Abstract
Background: Type 1 diabetes mellitus (TIDM) results from an immune-mediated destruction of insulin-producing-cells in the pancreatic islets of Langerhans. There are clear differences in immunogenetic predisposition to type1 diabetes among countries. Studies have indicated that vitamin D supplementation in early childhood decreases the risk of TIDM. Vitamin D exerts its action via the nuclear vitamin D receptor (VDR), which shows an extensive polymorphism. VDR gene polymorphisms have been associated with altered gene expression or gene function. Four single nucleotide polymorphisms (SNPs) in the VDR gene produce variation in four recognition sites. These recognition sites variants include Fok I, Bsm I, Apa I and Taq I. Aim of the study: TO investigate the relationship between VDR gene polymorphisms (at positions Taq I and Apa I) and the incidence of TIDM in Egyptian peoples. Subjects and methods: This study included 74 patients with type 1 DM in addition to 28 healthy age and sex matched control subjects. All of them were subjected to full history taking and clinical examination. Three ml of venous blood were withdrawn from each patient at fasting and postprandial times and used for genomic DNA extraction, estimation of Hb A1C, as well as, fasting and postprandial C-peptide and blood glucose levels. Results: Apa I recognition site was found in low frequency in diabetic patients (14/74) 18.9% while, its frequency was high (16/28) 57.1% among normal subjects. Taq I has two recognition sites. The first was found at nucleotide number 293 that was found in a frequency of (2/28) 7.1% in normal non-diabetic individuals while it was detected in (14/74) 18.9% in diabetic patients. The second Taq I recognition site was found at nucleotide number 494 without any differences between diabetic and normal individuals. Conclusion: This study indicates that there is an association between VDR genetic polymorphism and incidence of TIDM in Egyptian patients.
Keywords: Vitamin D receptor (VDR), polymorphism, type 1 diabetes mellitus (TIDM)
Introduction
Type 1 diabetes mellitus (TIDM) is the most common form of diabetes in childhood and it is characterized by the destruction of pancreatic beta cells resulting in the absence of insulin secretion, thus requiring exogenous insulin for survival [1]. The activation of autoreactive lymphocytes and the cytokine induced apoptosis of pancreatic-cells play a major role in the etiology of type 1 diabetes. There are clear differences in the immunogenetic predisposition to type 1 diabetes between countries and the disease incidence seems to vary along with the differences in the predisposition [2].
Global incidence of diabetes mellitus has been increased in recent decades; there are strong differences between different geographical areas and population groups [3]. 1, 25-Dihydroxyvitamin D3 inhibits lymphocyte activation and affects other elements of the immune system, such as cytokine and immunoglobulin production, as well as major histocompatibility complex (MHC) class II and the cluster differentiation 4 (CD-4) expression [4,5].
Studies in humans have indicated that vitamin D supplementation in early childhood decreases the risk of TIDM and the intake of vitamin D in pregnancy may prevent the appearance of islet autoantibodies in the offspring [6]. Moreover, supplementing infants with vitamin D was suggested to be safe and effective strategy for reducing the risk of TIDM [7].
Vitamin D exerts its action via the nuclear vitamin D receptor (VDR), which shows an extensive polymorphism. The VDR belongs to the steroid receptor super-family and is widely expressed in many cell types, including lymphocytes, macrophages, and pancreatic cells [8]. VDR is located in the q13 region of chromosome 12 [9]. Polymorphisms within the VDR gene have been associated with altered gene expression or gene function [10].
The role of VDR polymorphisms in TIDM pathogenesis has been unclear. Several studies have suggested association between one or more of these SNPs and TIDM [11-13], but others have failed to confirm this finding [3]. Moreover, this inconsistency was attributed to the environmental factors that potentially interfere with the VDR genotype [14]. The interactions of the genetic background with the development of TIDM are well documented in various populations as the incidence of childhood TIDM is known to vary widely between and within countries [11-13].
