Abstract
Background: Imbalance between protease-antiprotease plays an important role in the pathogenesis of chronic obstructive pulmonary disease (COPD). Cystatin C in circulating blood is a cysteine protease inhibitor and contributes to elastolysis and tissue destruction. Objectives: The aims of the present study were to investigate whether cystatin C was a promising biomarker for the evaluation and follow-up of patients with COPD. Methods: Serum cystatin C level was determined in groups of acute exacerbation of chronic obstructive pulmonary disease (AECOPD) (n = 93), stable COPD (SCOPD) (n = 299) and healthy controls (n = 151). The influences of smoking on the level of serum cystatin C and the correlation of cystatin C with lung functional parameters were further analyzed. Results: Serum cystatin C level was significantly higher in COPD patients than that of healthy controls. Smoking increased serum cystatin C level in patients with SCOPD but not in AECOPD and control. In SCOPD group, serum cystatin C level was positively correlated with RV%TLC and negatively correlations with FEV1% predicted, FEV1%FVC, MMEF75/25% predicted, MVV% predicted and DLco% predicted. In multiple line analysis, FEV1% predicted and age were found to be independent predictors of serum cystatin C levels, but not smoking statue, sex and BMI. Conclusions: COPD had a higher level of serum cystatin C, smoking only increased cystatin C level in SCOPD. Serum cystatin C level was negatively correlated with FEV1% predicted. These results suggest that cystatin C might be a potential biomarker for lung tissue destruction and severity of COPD.
Keywords: COPD, cystatin C, cathepsins, lung function, biomarker
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by progressive irreversible airway obstruction with destruction of parenchyma caused by airway inflammation and remodeling, which is an increasing and major global public health problem [1]. An imbalance between endogenous proteases and inhibitor of proteases in the lung is considered as one of the critical mechanisms underlying the development of COPD [2]. Proteinases are classified into four groups based on the chemical nature of their active sites: serine, metallo, cysteine and aspartic [3]. The cathepsin family of papain-like cysteine proteases is mainly involved in the degradation of peptides and proteins in the lysosome. Extracellular cathepsins secreted by alveolar macrophages and neutrophils degrade lung extracellular matrix, especially lung elastin during the inflammatory processes [4]. Furthermore, the degraded products of elastin fibers have chemotactic effects on monocytes and promote monocytes infiltration and exacerbate the inflammatory responses in lung. This indicates that there is a positive feedback loop between cathepsins and monocytes (macrophge) in lung to amplify the inflammation [5,6]. At the meantime, reduction of lung elastin fibers causes the airflow limitation by reducing the elastic recoil pressure available to drive air out of the lung during forced expiration [7,8].
The levels of cathepsins are measurable in the serum, however, serum levels don’t reflect faithfully the activities of cathepsins in lung tissue. Despite the samples obtained from the lung can be used for the measurement of activities of cathepsins, the cathepsins are easily inactivated by invasive processes and environmental change [9], furthermore, it is difficult and costive in the process of measurement. Enhanced protease activity of cathepsins increases lung elastic tissue destruction in COPD [2], it makes transforming growth factor-β1 (TGF-β1) released from proteoglycan storage sites [10], TGF-β1 up-regulates cystatin C production [11,12]. Cystatin C is one of major human extracellular cathepsins inhibitor [13], it is secreted into the bloodstream by inflammatory cells, particularly alveolar macrophages [14-16], and the level of cystatin C may indirectly reflect cathepsin activity. It has been well-known that constant serum cystatins C is a sensitive marker for glomerular filtration rate and cardiovascular events [17,18]. A recent study by Haala K et al showed that cystatin C level in the emphysema is significantly increased than that in healthy subjects [19]. Study by Takeyabu K further suggested that level of cystatin C is dramatically elevated in bronchoalveolar lavage fluid (BALF) in smokers with emphysema compare with smokers without emphysema [20]. Based on the above observations, it is interesting to know whether the level of cystatin C rise in patients with COPD, and whether it correlates with lung function in COPD patients. This study aims to address these issues.
