Abstract
In the present study, we aimed to detect microRNA-9 (miR-9) expression level and its clinical significance in laryngeal squamous cell carcinomas (LSCC). 103 patients who were diagnosed with LSCC and treated between March 2010 and June 2013 were enrolled in this study. Expression levels of miR-9 were detected by real-time quantitative RT-PCR assay. Survival curves were estimated using the Kaplan-Meier method, and differences between them were evaluated by the log-rank test. Cox proportional hazard regression test was used to estimate univariate and multivariate hazard ratios for prognosis. We found that miR-9 levels were significantly higher in LSCC tissues compared with matched non-cancerous tissues [4.55 (164-6.75) vs. 2.07 (0.89-2.65), P < 0.05]. The level of miR-9 in LSCC was strongly correlated with tumor differentiation (P = 0.031), thyroid cartilage invasion (P = 0.024), lymph node metastasis (P = 0.009) and clinical TNM stage (P = 0.011). The log-rank test showed that the survival time was significantly different between groups with high and low expression of miR-9 (Log Rank test, P = 0.014). Furthermore, Cox regression multivariate analysis demonstrated that miR-9 expression was an independent prognostic factor of outcomes in patients with LSCC after tumour resection (HR = 3.18, 95% CI = 2.19-11.91, P = 0.012). In conclusion, miR-9 expression was up-regulated in LSCC and was significantly associated with the progression and poorer prognosis of LSCC. Therefore, it might be utilized as a useful prognostic biomarker for LSCC.
Keywords: MicroRNA, miR-9, prognostic biomarker, laryngeal squamous cell carcinoma
Introduction
Laryngeal cancer is currently the eleventh most common cancer worldwide, and laryngeal squamous cell carcinomas (LSCC) represents approximately 85-90% of all the malignant tumors of the larynx [1]. Since the survival of patients with LSCC is poor, prognostic assessment of the patient is essential for the choice of better therapeutic strategies. The current challenge demands the discovery of accurate and non-invasive biomarkers for diagnosis, prognosis and prediction of recurrence to improve the clinical management of LSCC patients.
MicroRNAs (miRNAs) are a family of small non-coding RNA molecules [2]. MiRNA can regulate gene expression at a post-transcriptional level and play a pivotal role in the regulation of cell development, metabolism, immunity, proliferation, differentiation, and apoptosis [3]. Published data finds that miRNA is involved in carcinogenesis as either oncogene or tumor suppressor and many cancer-related miRNAs have been identified functionally. MicroRNA-9 (miR-9) has been reported to be highly expressed in many types of malignant tumors, and it can promote cancer cell proliferation and metastasis [4-7]. However, the expression level of miR-9 in LSCC, and its clinical significance, as well as prognostic value have not been reported previously. Therefore, in the present study, we aimed to detect miR-9 expression level and its clinical significance in LSCC.
Material and methods
Patients and specimens
The present study was conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent for the collection of samples and subsequent analysis. The study was approved by the Ethics Committee of the 4th Affiliated Hospital of Harbin Medical University. 103 patients who were diagnosed with LSCC and treated between March 2010 and June 2013 at the 4th Affiliated Hospital of Harbin Medical University, were enrolled in this study. All of the patients with LSCC in our study had the following inclusion criteria: no history of radiotherapy or chemotherapy and a diagnosis of primary squamous cell carcinoma of the larynx without other malignancies. Tumor stage was classified according to the 2002 TNM staging system of the Union for International Cancer Control (UICC). After surgery, the matched specimens of LSCC and the corresponding adjacent nonneoplastic tissues obtained from patients were preserved in liquid nitrogen within 5 minutes of excision and then were transported frozen to the laboratory and stored at -80°C. These patients received surgery in our department and were followed for at least 2 years. Clinical follow-up data were obtained by telephone or from outpatient records.
