Abstract
The prognostic significance of serum human epididymis protein 4 (HE4) levels in human NSCLC among a Chinese population has not been investigated. The purpose of this study was to evaluate the prognostic significance of serum HE4 level in patients with NSCLC among a Chinese population. Serum HE4 expression levels were measured by enzyme-linked immunosorbent assay (ELISA). The overall survival (OS) analyzed by log-rank test, and survival curves was plotted according to Kaplan-Meier. The COX proportional hazards regression model was used to determine the joint effects of several variables on survival. Serum HE4 level was found to be significantly higher in patients with NSCLC than that of controls (13.76 ± 5.01 ng/ml vs. 5.09 ± 1.25 ng/ml, P < 0.01). High HE4 expression was correlated with TNM stage (P = 0.003), lymph node metastases (P = 0.007), and distant metastases (P < 0.001). Furthermore, patients with high serum HE4 level had a significantly lower 5-year OS rate (34.0% vs. 59.7%; P = 0.022) than those with low serum HE4 level. In a multivariate Cox model, we found that HE4 expression was an independent poor prognostic factor for 5-year OS (hazards ratio [HR] = 3.654, 95% confidence interval [CI] = 2.753-11.981, P = 0.019) in NSCLC. In conclusion, the detection of HE4 levels in the serum might serve as a new tumor biomarker in the prognosis of NSCLC among Chinese population.
Keywords: Lung cancer, prognosis, HE4, serum, marker
Introduction
Lung cancer still remains the most common cancer and the most common cause of cancer-related death worldwide [1,2]. About 85% lung cancers were non-small cell lung cancers (NSCLC), and approximately two thirds of NSCLC cases were diagnosis at locally advanced or metastatic disease as the typically asymptomatic at early stages [3]. It is well known that pathologic TNM category, age, sex, and cell type are all important prognostic factors for the patients with NSCLC [4]. The advances in molecular biology have enabled researchers to focus on molecular or biological markers in NSCLC.
Human epididymis protein 4 (HE4) is an 11 kDa protein that is a precursor to the epididymal secretory protein E4 and has since been reported to be expressed in several normal tissues including the epithelia of respiratory and reproductive tissues [5,6]. Previously, Paek et al. demonstrated that an elevated serum HE4 level was related to the advanced stage of epithelial ovarian cancer. An elevated serum level of HE4 was a poor prognostic factor for progression-free survival in patients with epithelial ovarian cancer who were treated with debulking surgery and adjuvant taxane and platinum-based chemotherapy [7]. Iwahori et al. found that serum HE4 levels were elevated in lung cancer, and it might be a potential prognostic marker for lung cancer patients [8]. However, the prognostic significance of serum HE4 levels in human NSCLC among Chinese population has not been investigated. The purpose of this study was to evaluate the prognostic significance of serum HE4 level in patients with NSCLC among Chinese population.
Materials and methods
Patients and serum samples
The selection criteria for patients with NSCLC were as follows: (1) pathologically confirmed patients with NSCLC; (2) the patients had no previous history of other cancers. 100 patients with recently diagnosed NSCLC in the Department of Thoracic Surgery, Provincial Hospital Affiliated to Shandong University between October 2007 and February 2013 were enrolled in the present study. Control serum samples were collected from 100 healthy volunteers. All subjects underwent clinical examination; plain chest radiograph; CT scan of the chest, upper abdomen, and brain; fiberoptic bronchoscopy; and bone scan. Blood samples were collected from the patients at the time of diagnosis, before any kind of treatment (surgery, radiation, or chemotherapy). The demographic and pathological data, including age, gender, and the tumor stage, were obtained by a review of the patients’ medical records (the data was used with the consent of the patients as well as the approval of the Ethics Committee of Provincial Hospital Affiliated to Shandong University). Tumor stage was determined according to the 2009 TNM staging classification system. The clinical characteristic of 100 patients with NSCLC was summarized in Table 1. Fasting blood was taken for all participants and serum was collected and stored at -80°C.
Table 1.