Methods
Subjects
The present research was approved by the Research Committee of Human Investigation of Medical College of Xi’an Jiaotong University. All participants gave informed consent. The diagnosis of COPD was in accordance with Gold guidelines. AECOPD was defined as the worsening of respiratory symptoms, and based on the presence of an increase in any two major symptoms (dyspnoea, sputum purulence and sputum quantity), or an increase in one major and one minor symptom (wheeze, sore throat, cough and nasal congestion/discharge) for at least two consecutive days according to previously accepted criteria [21]. Healthy volunteers were recruited from health examination center of Second Affiliated Hospital of Medical College of Xi’an Jiaotong University. All participants’ main diagnosis, medical history and smoking habits were collected. As shown in Figure 1, Patients with chronic kidney disease, coronary artery disease, cancer, diabetes, acute inflammatory disease and thyroid dysfunction which could affect cystatins C level were excluded. 1, 685 subjects were screened for the study and 543 met the required inclusion and exclusion criteria, the subjects were divided into three groups, including 93 patients with AECOPD, 299 patients with SCOPD, and 151 healthy controls.
Figure 1.
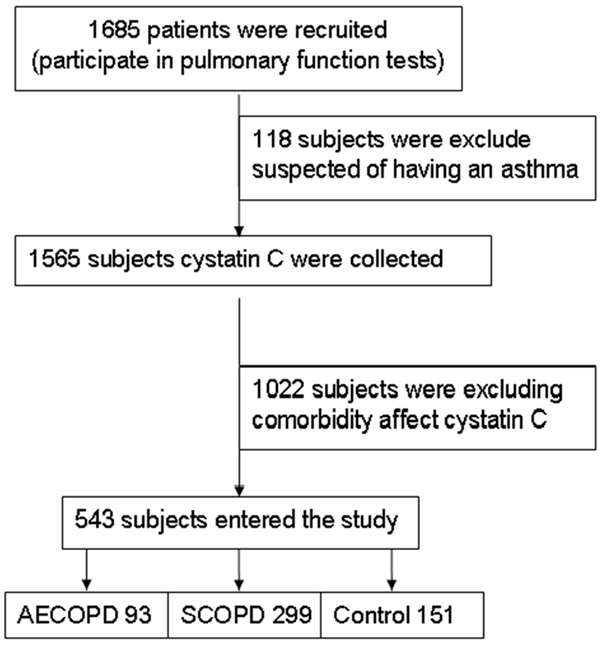
Flow chart diagram of study participants and reasons for exclusion from the study.
Pulmonary function test
Spirometry was performed on each subject except patient with AECOPD, since it is often difficult to perform a complete set ofpulmonary function tests during an acute exacerbation. Reversibility assessments were conducted in SCOPD patients by making them inhale a short-acting β2 agonist equivalents 200 μg salbutamol.
BMI and cystatin C measurements
Body weight and height were measured in bare feet and light clothing in the morning. Body mass index (BMI) was calculated as weight (kilograms)/height square (square meters). Serum cystatin C level was measured immediately by the N latex cystatin C assay on the automatic biochemical analyzer (Olympus AU2707. Japan) in clinical laboratory.
Statistical analyses
Statistical analysis was performed using SPSS 13.0 package for Windows, normality of distribution of data was examined with Kolmogorove-Smirnov test. Normally distributed data were expressed as mean ± standard deviation (SD), non-normally distributed data were expressed as median (range). The level of statistical significance was set at P < 0.05. The differences of normally distributed data among the three groups (Control, SCOPD, and AECOPD) were tested by one-way ANOVA with Tukey’s post hoc test. Analysis of non-normally distributed variables among the three groups was performed using Kruskal-Wallis test. Comparisons of the difference between two groups of data were performed by indepentdent-sample T test or Mann-Whitney U test. Frequency data (for example, sex) were analyzed by χ2 test. In the SCOPD group, correlations between cystatin C and age, body mass index, lung function parameters were examined using Spearman’s rank-correlation, multivariable linear regression analysis was performed, with cystatin C as the dependent factor, to evaluate the relationship between cystatin C and FEV1% predicted, age, sex, BMI and smoking statue.
