOI] [PubMed] [Google Scholar]
- 3.Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 2006;55:1537–1545. doi: 10.2337/db06-0263. [DOI] [PubMed] [Google Scholar]
- 4.Ahima RS, Flier JS. Adipose tissue as an endocrine organ. Trends Endocrinol Metab. 2000;11:327–332. doi: 10.1016/s1043-2760(00)00301-5. [DOI] [PubMed] [Google Scholar]
- 5.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92:347–355. doi: 10.1079/bjn20041213. [DOI] [PubMed] [Google Scholar]
- 6.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fantuzzi G, Mazzone T. Adipose tissue and atherosclerosis: exploring the connection. Arterioscler Thromb Vasc Biol. 2007;27:996–1003. doi: 10.1161/ATVBAHA.106.131755. [DOI] [PubMed] [Google Scholar]
- 8.Steffens S, Mach F. Adiponectin and adaptive immunity: linking the bridge from obesity to atherogenesis. Circ Res. 2008;102:140–142. doi: 10.1161/CIRCRESAHA.107.170274. [DOI] [PubMed] [Google Scholar]
- 9.Lau DC, Dhillon B, Yan H, Szmitko PE, Verma S. Adipokines: molecular links between obesity and atheroslcerosis. Am J Physiol Heart Circ Physiol. 2005;288:H2031–2041. doi: 10.1152/ajpheart.01058.2004. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura Y, Shimada K, Fukuda D, Shimada Y, Ehara S, Hirose M, Kataoka T, Kamimori K, Shimodozono S, Kobayashi Y, Yoshiyama M, Takeuchi K, Yoshikawa J. Implications of plasma concentrations of adiponectin in patients with coronary artery disease. Heart. 2004;90:528–533. doi: 10.1136/hrt.2003.011114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parhami F, Tintut Y, Ballard A, Fogelman AM, Demer LL. Leptin enhances the calcification of vascular cells: artery wall as a target of leptin. Circ Res. 2001;88:954–960. doi: 10.1161/hh0901.090975. [DOI] [PubMed] [Google Scholar]
- 12.Ingelsson E, Sundstrom J, Melhus H, Michaëlsson K, Berne C, Vasan RS, Risérus U, Blomhoff R, Lind L, Arnlöv J. Circulating retinol-binding protein 4, cardiovascular risk factors and prevalent cardiovascular disease in elderly. Atherosclerosis. 2009;206:239–244. doi: 10.1016/j.atherosclerosis.2009.02.029. [DOI] [PubMed] [Google Scholar]
- 13.Pischon T, Bamberger CM, Kratzsch J, Zyriax BC, Algenstaedt P, Boeing H, Windler E. Association of plasma resistin levels with coronary heart disease in women. Obes Res. 2005;13:1764–1771. doi: 10.1038/oby.2005.215. [DOI] [PubMed] [Google Scholar]
- 14.Kutzleb C, Busmann A, Wendland M, Maronde E. Discovery of novel regulatory peptides by reverse pharmacology: spotlight on chemerin and the RF-amide peptides metastin and QRFP. Curr Protein Pept Sci. 2005;6:265–278. doi: 10.2174/1389203054065419. [DOI] [PubMed] [Google Scholar]
- 15.Goralski KB, McCarthy TC, Hanniman EA, Zabel BA, Butcher EC, Parlee SD, Muruganandan S, Sinal CJ. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J Biol Chem. 2007;282:28175–28188. doi: 10.1074/jbc.M700793200. [DOI] [PubMed] [Google Scholar]
- 16.Ernst MC, Sinal CJ. Chemerin: at the crossroads of inflammation and obesity. Trends Endocrinol Metab. 2010;21:660–667. doi: 10.1016/j.tem.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Lehrke M, Becker A, Greif M, Stark R, Laubender RP, von Ziegler F, Lebherz C, Tittus J, Reiser M, Becker C, Göke B, Leber AW, Parhofer KG, Broedl UC. Chemerin is associated with markers of inflammation and components of the metabolic syndrome but does not predict coronary atherosclerosis. Eur J Endocrinol. 2009;161:339–344. doi: 10.1530/EJE-09-0380. [DOI] [PubMed] [Google Scholar]
- 18.Bozaoglu K, Bolton K, McMillan J, Zimmet P, Jowett J, Collier G, Walder K, Segal D. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology. 2007;148:4687–4694. doi: 10.1210/en.2007-0175. [DOI] [PubMed] [Google Scholar]