It is known that type 1 diabetes is a multi-factorial disease, with genetic and environmental factors that could explain the incidence rates that have been found in different ethnic groups and countries. Taking into consideration the environmental influence on development of this disease and its relation with genetic factors, we examined the VDR polymorphisms that interact with vitamin D.
This study was conducted to investigate the relationship between VDR gene polymorphisms (at positions Taq I and Apa I) and the incidence of TIDM in Egyptian peoples.
Subjects and methods
Subjects
This study included 74 patients with TIDM included 48 males and 26 females with an age range of 3 to 26 years. All patients were considered as affected by TIDM according to American Diabetes Association criteria [15]. Patients with pancreatitis and other immune mediated diseases were excluded. Twenty eight healthy persons (control group) were involved in this study, with an age range of 5 to 29 years, the control individuals with no history of diabetes or other autoimmune diseases. Cases were selected from the Pediatric and Internal Medicine departments, Faculty of Medicine, Cairo University, at the period from April, 2012 to January 2014.
Ethical approval
This study was approved by the ethical committee of Faculty of Medicine, Cairo University. Written informed consents were obtained from all participants involved in our study.
Methods
All subjects involved in this study were subjected to the following:
Full clinical examination
Full history taking and clinical examination, including the onset of disease, insulin treatment, blood sugar level and complication of the disease were recorded. Data about age, sex, weight, height, liver, spleen, kidney, neurological and eye examination were collected.
Sampling
3 mL of venous blood were withdrawn from each patient at fasting time and postprandial by sterile heparinized tubes. These samples were used for genomic DNA extraction, and estimation of Hb A1C (was measured by high-performance liquid chromatography (normal range, 3.5-6.0%), as well as fasting and postprandial C-peptide, fasting and postprandial blood glucose level were measured using an immunofluorometric assay with detection limit of 0.15 ng/ml.
Separation of peripheral blood polymorph nuclear leukocytes (PMNs)
PMNs were separated using RPMI solutions and Ficoll-PM (Sigma-Aldrich) according to the manufacturer’s instruction.
DNA extraction and polymerase chain reaction (PCR)
Genomic DNA was extracted from peripheral blood polymorph nuclear (PMNs) cells using a genomic DNA Isolation System (Koma Bioteck. Inc., Seoul, Korea). DNA samples concentration and quality were detected spectrophotometrically at 260/280 nm. PCR was performed using Taq/Apa-for (5’ -CAG AGC ATG GAC AGG GAG CAA-3’) and Taq/Apa-rev (5’ -GCA ACT CCT CAT GGC TGA GGT CTC-3’) primers that flank a 740-bp fragment of Vitamin D intron 8/exon 9 as previously described [5]. The PCR conditions were; an initial denaturation for 5 min at 94°C, 35 cycles each of which consisted of (denaturation at 94°C for 1 min, annealing at 60°C for 1 min and extension at 72°C for 1 min), and final extension for 7 min at 72°C.
Single nucleotide polymorphisms of the vitamin D by direct sequencing
PCR products were subjected to electrophoresis in 1% agarose gel with ethidium bromide staining. VDR specific bands were excised and purified with the QIAquick gel extraction kit (Koma Bioteck, Seoul, Korea). Purified PCR products were sequenced directly using specific primer pairs (Macrogen, Korea). Different sequences of the VDR gene were submitted to GenBank (accession numbers KF054040-KF054055).
Multiple sequence analysis and phylogenetic tree
Comparative analyses were performed using the CLUSTAL W multiple sequence alignment program, Mega 4.1 [16]. VDR sequences obtained in the current study were used for the alignments. The phylogenetic tree was constructed by using the neighbor-joining method with Kimura two-parameter distances by using the Mega 4.1. The reliability of internal branches was assessed by 1000 bootstrap replications and the p-distance substitution model.