Quantitative RT-PCR analysis
Total RNA was extracted from specimens of LSCC and the corresponding adjacent nonneoplastic tissues by homogenizing tissue in Trizol reagent (Invitrogen, Carlsbad, California, USA) according to the manufacturer’s instructions. Primers for miR-9 and endogenous control U6 snRNA were obtained from Applied Biosystems (Foster City, California, USA). The concentration and purity of RNA were determined spectrophotometrically using the NanoDrop ND-1000 (NanoDrop Technologies, Wilmington, Delaware, USA). cDNA was generated using the Prime-Script RT reagent kit (Takara Co. Ltd, Dalian, China) in a 20 μl final reaction volume containing 0.5 μg of RNA, 0.5 μl Prime-Script RT enzyme mix, and 4 μl 5× PrimeScript buffer, and 1 μl RT primer, and incubated at 42°C for 60 min and at 85°C for 5 min. Quantitative real-time PCR assay was performed to evaluate miR-9 expression using SYBR Premix Ex Taq (Takara Co. Ltd) and measured in a LightCycler 480 System (Roche, Basel, Switzerland). The amplification profile was denatured at 95°C for 10 min, followed by 45 cycles of denaturation at 95°C for 15 s, annealing at 60°C for 30 s, and extension at 72°C for 1 min. Relative quantification of miRNA expression was performed using the 2-ΔΔCT. The raw data were presented as the relative quantity of target miRNA, normalized with respect to U6 snRNA and relative to a calibrator sample. Primers for real-time PCR analysis were listed as follows: miR-9_F, 5’-GTGCAGGGTCCGAGGT; miR-9_R, 5’-GCGCTCTTTGGTTATCTAGC; U6_F, 5’-CTCGCTTCGGCAGCACA; and U6_R, 5’-AACGCTTCACGAATTTGCGT.
Statistical analysis
The differences between groups were estimated using Mann-Whitney U test or Kruskal-Wallis test, as appropriate. Overall survival (OS) was measured for each patient. Survival curves were estimated using the Kaplan-Meier method, and differences between them were evaluated by the log-rank test. Cox proportional hazard regression test was used to estimate univariate and multivariate hazard ratios for prognosis. Difference was considered statistically significant when the P value was < 0.05. All statistical analyses were done using SPSS 18.0 (SPSS Inc, Chicago, IL).
Results
Increased expression of miR-9 in LSCC
To reveal the role of miR-9 in LSCC, qRT-PCR was performed to measure miR-9 levels in 103 pairs of LSCC tissues and adjacent non-cancerous tissues. Median miR-9 levels were significantly higher in LSCC tissues compared with matched non-cancerous tissues [4.55 (164-6.75) vs. 2.07 (0.89-2.65), P < 0.05] (shown in Figure 1).
Figure 1.
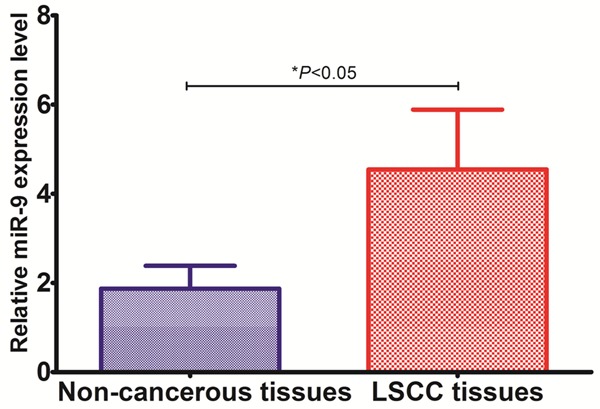
Expression level of miR-9 in LSCC tissues and the corresponding adjacent nonneoplastic tissues.
Correlations between miR-9 expression and LSCC clinicopathologic characteristics
The relationship between miR-9 expression and clinicopathologic parameters of 103 patients with LSCC was evaluated. As shown in Table 1, the level of miR-9 in LSCC was strongly correlated with tumor differentiation (P = 0.031), thyroid cartilage invasion (P = 0.024), lymph node metastasis (P = 0.009) and clinical TNM stage (P = 0.011). However, there were no significant associations between miR-9 expression and other clinical features including sex (P = 0.63), age (P = 0.31), primary location (P = 0.62) and T classification (P = 0.066).