Results
Characteristics of the subjects
Table 1 summarizes the clinical characteristics of patients with AECOPD, SCOPD and healthy control. There was no significant difference in the age among three groups. BMI of healthy controls was slightly higher than that of patients with SCOPD and was similar with that of AECOPD, no significant difference was observed in BMI between AECOPD and SCOPD. The proportion of male was significantly greater in COPD patients compared with control subjects, tobacco exposure was the greatest in AECOPD patients, followed by the SCOPD and then by healthy control. The parameters of lung function were worse in SCOPD patients compared with healthy control.
Table 1.
Basic information about the study participants
| Parameters | Controls | SCOPD | AECOPD |
|---|---|---|---|
| Number (male/female) | 151 (83/68) | 299 (220/79)* | 93 (68/25)*,# |
| Ages (year) | 63 (51-87) | 65 (36-86) | 65 (44-81) |
| BMI (kg/m2) | 25.1 (15.3-47.6) | 23.8 (15.4-37.9)* | 24 (13.1-34.6) |
| Current/former/never smoker | 35/6/110 | 96/26/177* | 42/11/40*,# |
| FEV1%pred (%) | 107.2±19.9 | 72.1±23.7* | N/A |
| FEV1%FVC (%) | 79.2 (70.1-98.1) | 61.7 (26.8-69.8)* | N/A |
| MMEF75/25%pred (%) | 77.8 (18.7-168.5) | 27.2 (6.3-69.5)* | N/A |
| MVV%pred (%) | 95.8±21.9 | 61.2 (20.2-135.7)* | N/A |
| RV%TLC (%) | 36.4 (13.7-91.8) | 40.1 (23.0-76.1)* | N/A |
| DLco/%pred (%) | 92.0±25.3 | 86.4±23.1 | N/A |
| Cystatin C (mg/L) | 0.84±0.18 | 1.03±0.21* | 1.09±0.22* |
AECOPD = acute exacerbation of COPD; SCOPD = stable chronic obstructive pulmonary disease; BMI = body mass index; FEV1 = forced expiratory volume at 1 s; % pred = percent of predicted value; FVC = forced vital capacity; MMEF75/25 = maximal midexpiratory flow between 75 and 25 percent of FVC; MVV = maximum ventilatory volume/minute; RV = residual volume; TLC = total lung capacity; DLco = diffusing capacity of the lung for carbon monoxide; N/A = not available.
P < 0.05, versus healthy controls;
P < 0.05, versus SCOPD; Values are mean ± SD or median (range).
Serum cystatin C levels
Serum cystatin C levels are shown in Figure 2, which indicates that cystatin C was significantly higher in AECOPD (1.09±0.22 mg/L) and SCOPD (1.03±0.21 mg/L) group than that of healthy control (0.84±0.18 mg/L) (P < 0.05), however, the level was no significant difference between AECOPD and SCOPD.
Figure 2.
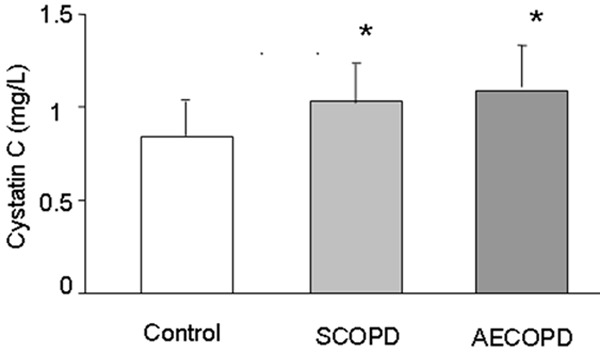
Serum cystatin C levels for healthy control subjects, patients with SCOPD and patients with AECOPD are shown. Analysis (mean ± SD) of the serum cystatin C level showed that COPD including SCOPD and AECOPD has a higher level than that of healthy controls, while no difference was found between SCOPD and AECOPD. *: P < 0.05, versus healthy controls.