Statistical analysis
Data were analyzed using SPSS, version 11.5 statistical software. Comparisons of genotype frequencies between groups were performed using the t-test. The chi-squared test was used for analysis of the difference between the 2 groups. P-value < 0.05 was considered significant. The association between VDR variants and TIDM was assessed using logistic regression method by an additive model in PLINK. Omnibus and haplotype association test was also performed by PLINK. Linkage disequilibrium (LD) between two loci was performed by Haploview program. Genotypes, alleles and haplotypes in cases and healthy controls were compared by two-tailed Fisher’s exact test. Odds ratios (OR) and 95% confidence intervals (CI) were calculated for each allele, genotype and haplotype. Hardy-Weinberg equilibriums were tested to compare the observed and expected genotype and allele frequencies.
Results
Patients were studied at diagnosis and during the course of treatment. The study included 48 males (64.9%) and 26 females (35.1%), with an age range of 3 to 26 years, a mean ± SD of (12.7 ± 4.7) and median 10.5 years. Of the 74 patients, only 4 cases (5.4%) showed renal complication, 2 cases (2.7%) with eye complication and 12 cases (16.2%) with uncontrolled TIDM (Figure 1).
Figure 1.
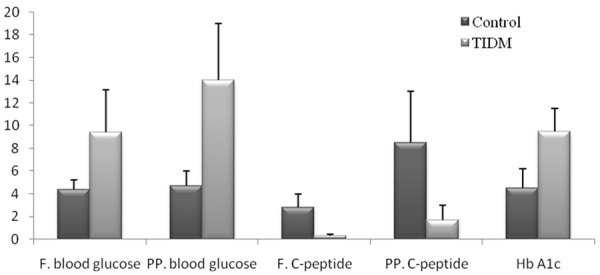
The clinical and laboratory data of the patients and the controls. (Fasting and postprandial blood glucose (mmol/L), C-peptide (ng/ml) and HbA1c (%)).
ApaI recognition site (GGGCC/C) was found at nucleotide number 213 in (14/74) 18.9% for aa in diabetic patient while it was found among normal subjects with high frequency; (16/28) 57.1%. The rest of individuals showed GTGCCC motif (Table 1). Taq I recognition sites (T/CGA) at nucleotide number 494 was found in amplicon from all tested individuals, however, a second site was found at nucleotide number 293 that was presented only in some individuals. We obtained allelic frequencies of (81.1% vs. 18.9%), (42.9% vs. 57.1%), for (A vs. a) in diabetic patients and control individuals respectively, while (81.1% vs. 18.9%), (92.9% vs. 7.1%) for (T vs. t) alleles in diabetic patients and control individuals respectively (Figure 2).
Table 1.
Vitamin D Receptor polymorphism
| Patients (74) | Control (28) | P-value | |
|---|---|---|---|
| Apa I | 14 (18.9%) | 16 (57.1%) | < 0.05 |
| Taq I (1st site) | 74 (100%) | 28 (100%) | ≥ 0.05 |
| Taq I (2nd site) | 14 (18.9%) | 2 (7.1%) | < 0.05 |
Figure 2.
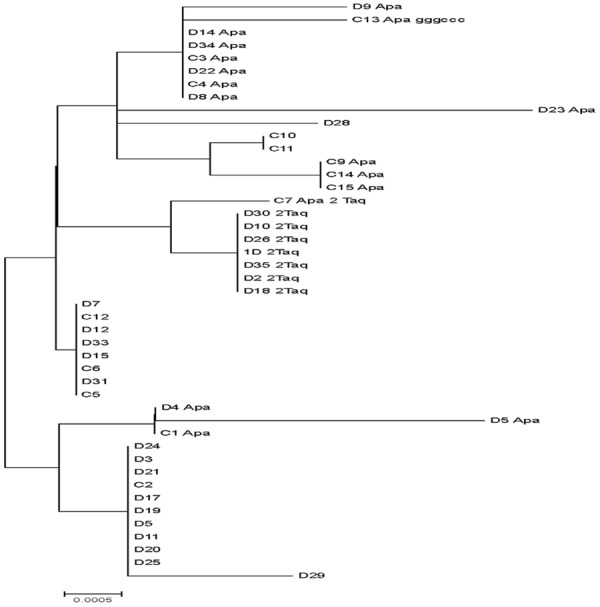
Phylogenetic tree of VDR sequences of the Egyptian nationals generated by neighbour-joining analysis. The robustness of individual nodes of the tree was assessed using a bootstrap of 1000 replications of bootstrap re-sampling of the originally-aligned nucleotide sequences. Scale bar represents 0.002 nucleotide substitutions.