Table 1.
Relationship between miR-9 expression level and clinical parameters
| Clinicopathological factors | Cases | Tissue miR-9 level | P value | |
|---|---|---|---|---|
|
|
||||
| High (n = 53) | Low (n = 50) | |||
| Age (years) | ||||
| < 60 | 41 | 20 | 21 | 0.31 |
| ≥ 60 | 62 | 33 | 29 | |
| Sex distribution | ||||
| Female | 49 | 25 | 24 | 0.63 |
| Male | 54 | 28 | 26 | |
| Primary location | ||||
| Supraglottic | 66 | 21 | 45 | 0.62 |
| Glottic | 37 | 32 | 5 | |
| Thyroid cartilage invasion | ||||
| Yes | 33 | 24 | 9 | 0.024 |
| No | 70 | 29 | 41 | |
| Pathological differentiation | ||||
| Moderately and highly differentiated | 69 | 32 | 37 | 0.031 |
| Poorly differentiated | 34 | 21 | 13 | |
| T classification | ||||
| T1-2 | 55 | 24 | 31 | 0.066 |
| T3-4 | 48 | 29 | 19 | |
| Lymph node metastasis | ||||
| Yes | 31 | 22 | 9 | 0.009 |
| No | 74 | 33 | 41 | |
| TNM stage | ||||
| I/II | 43 | 11 | 32 | 0.011 |
| III/IV | 60 | 42 | 18 | |
Correlation between miR-9 expression and patients’ survival
As determined by Kaplan-Meier method, the expression of miR-9 in LSCC tissues was significantly correlated with overall survival. The log-rank test showed that the survival time was significantly different between groups with high and low expression of miR-9 (Log Rank test, P = 0.014, shown in Figure 2), indicating that the high expression of miR-9 was correlated with a shorter survival time of patients with LSCC. Cox regression multivariate analysis including age, sex, tumour site, tumour differentiation, T classification, clinical TNM stage, lymph node metastasis and miR-9 expression was performed. Our results demonstrated that miR-9 expression had a significant correlation with LSCC prognosis, and it was found to be an independent prognostic factor of outcomes in patients with LSCC after tumor resection (HR = 3.18, 95% CI = 2.19-11.91, P = 0.012, shown in Table 2).
Figure 2.
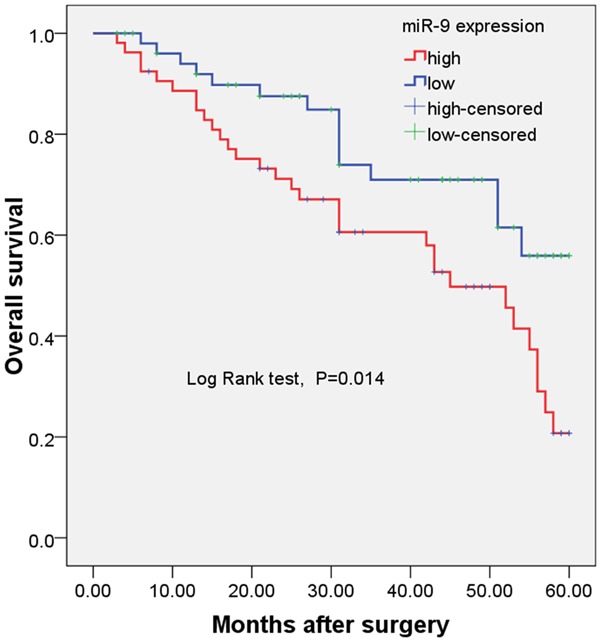
Kaplan-Meier overall survival curve for 103 patients with LSCC.
Table 2.
