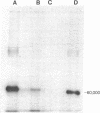
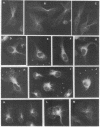
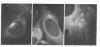
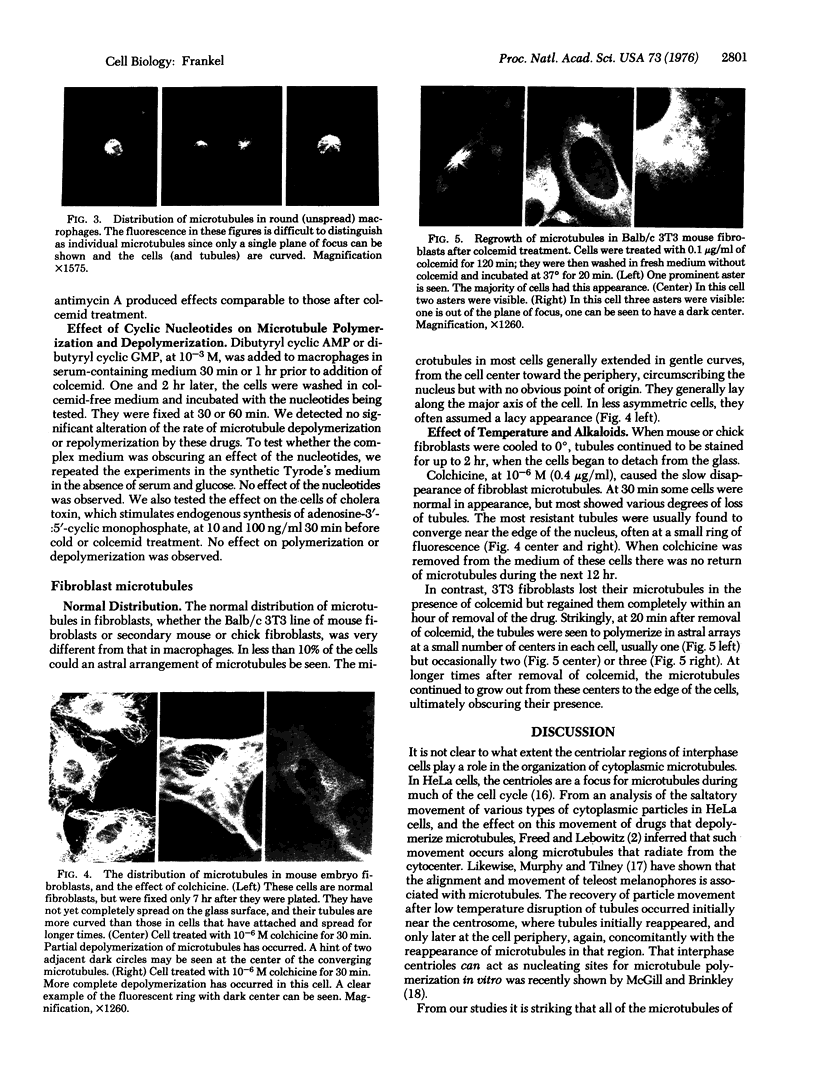
Abstract
The organization and growth of microtubules in cultured mouse macrophages and fibroblasts were examined by indirect immunofluorescence microscopy with antibodies to microtubule protein. In macrophages, microtubules converged at a samll region at the cytocenter. During depolymerization, and repolymerization, this region acted as a microtubule organizing center. Microtubule growth was energy-dependent, but unaffected by dibutyryl-adenosine 3':5'-cyclic monophosphate, cholera toxin, or dibutyryl-guanosine 3':5'-cyclic monophosphate. Fibroblasts, which did not show such a simple microtubule organization as macrophages, contained mainly one or two, but occasionally as many as four, organizing centers during repolymerization. These microtubule organizing centers often appeared as fluorescent rings with a dark center.