Discussion
Vitamin D receptor is known to modulate cell proliferation and differentiation, and calcium absorption from the gut. The action of VDR is regulated by vitamin D, parathyroid hormone, growth factors and protein kinase A. Defects in the VDR gene could modulate the metabolism of calcium thus increasing the risk of developing different diseases including calcium stones, prostate cancer, diabetes, osteoporosis, and many others [9]. The active form of vitamin D, 1, 25-Dihydroxyvitamin D, performs its effects via the VDR. Single nucleotide polymorphisms (SNPs), Apa I A > a (rs7975232), and Taq I T > t (rs731236), in the VDR gene have been investigated. The Apa I a allele corresponds to a T→G transition and the Taq I t allele results in a silent T→C transition in intron 8 and exon 9 respectively [17]. The PCR amplicon used in the reaction, 740 bp of the gene, flanked both the Apa I and Taq I (designated as rs7975232 and rs731236 SNP, respectively) polymorphic sites [5].
The Taq I polymorphism results in a silent mutation in the VDR gene [18], which would therefore not be expected to alter VDR function. The Apa I site is located within an intron of the VDR gene. Alterations in intronic sequences may influence protein expression [19]. Four common single SNPs in the VDR gene have been studied intensively [20]: FokI T > C (rs10735810), BsmI A > G (rs1544410), ApaI G > T (rs7975232) and TaqI C > T (rs731236). These SNPs have been screened for the association with various human traits and diseases [20]. Several studies reported association of type 1 diabetes with one of these SNPs [13,21], however, the reported associations were inconsistent among different studies [3,5,11,22-24].
In the present study, we have investigated the distribution of VDR (Taq-I and Apa-I) genotypes in Egyptian patients with type 1 diabetes mellitus and compared them with healthy controls. Here we reported a study of association between SNPs in the VDR gene region and type 1 diabetes and their Apa I and TaqI haplotypes to be associated with type 1 diabetes in the Egyptian population. SNPs of Apa I and Taq I with linkage disequilibrium were detected in the current study.
The VDR locus has been studied for the association with the susceptibility to immune-mediated diseases including TIDM, but findings have often been contradictory among different populations worldwide [25-28]. These could be due to ethnic differences, diverse genetic or environmental factors involved in the pathogenesis of TIDM. The VDR polymorphism may reflect linkage disequilibrium and act as marker for functional variants that affect expression levels of VDR rather than being the disease-affecting locus. Taq I is a silent SNP in exon 9, however, Apa I is located in the intron between exons 8-9 and does not affect VDR protein structure [25,29].
In addition to the allelic variation in relation to ApaI and TaqI recognition sites, deduced nucleotide alignment and phylogenic tree analysis revealed the presence of difference in nucleotide sequences of the tested individuals (Figure 2). The observed nucleotide substitutions possessed no uniform distribution among different diabetic and control individuals however, the impact of these substitutions on the gene structure and functions are unknown and need further investigation. Our results suggested that the frequency and distribution of the polymorphisms in type 1 diabetic Egyptian patients and normal person were substantially different from other populations. Thus the data suggests an impact of ethnicity and provides a basis for future epidemiological and clinical studies.