Influence of smoking status on serum cystatin C level in three groups
Table 2 shows that there was no significant difference in BMI between smoker and non-smoker in every group. Average age of smoker was younger compared with that of non-smoker only in the healthy control group. As shown in Table 2, there was no dramatic difference in serum cystatin C level between subjects with smoking and non-smoking in either AECOPD (P = 0.431) or healthy control group (P = 0.703). While the level of serum cystatin C was higher in SCOPD patients with smoking than that without smoking (P = 0.037).
Table 2.
Serum cystatin C levels different between smoker and no-smoker in three groups
| Group | Parameters | No-smoking | smoking | t/z | P |
|---|---|---|---|---|---|
| AECOPD (51/42) | Age (year) | 67.84±7.11 | 62.90±9.12 | 2.934 | 0.004 |
| BMI (kg/m2) | 24.42±4.15 | 23.60±3.96 | 0.965 | 0.337 | |
| Cystatin C (mg/L) | 1.11±0.22 | 1.07±0.23 | 0.791 | 0.431 | |
| SCOPD (211/98) | Age (year) | 65 (36-85) | 66 (38-86) | -0.335 | 0.735 |
| BMI (kg/m2) | 24.09±3.56 | 23.48±4.15 | 1.303 | 0.194 | |
| FEV1%pred (%) | 72.42±23.46 | 71.91±24.40 | 0.331 | 0.741 | |
| Cystatin C (mg/L) | 1.01±0.21 | 1.06±0.21 | -2.094 | 0.037 | |
| Control (115/36) | age (year) | 64 (42-84) | 59 (52-87) | -2.174 | 0.030 |
| BMI (kg/m2) | 25.40±4.55 | 24.50±3.97 | 1.067 | 0.288 | |
| FEV1%pred (%) | 107.7±20.20 | 105.6±19.75 | 0.531 | 0.596 | |
| Cystatin C (mg/L) | 0.85±0.19 | 0.83±0.16 | 0.382 | 0.703 |
BMI = body mass index; FEV1 = forced expiratory volume at 1 s; % pred = percent of predicted value. Values are mean ± SD or median (range).
Level of serum cystatin C correlates with age, lung functions in SCOPD
The Spearman’s correlation analysis indicates that serum cystatin C level was positively correlated with age (correlation coefficient r = 0.152, P = 0.008), but was not correlated with BMI (correlation coefficient r = -0.013, P = 0.819) in SCOPD. Serum cystatin C level was further found in SCOPD to positively correlate with RV%TLC (%) (correlation coefficient r = 0.265, P < 0.001), and negatively correlate with FEV1% predicted (%) (correlation coefficient r = -0.464, P < 0.001), FEV1%FVC (%) (correlation coefficient r = -0.390, P < 0.001), MMEF75/25% predicted (%) (correlation coefficient r = -0.431, P < 0.001), MVV% predicted (%) (correlation coefficient r = -0.492, P < 0.001), DLco% predicted (%) (correlation coefficient r = -0.238, P < 0.001) (Table 3).
Table 3.