- 19.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC Jr, Spertus JA, Costa F American Heart Association; National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 20.Gensini GG. A more meaningful scoring system for determining the severity of coronary artery disease. Am J Cardiol. 1983;51:606. doi: 10.1016/s0002-9149(83)80105-2. [DOI] [PubMed] [Google Scholar]
- 21.Quinones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA. Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002;15:167–84. doi: 10.1067/mje.2002.120202. [DOI] [PubMed] [Google Scholar]
- 22.Wittamer V, Bondue B, Guillabert A, Vassart G, Parmentier M, Communi D. Neutrophil-mediated maturation of chemerin: a link between innate and adaptive immunity. J Immunol. 2005;175:487–93. doi: 10.4049/jimmunol.175.1.487. [DOI] [PubMed] [Google Scholar]
- 23.Stejskal D, Karpisek M, Hanulova Z, Svestak M. Chemerin is an independent marker of the metabolic syndrome in a Caucasian population--a pilot study. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2008;152:217–221. doi: 10.5507/bp.2008.033. [DOI] [PubMed] [Google Scholar]
- 24.Dong B, Ji W, Zhang Y. Elevated serum chemerin levels are associated with presence of coronary artery disease in patients with metabolic syndrome. Intern Med. 2011;50:1093–1097. doi: 10.2169/internalmedicine.50.5025. [DOI] [PubMed] [Google Scholar]
- 25.Xiaotao L, Xiaoxia Z, Yue X, Liye W. Serum chemerin levels are associated with the presence and extent of coronary artery disease. Coron Artery Dis. 2012;23:412–416. doi: 10.1097/MCA.0b013e3283576a60. [DOI] [PubMed] [Google Scholar]
- 26.Yan Q, Zhang Y, Hong J, Gu W, Dai M, Shi J, Zhai Y, Wang W, Li X, Ning G. The association of serum chemerin level with risk of coronary artery disease in Chinese adults. Endocrine. 2012;41:281–288. doi: 10.1007/s12020-011-9550-6. [DOI] [PubMed] [Google Scholar]
- 27.Hah YJ, Kim NK, Kim MK, Kim HS, Hur SH, Yoon HJ, Kim YN, Park KG. Relationship between chemerin levels and cardiometabolic parameters and degree of coronary stenosis in Korean patients with coronary artery disease. Diabetes Metab J. 2011;35:248–254. doi: 10.4093/dmj.2011.35.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kostopoulos CG, Spiroglou SG, Varakis JN, Apostolakis E, Papadaki HH. Chemerin and CMKLR1 expression in human arteries and periadventitial fat: a possible role for local chemerin in atherosclerosis. BMC Cardiovascular Disorders. 2014;14:56. doi: 10.1186/1471-2261-14-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spiroglou SG, Kostopoulos CG, Varakis JN, Papadaki HH. Adipokines in periaortic and epicardial adipose tissue: differential expression and relation to atherosclerosis. J Atheroscler Thromb. 2010;17:115–130. doi: 10.5551/jat.1735. [DOI] [PubMed] [Google Scholar]
- 30.Hart R, Greaves DR. Chemerin contributes to inflammation by promoting macrophage adhesion to VCAM-1 and fibronectin through clustering of VLA-4 and VLA-5. J Immunol. 2010;185:3728–3739. doi: 10.4049/jimmunol.0902154. [DOI] [PubMed] [Google Scholar]
- 31.Kaur J, Adya R, Tan BK, Chen J, Randeva HS. Identification of chemerin receptor (ChemR23) in human endothelial cells: chemerin-induced endothelial angiogenesis. Biochem Biophys Res Commun. 2010;391:1762–1768. doi: 10.1016/j.bbrc.2009.12.150. [DOI] [PubMed] [Google Scholar]
- 32.Landgraf K, Friebe D, Ullrich T, Kratzsch J, Dittrich K, Herberth G, Adams V, Kiess W, Erbs S, Körner A. Chemerin as a mediator between obesity and vascular inflammation in children. J Clin Endocrinol Metab. 2012;97:E556–564. doi: 10.1210/jc.2011-2937. [DOI] [PubMed] [Google Scholar]
- 33.Bozaoglu K, Segal D, Shields KA, Cummings N, Curran JE, Comuzzie AG, Mahaney MC, Rainwater DL, VandeBerg JL, MacCluer JW, Collier G, Blangero J, Walder K, Jowett JB. Chemerin is associated with metabolic syndrome phenotypes in a Mexican- MetS patients American population. J Clin Endocrinol Metab. 2009;94:3085–3088. doi: 10.1210/jc.2008-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]