Conflicting results were found regarding the correlation of Vitamin D receptor and Bsm1, Fok1, Apa1, and Taq1 genotypes, some studies showed that the distributions were not different between patients with diabetes and control groups [30].
The apparent discrepancies between this study and others could be a result of the effect of ethnic differences related to the distribution of VDR polymorphisms in these populations, as well as the interactions with other genetic or environmental factors involved in the pathogenesis of type 1 diabetes mellitus [13].
It seems that environmental factors that influence levels of active vitamin D in humans are complex and a significant difference exists between vitamin D functions and VDR polymorphisms [21]. Interestingly, environmental factor(s) were described to alter the risk associated with VDR SNPs [31].
This study has been carried out on Egyptian TIDM patient and healthy control individuals possessing different environmental factors in comparison to other locations in other countries. The interaction of these special environmental conditions with the genetic constituents may increase or decrease the incidence of TID among Egyptian population in comparison to other countries.
On conclusion, the present study showed variability in the VDR gene on the basis of SNPs of the basic core sequence. There were differences in allele frequency and distribution of genotypes of VDR (Taq-I and Apa-I) in Egyptian diabetic patients than controls, as they represent different environmental factors in comparison to other countries. We recommend further studies with larger sample size in different population. In addition, future studies on the correlation between environmental factors and TIDM and to extend the analysis to gene SNPs in vitamin D metabolism genes on which there is consensus that they are associated with TIDM.
References
- 1.Sloka S, Grant M, Newhook LA. The geospatial relation between UV solar radiation and type 1 diabetes in Newfoundland. Acta Diabetol. 2010;47:73–8. doi: 10.1007/s00592-009-0100-0. [DOI] [PubMed] [Google Scholar]
- 2.Atkinson MA, MacLaren NK. Mechanisms of disease: thepathogenesis of insulin-dependent diabetes mellitus. N Engl J Med. 1994;331:1428–1436. doi: 10.1056/NEJM199411243312107. [DOI] [PubMed] [Google Scholar]
- 3.Turpeinen H, Hermann R, Vaara S, Laine AP, Simell O, Knip M, Veijola R, Ilonen J. Vitamin D receptor polymorphisms: no association with type 1 diabetes in the Finnish population. Eur J Endocrinol. 2003;149:591–596. doi: 10.1530/eje.0.1490591. [DOI] [PubMed] [Google Scholar]
- 4.Thomasset M. Vitamin D and the immune system. Pathol Biol. 1994;42:163–172. [PubMed] [Google Scholar]
- 5.Pani MA, Knapp M, Donner H, Braun J, Baur MP, Usadel KH, Badenhoop K. Vitamin D receptor allele combinations influence genetic susceptibility to type 1 diabetes in Germans. Diabetes. 2000;49:504–507. doi: 10.2337/diabetes.49.3.504. [DOI] [PubMed] [Google Scholar]
- 6.Fronczak CM, Baron AE, Chase HP. In utero dietary exposures and risk of islet autoimmunity in children. Diabetes Care. 2003;26:3237–3242. doi: 10.2337/diacare.26.12.3237. [DOI] [PubMed] [Google Scholar]