Correlation of serum cystatin C levels with age, BMI and lung function in SCOPD
| Parameter | r | P |
|---|---|---|
| Age (years) | 0.152 | 0.008 |
| BMI (Kg/m2) | -0.013 | 0.819 |
| FEV1%pred (%) | -0.464 | < 0.001 |
| FEV1%FVC (%) | -0.390 | < 0.001 |
| MMEF75/25% pred (%) | -0.431 | < 0.001 |
| MVV%pred (%) | -0.492 | < 0.001 |
| RV%TLC (%) | 0.265 | < 0.001 |
| DLco%pred (%) | -0.238 | < 0.001 |
BMI = body mass index; FEV1 = forced expiratory volume at 1 s; % pred = percent of predicted value; FVC = forced vital capacity; MMEF75/25 = maximal midexpiratory flow between 75 and 25 percent of FVC; MVV = maximum ventilatory volume/minute; RV = residual volume; TLC = total lung capacity; DLco = diffusing capacity of the lung for carbon monoxide.
Multiple linear regression analysis revealed that serum cystatin C level was inversely correlated with airflow limitation FEV1% predicted (beta coefficient = -0.533), and positively with age (beta coefficient = 0.196) in SCOPD (both P < 0.01). Whereas sex, BMI and smoking statue did not appear to be significant factors influencing level of cystatin C (Table 4).
Table 4.
Multiple linear regression of SCOPD with serum cystatin C as the dependent variable
| Parameter | B* | SD | β# | t | P |
|---|---|---|---|---|---|
| Constant | 0.944 | 0.103 | 9.682 | < 0.01 | |
| Age (years) | 0.004 | 0.001 | 0.196 | 3.997 | < 0.01 |
| FEV1% pred (%) | -0.005 | 0.000 | -0.533 | -10.784 | < 0.01 |
| BMI (Kg/m2) | 0.003 | 0.003 | 0.054 | 1.090 | 0.277 |
| never/former/Current smoker | 0.017 | 0.012 | 0.072 | 1.367 | 0.173 |
| Sex (male/female) | 0.038 | 0.025 | 0.080 | 1.499 | 0.135 |
FEV1 = forced expiratory volume at 1 s; % pred = percent of predicted value; Sex: male = 1, female = 2; Smoking statue: never smokers = 0, ex-smokers = 1, current smokers = 2; BMI = body mass index;
Nonstandardised coefficient;
standardized coefficient.
Discussion
This study demonstrates that the level of cystatin C is higher in COPD patients than that in healthy individuals, while cystatin C level is no difference between AECOPD and SCOPD. Independent analyses between smoking and no-smoking in healthy controls, SCOPD and AECOPD group indicate that SCOPD patients with smoking had higher level of cystatin C than SCOPD patients without smoking, there was no significant change between smoking and no-smoking in either healthy controls or AECOPD group. We also show that serum cystatin C level significantly and positively correlated with age and negatively with FEV1% predicted in SCOPD.
Macrophages are the predominant leukocytes in the normal airway and alveolar, its number is increased in the airway, lung parenchyma and sputum of smokers and patients with COPD, it is not further increased during the acute exacerbation of COPD. While the infiltration of neutrophils in lung tissues is dramatically found during the AECOPD [22]. This indicates that macrophages play an important role in chronic inflammatory process in SCOPD, while neutrophils are majorly involved in acute inflammatory responses in AECOPD [23]. Studies have shown that macrophages are major inflammatory cells to produce and secret cystatin C and cathepsins [14,24]. Furthermore, cathepsins degrade elastin and release trapped TGF-β1 which in turn causes an increase of cystatin C [10-12], suggesting that serum cystatin C level might be as a marker for lung tissue destruction of COPD. This explains that the serum level of cystatin C was not further elevated in patients with AECOPD.