Multivariate survival analyses for overall survival by the Cox proportional hazard model
| Variables | HR | 95% CI | P value |
|---|---|---|---|
| Age | 0.81 | 0.67-2.19 | 0.37 |
| Sex distribution | 1.27 | 0.23-1.91 | 0.81 |
| Primary location | 0.78 | 0.36-2.13 | 0.54 |
| Thyroid cartilage invasion | 1.82 | 1.03-5.33 | 0.033 |
| Pathological differentiation | 2.23 | 1.96-7.21 | 0.025 |
| T classification | 1.88 | 0.76-5.19 | 0.07 |
| Lymph node metastasis | 2.14 | 1.53-9.13 | 0.021 |
| TNM stage | 2.71 | 1.98-9.34 | 0.018 |
| miR-9 expression level | 3.18 | 2.19-11.91 | 0.012 |
Discussion
LSCC can develop in any part of the larynx, including the glottis, supraglottic, and subglottic areas. In China, the incidence of LSCC has gradually increased over the past several decades. Currently, LSCC is the second most common malignant tumor of the head and neck in China [8]. Although patients with LSCC benefit from advanced diagnostic and therapeutic management, survival remains poor and has not improved during the past 30 years [9]. Current tumor-node-metastasis (TNM) staging criteria and differentiation grade are the main factors used to predict outcome in patients with LSCC [10,11]. However, these parameters do not accurately predict the future course of early-stage LSCC. Moreover, the molecular mechanisms by which LSCC initiates and progresses remain unclear. Therefore, the identification of sensitive and specific molecular markers of LSCC would facilitate early prevention, diagnosis, and treatment. Identification of relevant biomarkers is essential for understanding the pathogenesis of LSCC and for developing new targeted treatment strategies for this tumor type.
Altered expression of tissue miRNAs has been found to be associated with many diseases, particularly cancer, and the use of tissue miRNA expression profiles as diagnostic or prognostic biomarkers in cancer has been demonstrated by several studies [12,13]. Emerging studies have revealed that miRNA is a promising biomarker associated with clinical outcomes in LSCC [14,15]. The miR-9, which was initially found to be selectively expressed in neuron tissues, was elevated in primary brain tumors and was described as an essential factor that functions in developing neurons, neural carcinogenesis, or other diseases of the nervous system [7,16,17]. Later research found that miR-9 was involved in the carcinogenesis of biliary tract carcinoma [4], colorectal cancer [5], Burkitt lymphoma [18], clear cell renal cell carcinoma [19], and gastric cancers [20]. In cell line studies, miR-9 has been observed to target junction protein E-cadherin, facilitating metastases and stimulating angiogenesis in breast cancer and hepatocellular carcinoma cells [21,22]. However, until now, the expression level of miR-9 in LSCC, and its clinical significance, as well as prognostic value have not been reported. Therefore, we aimed to detect miR-9 expression level and its clinical significance in LSCC. In the present study, we found that miR-9 levels were significantly higher in LSCC tissues compared with matched non-cancerous tissues, and the level of miR-9 in LSCC was strongly correlated with tumor differentiation, thyroid cartilage invasion, lymph node metastasis and clinical TNM stage. The log-rank test showed that the survival time was significantly different between groups with high and low expression of miR-9, indicating that the high expression of miR-9 was correlated with a shorter survival time of patients with LSCC. Furthermore, Cox regression multivariate analysis demonstrated that miR-9 expression had a significant correlation with LSCC prognosis, and it was found to be an independent prognostic factor of outcomes in patients with LSCC after tumour resection. To the best of our knowledge, this is the first study showing a significant correlation between miR-9 expression and the prognosis of LSCC.