- 7.Bener A, Alsaied A, Al-Ali M, Hassan AS, Basha B, Al-Kubaisi A, Abraham A, Mian M, Guiter G, Tewfik I. Impact of lifestyle and dietary habits on hypovitaminosis D in type 1 diabetes mellitus and healthy children from Qatar, a sun-rich country. Ann Nutr Metab. 2008;53:215–22. doi: 10.1159/000184439. [DOI] [PubMed] [Google Scholar]
- 8.Walters MR. Newly identified actions of the vitamin D endocrine system. Endocr Rev. 1992;13:719–764. doi: 10.1210/edrv-13-4-719. [DOI] [PubMed] [Google Scholar]
- 9.Bid HK, Mishra DK, Mittal RD. Vitamin-D receptor (VDR) gene (Fok-I, Taq-I and Apa-I) polymorphisms in healthy individuals from north Indian population. Asian Pac J Cancer Prev. 2005;6:147–52. [PubMed] [Google Scholar]
- 10.Van Etten E, Verliden L, Giulietti A, Ramos-Lopez E, Branisteanu DD, Ferreira GB. The vitamin D receptor gene FokI polymorphism: Functional impact on the immune system. Eur J Immunol. 2007;37:395–405. doi: 10.1002/eji.200636043. [DOI] [PubMed] [Google Scholar]
- 11.McDermott MF, Ramachandran A, Ogunkolade BW, Aganna E, Curtis D, Boucher BJ, Snehalatha C, Hitman GA. Allelic variation in the vitamin D receptor influences susceptibility to IDDM in Indian Asians. Diabetologia. 1997;40:971–975. doi: 10.1007/s001250050776. [DOI] [PubMed] [Google Scholar]
- 12.Ban Y, Taniyama M, Yanagawa T, Yamada S, Maruyama T, Kasuga A, Ban Y. Vitamin D receptor initiation codon polymorphism influences genetic susceptibility to type 1 diabetes mellitus in the Japanese population. BMC Med Genet. 2001;2:7. doi: 10.1186/1471-2350-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohammad Nejad Z, Ghanbari M, Ganjali R, Afshari JT, Heydarpour M, Taghavi SM, Fatemi S, Rafatpanah H. Association between vitamin D receptor gene polymorphisms and type 1 diabetes mellitus in Iranian population. Mol Biol Rep. 2012;39:831–837. doi: 10.1007/s11033-011-0805-3. [DOI] [PubMed] [Google Scholar]
- 14.Ponsonby AL, Pezic A, Ellis J, Morley R, Cameron F, Carlin J, Dwyer T. Variation in associations between allelic variants of the vitamin D receptor gene and onset of type 1 diabetes mellitus by ambient winter ultraviolet radiation levels: a meta-regression analysis. Am J Epidemiol. 2008;168:358–365. doi: 10.1093/aje/kwn142. [DOI] [PubMed] [Google Scholar]
- 15.American Diabetes Association. Clinical practice recommendations. Diab Care. 2013;36:S1–S110. [PubMed] [Google Scholar]
- 16.Kumar S, Tamura K, Jakobsen IB, Nei M. Molecular evolutionary genetics analysis software. Bioinformatics. 2001;17:1244–1245. doi: 10.1093/bioinformatics/17.12.1244. [DOI] [PubMed] [Google Scholar]
- 17.Panierakis C, Goulielmos G, Mamoulakis D, Petraki E, Papavasiliou E, Galanakis E. Vitamin D receptor gene polymorphisms and susceptibility to type 1 diabetes in Crete, Greece. Clin Immunol. 2009;133:276–81. doi: 10.1016/j.clim.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Nishimura A, Shinki T, Jin CH, Ohyama Y, Noshiro M, Okuda K, Suda T. Regulation of messenger ribonucleic acid expression of 1 alpha, 25-dihydroxyvitamin D3-24-hydroxylase in rat osteoblasts. Endocrinology. 1994;134:1794–1799. doi: 10.1210/endo.134.4.8137744. [DOI] [PubMed] [Google Scholar]
- 19.Mocharla H, Butch AW, Pappas AA, Flick JT, Weinstein RS, De Togni P, Jilka RL, Roberson PK, Parfitt AM, Manolagas SC. Quantification of vitamin D receptor mRNA by competitive polymerase chain reaction in PBMC: lack of correspondence with common allelic variants. J Bone Miner Res. 1997;12:726–733. doi: 10.1359/jbmr.1997.12.5.726. [DOI] [PubMed] [Google Scholar]