Patient’s characteristics and clinicopathologic correlation of serum HE4 expression levels
| Serum HE4 | ||||
|---|---|---|---|---|
|
|
||||
| Clinical variable | All cases | High expression | Low expression | P value |
| Gender | ||||
| Male | 54 | 40 | 14 | 0.79 |
| Female | 46 | 34 | 12 | |
| Age | ||||
| < 50 yr | 29 | 21 | 8 | 0.64 |
| 50-70 yr | 40 | 32 | 8 | |
| > 70 yr | 31 | 21 | 10 | |
| Smoking history | ||||
| Current | 53 | 39 | 14 | 0.31 |
| Former | 42 | 31 | 11 | |
| Never | 5 | 4 | 1 | |
| TNM stage | ||||
| I | 33 | 20 | 13 | 0.003 |
| II | 32 | 24 | 8 | |
| III | 23 | 19 | 4 | |
| IV | 12 | 11 | 1 | |
| Lymph node metastases | ||||
| No | 47 | 25 | 22 | 0.007 |
| Yes | 53 | 49 | 4 | |
| Distant metastases | ||||
| No | 89 | 64 | 25 | < 0.001 |
| Yes | 11 | 10 | 1 | |
Enzyme-linked immunosorbent assay
HE4 was measured by enzyme-linked immunosorbent assay (ELISA) with immunoassay kit (Miltenyi, Germany) according to the manufacturer’s directions. The optical density (OD) at 450 nm was determined. The standard curves were established with OD450 as Y axle and the concentration of standard substance as X axle. The level of protein was obtained through standard curve. Results are reported as concentration of HE4 ng/ml in samples.
Statistical analysis
Statistical analysis was performed using SPSS 18.0 (SPSS, IBM, Chicago, IL, USA). For continuous variables, the data were expressed as the means ± SD. The Mann-Whitney’s U test was used to analyze the relationship between serum HE4 expression and clinicopathological variables. The overall survival (OS) was calculated using the Kaplan-Meier method. The differences between the survival curves were tested by using the log-rank test. The COX proportional hazards regression model was used to determine the joint effects of several variables on survival. P values < 0.05 were considered to be significant.
Results
Serum level of HE-4 in patients with NSCLC and controls
Serum HE4 level was found to be significantly higher in patients with NSCLC than that of controls. Mean serum HE4 level was 13.76 ± 5.01 ng/ml in the NSCLC group and 5.09 ± 1.25 ng/ml in the control group (P < 0.01, shown in Figure 1). 95% of HE4 values of the control group were 7.26 ng/ml, which was used as the cutoff value.
Figure 1.
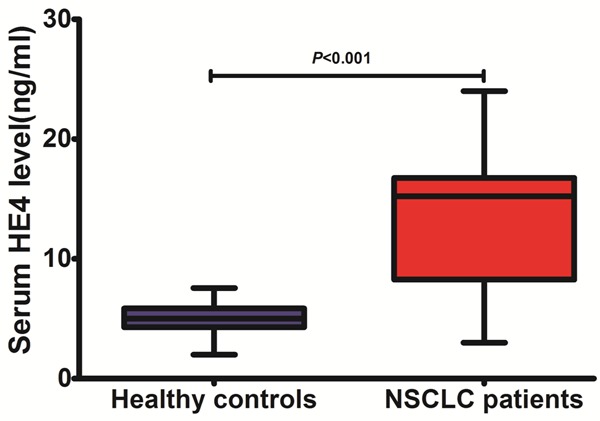
Serum level of HE-4 in patients with NSCLC and controls.
Increased serum HE4 expression level in NSCLC patients and its relationship with clinicopathological variables
In this study, NSCLC patients with values less than 95% of HE4 values of the control group (7.26 ng/ml) were assigned to the low expression group (n = 26), whereas those with values ≥ 7.26 ng/ml were assigned to the high expression group (n = 74). As shown in Table 1, high HE4 expression was correlated with TNM stage (P = 0.003), lymph node metastases (P = 0.007), and distant metastases (P < 0.001). However, high HE4 expression was not associated with other clinicopathological factors of patients, including age, sex, and smoking history (all P > 0.05).
Expression of HE4 in serum samples in relation to prognosis of patients with NSCLC
As shown in Figure 2, patients with high serum HE4 level had a significantly lower 5-year OS rate (34.0% vs. 59.7%; P = 0.022) than those with low serum HE4 level. Table 2 showed multivariate analyses of clinical variables and serum HE4 levels for their prognostic influence on OS. In a multivariate Cox model, we found that HE4 expression was an independent poor prognostic factor for 5-year OS (hazards ratio [HR] = 3.654, 95% confidence interval [CI] = 2.753-11.981, P = 0.019) in NSCLC.
Figure 2.