Smoking has been shown to evoke inflammatory responses in both human and animal [22]. Warfel’s study showed that smoking induces cystatin C release from cultured alveolar macrophages [16], Ishii found that cathepsin L and cystatin C level are elevated in BALF in smokers with emphysema compared to smokers without emphysema [25]. Consistent with the above findings, the present study indicated that the level of cystatin C was higher in smoker than non-smoker in SCOPD. Our study further shows there was no significant difference in serum cystatin C level between smoking and non-smoking in the group of either healthy control or AECOPD. As we discussed above, deterioration of inflammatory responses caused by neutrophils was the major pathogenesis of AECOPD, infections and sudden exacerbation of pollution are usually the inductive factors of AECOPD. Smoking as a risk factor only mediated inflammatory responses, which is not a major contributor to AECOPD [26]. Furthermore, patients with COPD usually choose to temporally pause smoking in the phase of acute exacerbation of COPD, however, not all smokers develop COPD, suggesting that genetic predisposition and other environmental factors also play a role in the pathogenesis of the disease [27]. However, our results also indicated that smoking and non-smoking in control group were no significant difference in serum cystatin C level, an explanation for this phenomenon may be age-related elevation of cystatin C [28], non-smoking was significantly older than smoking in control group of this study.
Our study indicated a significant association between serum cystatin C level and lung functional parameters as well as age in SCOPD. These results suggest that elevated cystatin C level may be a useful biochemical marker of declined pulmonary function in SCOPD. Previous studies have demonstrated a strong non-linear association of age with cystatin C level [28] and age-dependent decline in lung function [29]. It is now well accepted that the age may be confounded in the correlation between serum cystatin C level and lung function. Our results of multiple line regression analysis showed that sex, smoking statue and BMI were not the independent predictors of cystatin C level in SCOPD. We found an inverse correlation between serum cystatin C level and FEV1% predicted in SCOPD, and a positive correlation between cystatin C level and age was observed in SCOPD. Since the severity of airflow limitation of COPD can be determined by spirometry that measures the forced expiratory volume in one second (FEV1% predicted), which suggests that serum cystatin C level might be a better maker for COPD severity.
The current study has several limitations. First, there was no baseline serum cystatin C level in patients with AECOPD before the acute exacerbation. We did not determine the dynamic changes of serum cystatin C level from SCOPD to the onset of AECOPD. Second, BALF was not obtained in our study. We are not aware of changes of cystatin C in BALF and how the cystatin C in BALF is related to serum cystatin C. Third, patients with AECOPD were received systemic drug therapy, which may influence the serum cystatin C level. Finally, most of the patients in our study had severe COPD, the results and conclusions based on such patients may not be generalized to patients with mild or moderate disease. Further studies are needed to address these issues.
In conclusion, we have demonstrated that serum cystatin C level was elevated in patients with COPD, smoking increased serum cystatin C level in patients with SCOPD and that cystatin C was significantly associated with the severity of airflow obstruction as measured by FEV1% predicted. These findings imply that cystatin C may be a potential biomarker for lung parenchymal destruction in COPD and can reflect the overall severity of SCOPD. Future longitudinal study is needed to ascertain whether cystatin C level can assess the disease progression and comorbidities and mortality in COPD.
Acknowledgements
This work was supported by the Key Clinical Project for Affiliated Hospital of Ministry of Public Health of China (No. 111).
Disclosure of conflict of interest
None.