- 20.Zmuda JM, Cauley JA, Ferrell RE. Molecular epidemiology of vitamin D receptor gene variants. Epidemiol Rev. 2000;22:203–217. doi: 10.1093/oxfordjournals.epirev.a018033. [DOI] [PubMed] [Google Scholar]
- 21.Bonakdaran S, Abbaszadegan MR, Dadkhah E, Khajeh-Dalouie M. Vitamin D receptor gene polymorphisms in type 1 diabetes mellitus: a new pattern from Khorasan province, Islamic Republic of Iran. East Mediterr Health J. 2012;18:614–619. doi: 10.26719/2012.18.6.614. [DOI] [PubMed] [Google Scholar]
- 22.Koeleman BP, Valdigem G, Eerligh P, Giphart MJ, Roep BO. Seasonality of birth in patients with type 1 diabetes. Lancet. 2002;359:1246–1247. doi: 10.1016/S0140-6736(02)08228-4. [DOI] [PubMed] [Google Scholar]
- 23.Guja C, Marshall S, Welsh K, Merriman M, Smith A, Todd JA, Ionescu Tirgoviste C. The study of CTLA-4 and vitamin D receptor polymorphisms in the Romanian type 1 diabetes population. J Cell Mol Med. 2002;6:75–81. doi: 10.1111/j.1582-4934.2002.tb00312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fassbender WJ, Goertz B, Weismuller K, Steinhauer B, Stracke H, Auch D, Linn T, Bretzel RG. VDR gene polymorphisms are overrepresented in German patients with type 1 diabetes compared to healthy controls without effect on biochemical parameters of bone metabolism. Horm Metab Res. 2002;34:330–337. doi: 10.1055/s-2002-33262. [DOI] [PubMed] [Google Scholar]
- 25.Uitterlinden AG, Fang Y, Van Meurs JB, Pols HA, Van Leeuwen JP. Genetics and biology of vitamin D receptor polymorphisms. Gene. 2004;338:143–156. doi: 10.1016/j.gene.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 26.Wang G, Zhang Q, Xu N, Xu K, Wang J. Associations between Two Polymorphisms (FokI and BsmI) of Vitamin D Receptor Gene and Type 1 Diabetes Mellitus in Asian Population: A Meta-Analysis. PLoS One. 2014;9:e89325. doi: 10.1371/journal.pone.0089325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo SW, Magnuson VL, Schiller JJ. Meta-analysis of vitamin D receptor polymorphisms and type 1 diabetes: a HuGE review of genetic association studies. Am J Epidemiol. 2006;164:711–724. doi: 10.1093/aje/kwj278. [DOI] [PubMed] [Google Scholar]
- 28.Bianco MG, Minicucci L, Calevo MG. Vitamin D receptor polymorphisms: are they really associated with type 1 diabetes. Eur J Endocrinol. 2004;151:641–642. doi: 10.1530/eje.0.1510641. [DOI] [PubMed] [Google Scholar]
- 29.Obi-Tabot ET, Tian XQ, Chen TC, Holick MF. A human skin equivalent model that mimics the photoproduction of vitamin D3 in human skin. In Vitro Cell Dev Biol Anim. 2000;36:201–204. doi: 10.1290/1071-2690(2000)036<0201:AHSEMT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 30.Gogas Yavuz D, Keskin L, Kıyıcı S, Sert M, Yazıcı D, Sahin I, Yüksel M, Deyneli O, Aydın H, Tuncel E, Akalın S. Vitamin D receptor gene BsmI, FokI, ApaI, TaqI polymorphisms and bone mineral density in a group of Turkish type 1 diabetic patients. Acta Diabetol. 2011;48:329–36. doi: 10.1007/s00592-011-0284-y. [DOI] [PubMed] [Google Scholar]
- 31.Zittermann A. Vitamin D in preventive medicine: are we ignoring the evidence? Br J Nutr. 2003;89:552–572. doi: 10.1079/BJN2003837. [DOI] [PubMed] [Google Scholar]