Previously, the expression level of miR-9 and its clinical significance have been investigated in other types of cancers. For example, Zhou et al found that higher expression of miR-9 was significantly associated with breast cancer local recurrence in all cases as well as the subset of estrogen receptor positive cases (P = 0.02), and the Kaplan-Meier analysis showed that patients with lower miR-9 levels had significantly better 10-year local recurrence-free survival (67.9% vs 30.8%, P = 0.02) in estrogen receptor positive cases [23]. Xu et al found that miR-9 was up-regulated in non-small cell lung cancer tissues and correlated with adverse clinical features as well as unfavorable survival, indicating that miR-9 might be involved in non-small lung cancer progression and could serve as a promising biomarker for further risk stratification in the treatment of this cancer [24]. Wu et al found that the increased expression of miR-9 might play an important role in tumor progression in human gliomas, and miR-9 might be a useful marker for predicting the clinical outcome of glioma patients, especially for advanced subtypes [25]. Our findings are in line with theirs, however, in the present study, we have not investigated the detailed mechanism of miR-9 in the occurrence, development, and metastasis of LSCC. In conclusion, miR-9 expression was up-regulated in LSCC and was significantly associated with the progression and poorer prognosis of LSCC. Therefore, it might be utilized as a useful prognostic biomarker for LSCC.
Disclosure of conflict of interest
None.
References
- 1.Genden EM, Ferlito A, Silver CE, Jacobson AS, Werner JA, Suarez C, Leemans CR, Bradley PJ, Rinaldo A. Evolution of the management of laryngeal cancer. Oral Oncol. 2007;43:431–439. doi: 10.1016/j.oraloncology.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 3.Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 4.Shigehara K, Yokomuro S, Ishibashi O, Mizuguchi Y, Arima Y, Kawahigashi Y, Kanda T, Akagi I, Tajiri T, Yoshida H, Takizawa T, Uchida E. Real-time PCR-based analysis of the human bile microRNAome identifies miR-9 as a potential diagnostic biomarker for biliary tract cancer. PLoS One. 2011;6:e23584. doi: 10.1371/journal.pone.0023584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu L, Chen H, Zhou D, Li D, Bai R, Zheng S, Ge W. MicroRNA-9 up-regulation is involved in colorectal cancer metastasis via promoting cell motility. Med Oncol. 2012;29:1037–1043. doi: 10.1007/s12032-011-9975-z. [DOI] [PubMed] [Google Scholar]
- 6.Ma L, Young J, Prabhala H, Pan E, Mestdagh P, Muth D, Teruya-Feldstein J, Reinhardt F, Onder TT, Valastyan S, Westermann F, Speleman F, Vandesompele J, Weinberg RA. miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat Cell Biol. 2010;12:247–256. doi: 10.1038/ncb2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nass D, Rosenwald S, Meiri E, Gilad S, Tabibian-Keissar H, Schlosberg A, Kuker H, Sion-Vardy N, Tobar A, Kharenko O, Sitbon E, Lithwick Yanai G, Elyakim E, Cholakh H, Gibori H, Spector Y, Bentwich Z, Barshack I, Rosenfeld N. MiR-92b and miR-9/9* are specifically expressed in brain primary tumors and can be used to differentiate primary from metastatic brain tumors. Brain Pathol. 2009;19:375–383. doi: 10.1111/j.1750-3639.2008.00184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang SY, Lu ZM, Luo XN, Chen LS, Ge PJ, Song XH, Chen SH, Wu YL. Retrospective analysis of prognostic factors in 205 patients with laryngeal squamous cell carcinoma who underwent surgical treatment. PLoS One. 2013;8:e60157. doi: 10.1371/journal.pone.0060157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Almadori G, Bussu F, Cadoni G, Galli J, Paludetti G, Maurizi M. Molecular markers in laryngeal squamous cell carcinoma: towards an integrated clinicobiological approach. Eur J Cancer. 2005;41:683–693. doi: 10.1016/j.ejca.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 10.Gourin CG, Conger BT, Sheils WC, Bilodeau PA, Coleman TA, Porubsky ES. The effect of treatment on survival in patients with advanced laryngeal carcinoma. Laryngoscope. 2009;119:1312–1317. doi: 10.1002/lary.20477. [DOI] [PubMed] [Google Scholar]