References
- 1.Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, Stockley RA, Sin DD, Rodriguez-Roisin R. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 2.Abboud RT, Vimalanathan S. Pathogenesis of COPD. Part I. The role of protease-antiprotease imbalance in emphysema. Int J Tuberc Lung Dis. 2008;12:361–367. [PubMed] [Google Scholar]
- 3.Owen CA. Roles for proteinases in the pathogenesis of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2008;3:253–268. doi: 10.2147/copd.s2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Novinec M, Grass RN, Stark WJ, Turk V, Baici A, Lenarcic B. Interaction between human cathepsins K, L, and S and elastins: mechanism of elastinolysis and inhibition by macromolecular inhibitors. J Biol Chem. 2007;282:7893–7902. doi: 10.1074/jbc.M610107200. [DOI] [PubMed] [Google Scholar]
- 5.Hunninghake GW, Davidson JM, Rennard S, Szapiel S, Gadek JE, Crystal RG. Elastin fragments attract macrophage precursors to diseased sites in pulmonary emphysema. Science. 1981;212:925–927. doi: 10.1126/science.7233186. [DOI] [PubMed] [Google Scholar]
- 6.Fulop T, Khalil A, Larbi A. The role of elastin peptides in modulating the immune response in aging and age-related diseases. Pathol Biol (Paris) 2012;60:28–33. doi: 10.1016/j.patbio.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Black PN, Ching PS, Beaumont B, Ranasinghe S, Taylor G, Merrilees MJ. Changes in elastic fibres in the small airways and alveoli in COPD. Eur Respir J. 2008;31:998–1004. doi: 10.1183/09031936.00017207. [DOI] [PubMed] [Google Scholar]
- 8.Mead J, Turner JM, Macklem PT, Little JB. Significance of the relationship between lung recoil and maximum expiratory flow. J Appl Physiol. 1967;22:95–108. doi: 10.1152/jappl.1967.22.1.95. [DOI] [PubMed] [Google Scholar]
- 9.Jeffery P, Holgate S, Wenzel S. Methods for the assessment of endobronchial biopsies in clinical research: application to studies of pathogenesis and the effects of treatment. Am J Respir Crit Care Med. 2003;168:S1–17. doi: 10.1164/rccm.200202-150WS. [DOI] [PubMed] [Google Scholar]
- 10.Buczek-Thomas JA, Lucey EC, Stone PJ, Chu CL, Rich CB, Carreras I, Goldstein RH, Foster JA, Nugent MA. Elastase mediates the release of growth factors from lung in vivo. Am J Respir Cell Mol Biol. 2004;31:344–350. doi: 10.1165/rcmb.2003-0420OC. [DOI] [PubMed] [Google Scholar]
- 11.Solem M, Rawson C, Lindburg K, Barnes D. Transforming growth factor beta regulates cystatin C in serum-free mouse embryo (SFME) cells. Biochem Biophys Res Commun. 1990;172:945–951. doi: 10.1016/0006-291x(90)90767-h. [DOI] [PubMed] [Google Scholar]
- 12.Kotajima N, Yanagawa Y, Aoki T, Tsunekawa K, Morimura T, Ogiwara T, Nara M, Murakami M. Influence of thyroid hormones and transforming growth factor-beta1 on cystatin C concentrations. J Int Med Res. 2010;38:1365–1373. doi: 10.1177/147323001003800418. [DOI] [PubMed] [Google Scholar]
- 13.Barrett AJ, Davies ME, Grubb A. The place of human gamma-trace (cystatin C) amongst the cysteine proteinase inhibitors. Biochem Biophys Res Commun. 1984;120:631–636. doi: 10.1016/0006-291x(84)91302-0. [DOI] [PubMed] [Google Scholar]
- 14.Chapman HA Jr, Reilly JJ Jr, Yee R, Grubb A. Identification of cystatin C, a cysteine proteinase inhibitor, as a major secretory product of human alveolar macrophages in vitro. Am Rev Respir Dis. 1990;141:698–705. doi: 10.1164/ajrccm/141.3.698. [DOI] [PubMed] [Google Scholar]
- 15.Kos J, Lah TT. Cysteine proteinases and their endogenous inhibitors: target proteins for prognosis, diagnosis and therapy in cancer (review) Oncol Rep. 1998;5:1349–1361. doi: 10.3892/or.5.6.1349. [DOI] [PubMed] [Google Scholar]