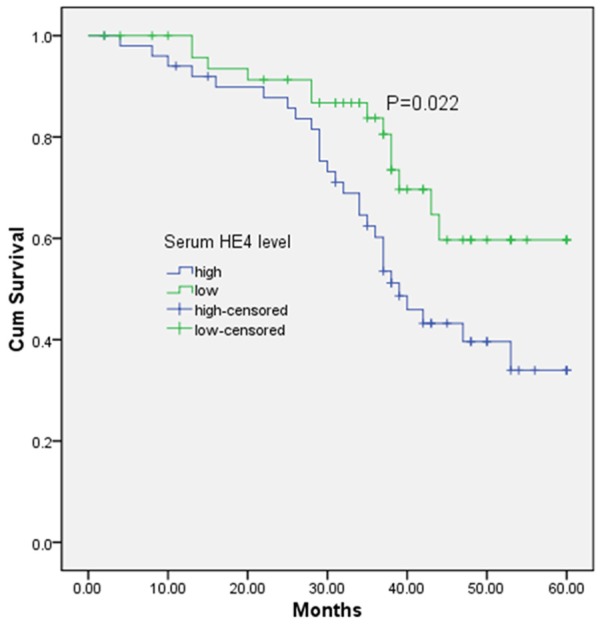
Expression of HE4 in serum samples in relation to prognosis of patients with NSCLC by the Kaplan-Meier method.
Table 2.
Multivariate analyses for prognostic factors in patients with NSCLC
| Variables | HR | 95% CI | P value |
|---|---|---|---|
| Age | 0.981 | 0.287-1.826 | 0.387 |
| Sex | 0.822 | 0.376-2.118 | 0.467 |
| Smoking history | 1.782 | 0.763-3.734 | 0.101 |
| TNM stage | 4.945 | 2.345-9.344 | 0.014 |
| Lymph node involvement | 4.563 | 3.643-10.332 | 0.007 |
| Distant metastases | 5.981 | 3.643-12.355 | 0.001 |
| Serum HE4 expression level | 3.654 | 2.753-11.981 | 0.019 |
HR = hazard ratio; CI = confidence interval; HE4 = human epididymis protein 4.
Discussion
The best prognostic system for overall survival in NSCLC is still the TNM staging system. We are now in an era where personalized medicine and targeted therapies may give new hope for this patient group [9]. Identification of novel molecular markers which can improve diagnosis and prognostic stratification and serve as possible therapeutic targets will be of great importance in the near future. Circulating biomarkers are a promising means of prognosis, as serum and plasma samples are easily obtainable. A number of novel markers for lung cancer have been identified in recent years, such as carcinoembryonic antigen (CEA), serum cytokeratin 19 fragment (CYFRA 21-1) and progastrin releasing peptide (pro-GRP), however, they are not satisfactory for monitoring the disease because of their relatively low sensitivity and specificity [10-12].
HE4 is one of the most intensively studied of the novel biomarkers, which was first described as an epididymis specific gene using northern blot analysis and in situ transcript hybridization [13,14]. HE4 is a member of the WAP domain family, and this domain shows fifty well-conserved amino acid motifs. This protein has a variety of functions, such as antiproteinases, SLPI, and elafin, which shows antibacterial activities and anti-inflammatory effects [15,16]. In the previous reports, HE4 is overexpressed in ovarian cancer cells and secreted to the sera in the patients with ovarian cancer [17-19]. Moore and colleagues analyzed the serum samples for levels of CA125, soluble mesothelin-related peptide, HE4, CA72-4, activin, inhibin, osteopontin, and epidermal growth factor. As a single tumor marker, HE4 had the highest sensitivity for detecting ovarian cancer and combined CA125 and HE4 was a more accurate predictor of malignancy than either alone [20]. On the other hand, HE4 is less frequently elevated in patients with benign gynecologic disease compared with CA125 [21]. In addition, Andersen et al. demonstrated that combining the symptom index with a serum HE4 or CA125 test could improve the prediction of ovarian cancer in a prospective case-control study [22].
Previously, Iwahori et al. found that serum HE4 levels were elevated in lung cancer, and it might be a potential prognostic marker for lung cancer patients [8]. However, the prognostic significance of serum HE4 levels in human NSCLC among Chinese population has not been investigated. The purpose of this study was to evaluate the prognostic significance of serum HE4 level in patients with NSCLC among Chinese population. In the present study, we had three findings. Firstly, the serum HE4 level was significantly higher in patients with NSCLC than that of controls, and high HE4 expression was correlated with TNM stage, lymph node metastases, and distant metastases. Secondly, Kaplan-Meier method found that patients with high serum HE4 level had a significantly lower 5-year OS rate (34.0% vs. 59.7%; P = 0.022) than those with low serum HE4 level. Thirdly, in a multivariate Cox model, we found that HE4 expression was an independent poor prognostic factor for 5-year OS in NSCLC. All the results suggested that serum HE4 level was befitting to predict prognosis of NSCLC patients.