- 11.Lohynska R, Slavicek A, Bahanan A, Novakova P. Predictors of local failure in early laryngeal cancer. Neoplasma. 2005;52:483–488. [PubMed] [Google Scholar]
- 12.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 13.Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 14.Sun X, Song Y, Tai X, Liu B, Ji W. MicroRNA expression and its detection in human supraglottic laryngeal squamous cell carcinoma. Biomed Rep. 2013;1:743–746. doi: 10.3892/br.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu TY, Zhang TH, Qu LM, Feng JP, Tian LL, Zhang BH, Li DD, Sun YN, Liu M. MiR-19a is correlated with prognosis and apoptosis of laryngeal squamous cell carcinoma by regulating TIMP-2 expression. Int J Clin Exp Pathol. 2014;7:56–63. [PMC free article] [PubMed] [Google Scholar]
- 16.Laneve P, Gioia U, Andriotto A, Moretti F, Bozzoni I, Caffarelli E. A minicircuitry involving REST and CREB controls miR-9-2 expression during human neuronal differentiation. Nucleic Acids Res. 2010;38:6895–6905. doi: 10.1093/nar/gkq604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Packer AN, Xing Y, Harper SQ, Jones L, Davidson BL. The bifunctional microRNA miR-9/miR-9* regulates REST and CoREST and is do-wnregulated in Huntington’s disease. J Neurosci. 2008;28:14341–14346. doi: 10.1523/JNEUROSCI.2390-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Onnis A, De Falco G, Antonicelli G, Onorati M, Bellan C, Sherman O, Sayed S, Leoncini L. Alteration of microRNAs regulated by c-Myc in Burkitt lymphoma. PLoS One. 2010:5. doi: 10.1371/journal.pone.0012960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hildebrandt MA, Gu J, Lin J, Ye Y, Tan W, Tamboli P, Wood CG, Wu X. Hsa-miR-9 methylation status is associated with cancer development and metastatic recurrence in patients with clear cell renal cell carcinoma. Oncogene. 2010;29:5724–5728. doi: 10.1038/onc.2010.305. [DOI] [PubMed] [Google Scholar]
- 20.Luo H, Zhang H, Zhang Z, Zhang X, Ning B, Guo J, Nie N, Liu B, Wu X. Down-regulated miR-9 and miR-433 in human gastric carcinoma. J Exp Clin Cancer Res. 2009;28:82. doi: 10.1186/1756-9966-28-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khew-Goodall Y, Goodall GJ. Myc-modulated miR-9 makes more metastases. Nat Cell Biol. 2010;12:209–211. doi: 10.1038/ncb0310-209. [DOI] [PubMed] [Google Scholar]
- 22.Tan HX, Wang Q, Chen LZ, Huang XH, Chen JS, Fu XH, Cao LQ, Chen XL, Li W, Zhang LJ. MicroRNA-9 reduces cell invasion and E-cadherin secretion in SK-Hep-1 cell. Med Oncol. 2010;27:654–660. doi: 10.1007/s12032-009-9264-2. [DOI] [PubMed] [Google Scholar]
- 23.Zhou X, Marian C, Makambi KH, Kosti O, Kallakury BV, Loffredo CA, Zheng YL. MicroRNA-9 as potential biomarker for breast cancer local recurrence and tumor estrogen receptor status. PLoS One. 2012;7:e39011. doi: 10.1371/journal.pone.0039011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu T, Liu X, Han L, Shen H, Liu L, Shu Y. Up-regulation of miR-9 expression as a poor prognostic biomarker in patients with non-small cell lung cancer. Clin Transl Oncol. 2014;16:469–475. doi: 10.1007/s12094-013-1106-1. [DOI] [PubMed] [Google Scholar]
- 25.Wu Z, Wang L, Li G, Liu H, Fan F, Li Z, Li Y, Gao G. Increased expression of microRNA-9 predicts an unfavorable prognosis in human glioma. Mol Cell Biochem. 2013;384:263–268. doi: 10.1007/s11010-013-1805-5. [DOI] [PubMed] [Google Scholar]