Full text
PDF

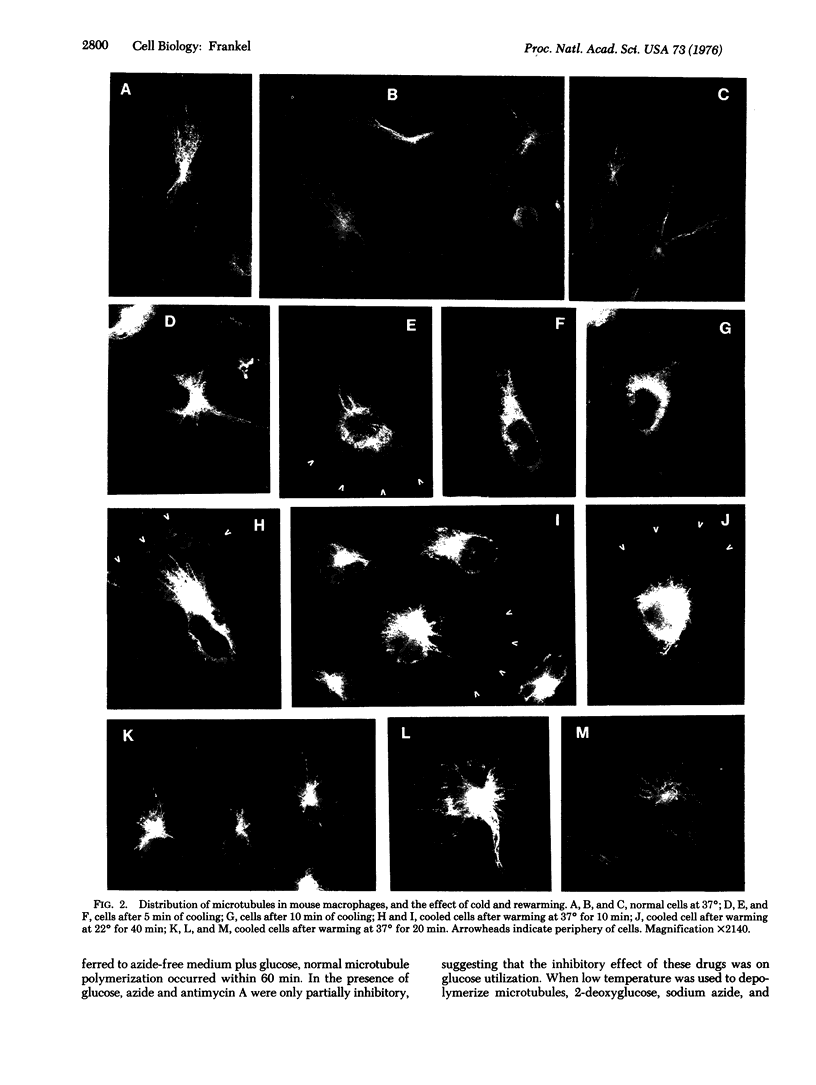

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C. The role of microfilaments and microtubules in cell movement, endocytosis and exocytosis. Ciba Found Symp. 1973;14:109–148. doi: 10.1002/9780470719978.ch6. [DOI] [PubMed] [Google Scholar]
- Bhisey A. N., Freed J. J. Ameboid movement induced in cultured macrophages by colchicine or vinblastine. Exp Cell Res. 1971 Feb;64(2):419–429. doi: 10.1016/0014-4827(71)90096-6. [DOI] [PubMed] [Google Scholar]
- Brinkley B. R., Fuller E. M., Highfield D. P. Cytoplasmic microtubules in normal and transformed cells in culture: analysis by tubulin antibody immunofluorescence. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4981–4985. doi: 10.1073/pnas.72.12.4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., FREEMAN G. Plaque production by the polyoma virus. Virology. 1959 Jul;8(3):396–397. doi: 10.1016/0042-6822(59)90043-1. [DOI] [PubMed] [Google Scholar]
- Daniels M. P. Fine structural changes in neurons and nerve fibers associated with colchicine inhibition of nerve fiber formation in vitro. J Cell Biol. 1973 Aug;58(2):463–470. doi: 10.1083/jcb.58.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey M. J., Wiberg J. S., Frankel F. R. Genetic control of whisker antigen of bacteriophage T4D. J Mol Biol. 1974 Apr 25;84(4):625–634. doi: 10.1016/0022-2836(74)90120-x. [DOI] [PubMed] [Google Scholar]
- Freed J. J., Lebowitz M. M. The association of a class of saltatory movements with microtubules in cultured cells. J Cell Biol. 1970 May;45(2):334–354. doi: 10.1083/jcb.45.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller G. M., Brinkley B. R., Boughter J. M. Immunofluorescence of mitotic spindles by using monospecific antibody against bovine brain tubulin. Science. 1975 Mar 14;187(4180):948–950. doi: 10.1126/science.1096300. [DOI] [PubMed] [Google Scholar]
- Goldman R. D., Berg G., Bushnell A., Chang C. M., Dickerman L., Hopkins N., Miller M. L., Pollack R., Wang E. Fibrillar systems in cell motility. Ciba Found Symp. 1973;14:83–107. doi: 10.1002/9780470719978.ch5. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Kirkland W. L., Burton P. R. Cyclic adenosine monophosphate-mediated stabilization of mouse neuroblastoma cell neuritis microtubules exposed to low temperature. Nat New Biol. 1972 Dec 13;240(102):205–207. doi: 10.1038/newbio240205a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Weber K. Actin antibody: the specific visualization of actin filaments in non-muscle cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2268–2272. doi: 10.1073/pnas.71.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malawista S. E. Microtubules and the mobilization of lysosomes in phagocytizing human leukocytes. Ann N Y Acad Sci. 1975 Jun 30;253:738–749. doi: 10.1111/j.1749-6632.1975.tb19242.x. [DOI] [PubMed] [Google Scholar]
- McGill M., Brinkley B. R. Human chromosomes and centrioles as nucleating sites for the in vitro assembly of microtubules from bovine brain tubulin. J Cell Biol. 1975 Oct;67(1):189–199. doi: 10.1083/jcb.67.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D. B., Tilney L. G. The role of microtubules in the movement of pigment granules in teleost melanophores. J Cell Biol. 1974 Jun;61(3):757–779. doi: 10.1083/jcb.61.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J. M., Zurier R. B., Berlin R. D. Concanavalin a cap formation on polymorphonuclear leukocytes of normal and beige (chediak-higashi) mice. Nature. 1975 Feb 6;253(5491):471–473. doi: 10.1038/253471a0. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B., Borisy G. G. Microtubules. Annu Rev Biochem. 1973;42:507–540. doi: 10.1146/annurev.bi.42.070173.002451. [DOI] [PubMed] [Google Scholar]
- Porter K. R., Puck T. T., Hsie A. W., Kelley D. An electron microscopy study of the effects on dibutyryl cyclic AMP on Chinese hamster ovary cells. Cell. 1974 Jul;2(3):145–162. doi: 10.1016/0092-8674(74)90089-0. [DOI] [PubMed] [Google Scholar]
- Robbins E., Jentzsch G., Micali A. The centriole cycle in synchronized HeLa cells. J Cell Biol. 1968 Feb;36(2):329–339. doi: 10.1083/jcb.36.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Pollack R., Bibring T. Antibody against tuberlin: the specific visualization of cytoplasmic microtubules in tissue culture cells. Proc Natl Acad Sci U S A. 1975 Feb;72(2):459–463. doi: 10.1073/pnas.72.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann G., Goldstein I., Hoffstein S., Tsung P. K. Reciprocal effects of cAMP and cGMP on microtubule-dependent release of lysosomal enzymes. Ann N Y Acad Sci. 1975 Jun 30;253:750–762. doi: 10.1111/j.1749-6632.1975.tb19243.x. [DOI] [PubMed] [Google Scholar]