- 16.Warfel AH, Cardozo C, Yoo OH, Zucker-Franklin D. Cystatin C and cathepsin B production by alveolar macrophages from smokers and nonsmokers. J Leukoc Biol. 1991;49:41–47. doi: 10.1002/jlb.49.1.41. [DOI] [PubMed] [Google Scholar]
- 17.Hojs R, Bevc S, Ekart R, Gorenjak M, Puklavec L. Serum cystatin C as an endogenous marker of renal function in patients with chronic kidney disease. Ren Fail. 2008;30:181–186. doi: 10.1080/08860220701810315. [DOI] [PubMed] [Google Scholar]
- 18.Angelidis C, Deftereos S, Giannopoulos G, Anatoliotakis N, Bouras G, Hatzis G, Panagopoulou V, Pyrgakis V, Cleman MW. Cystatin C: an emerging biomarker in cardiovascular disease. Curr Top Med Chem. 2013;13:164–179. doi: 10.2174/1568026611313020006. [DOI] [PubMed] [Google Scholar]
- 19.Rokadia HK, Agarwal S. Serum cystatin C and emphysema: results from the National Health and Nutrition Examination Survey (NHANES) Lung. 2012;190:283–290. doi: 10.1007/s00408-012-9374-z. [DOI] [PubMed] [Google Scholar]
- 20.Takeyabu K, Betsuyaku T, Nishimura M, Yoshioka A, Tanino M, Miyamoto K, Kawakami Y. Cysteine proteinases and cystatin C in bronchoalveolar lavage fluid from subjects with subclinical emphysema. Eur Respir J. 1998;12:1033–1039. doi: 10.1183/09031936.98.12051033. [DOI] [PubMed] [Google Scholar]
- 21.Vestbo J, Hurd SS, Rodriguez-Roisin R. [An overview of Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease (GOLD) (revised 2011)] . Zhonghua Yi Xue Za Zhi. 2012;92:937–938. [PubMed] [Google Scholar]
- 22.Tetley TD. Inflammatory cells and chronic obstructive pulmonary disease. Curr Drug Targets Inflamm Allergy. 2005;4:607–618. doi: 10.2174/156801005774912824. [DOI] [PubMed] [Google Scholar]
- 23.Sarir H, Henricks PA, van Houwelingen AH, Nijkamp FP, Folkerts G. Cells, mediators and Toll-like receptors in COPD. Eur J Pharmacol. 2008;585:346–353. doi: 10.1016/j.ejphar.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 24.Rintahaka J, Lietzen N, Ohman T, Nyman TA, Matikainen S. Recognition of cytoplasmic RNA results in cathepsin-dependent inflammasome activation and apoptosis in human macrophages. J Immunol. 2011;186:3085–3092. doi: 10.4049/jimmunol.1002051. [DOI] [PubMed] [Google Scholar]
- 25.Ishii T, Abboud RT, Wallace AM, English JC, Coxson HO, Finley RJ, Shumansky K, Pare PD, Sandford AJ. Alveolar macrophage proteinase/antiproteinase expression and lung function/emphysema. Eur Respir J. 2014;43:82–91. doi: 10.1183/09031936.00174612. [DOI] [PubMed] [Google Scholar]
- 26.MacDonald M, Korman T, King P, Hamza K, Bardin P. Exacerbation phenotyping in chronic obstructive pulmonary disease. Respirology. 2013;18:1280–1281. doi: 10.1111/resp.12197. [DOI] [PubMed] [Google Scholar]
- 27.Zeng G, Sun B, Zhong N. Non-smoking-related chronic obstructive pulmonary disease: a neglected entity? Respirology. 2012;17:908–912. doi: 10.1111/j.1440-1843.2012.02152.x. [DOI] [PubMed] [Google Scholar]
- 28.Odden MC, Tager IB, Gansevoort RT, Bakker SJ, Katz R, Fried LF, Newman AB, Canada RB, Harris T, Sarnak MJ, Siscovick D, Shlipak MG. Age and cystatin C in healthy adults: a collaborative study. Nephrol Dial Transplant. 2010;25:463–469. doi: 10.1093/ndt/gfp474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ren WY, Li L, Zhao RY, Zhu L. Age-associated changes in pulmonary function: a comparison of pulmonary function parameters in healthy young adults and the elderly living in Shanghai. Chin Med J (Engl) 2012;125:3064–3068. [PubMed] [Google Scholar]