In conclusion, our results indicated that serum HE4 was overexpressed in NSCLC and high expression of HE4 was associated with poor prognosis in patients with NSCLC. The data suggests that serum HE41 may be useful molecular markers in NSCLC among Chinese population.
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Thun MJ, Ries LA, Howe HL, Weir HK, Center MM, Ward E, Wu XC, Eheman C, Anderson R, Ajani UA, Kohler B, Edwards BK. Annual report to the nation on the status of cancer, 1975-2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst. 2008;100:1672–1694. doi: 10.1093/jnci/djn389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Subramaniam S, Thakur RK, Yadav VK, Nanda R, Chowdhury S, Agrawal A. Lung cancer biomarkers: State of the art. J Carcinog. 2013;12:3. doi: 10.4103/1477-3163.107958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgensztern D, Ng SH, Gao F, Govindan R. Trends in stage distribution for patients with non-small cell lung cancer: a National Cancer Database survey. J Thorac Oncol. 2010;5:29–33. doi: 10.1097/JTO.0b013e3181c5920c. [DOI] [PubMed] [Google Scholar]
- 4.Chansky K, Sculier JP, Crowley JJ, Giroux D, Van Meerbeeck J, Goldstraw P, International Staging C, Participating I. The International Association for the Study of Lung Cancer Staging Project: prognostic factors and pathologic TNM stage in surgically managed non-small cell lung cancer. J Thorac Oncol. 2009;4:792–801. doi: 10.1097/JTO.0b013e3181a7716e. [DOI] [PubMed] [Google Scholar]
- 5.Galgano MT, Hampton GM, Frierson HF Jr. Comprehensive analysis of HE4 expression in normal and malignant human tissues. Mod Pathol. 2006;19:847–853. doi: 10.1038/modpathol.3800612. [DOI] [PubMed] [Google Scholar]
- 6.Wang K, Gan L, Jeffery E, Gayle M, Gown AM, Skelly M, Nelson PS, Ng WV, Schummer M, Hood L, Mulligan J. Monitoring gene expression profile changes in ovarian carcinomas using cDNA microarray. Gene. 1999;229:101–108. doi: 10.1016/s0378-1119(99)00035-9. [DOI] [PubMed] [Google Scholar]
- 7.Paek J, Lee SH, Yim GW, Lee M, Kim YJ, Nam EJ, Kim SW, Kim YT. Prognostic significance of human epididymis protein 4 in epithelial ovarian cancer. Eur J Obstet Gynecol Reprod Biol. 2011;158:338–342. doi: 10.1016/j.ejogrb.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 8.Iwahori K, Suzuki H, Kishi Y, Fujii Y, Uehara R, Okamoto N, Kobayashi M, Hirashima T, Kawase I, Naka T. Serum HE4 as a diagnostic and prognostic marker for lung cancer. Tumour Biol. 2012;33:1141–1149. doi: 10.1007/s13277-012-0356-9. [DOI] [PubMed] [Google Scholar]
- 9.Ma PC. Personalized targeted therapy in advanced non-small cell lung cancer. Cleve Clin J Med. 2012;79(Electronic Suppl 1):eS56–60. doi: 10.3949/ccjm.79.s2.12. [DOI] [PubMed] [Google Scholar]
- 10.Aoyagi K, Miyake Y, Urakami K, Kashiwakuma T, Hasegawa A, Kodama T, Yamaguchi K. Enzyme immunoassay of immunoreactive progastrin-releasing peptide(31-98) as tumor marker for small-cell lung carcinoma: development and evaluation. Clin Chem. 1995;41:537–543. [PubMed] [Google Scholar]
- 11.Pujol JL, Grenier J, Daures JP, Daver A, Pujol H, Michel FB. Serum fragment of cytokeratin subunit 19 measured by CYFRA 21-1 immunoradiometric assay as a marker of lung cancer. Cancer Res. 1993;53:61–66. [PubMed] [Google Scholar]
- 12.Shinkai T, Saijo N, Tominaga K, Eguchi K, Shimizu E, Sasaki Y, Fujita J, Futami H, Ohkura H, Suemasu K. Serial plasma carcinoembryonic antigen measurement for monitoring patients with advanced lung cancer during chemotherapy. Cancer. 1986;57:1318–1323. doi: 10.1002/1097-0142(19860401)57:7<1318::aid-cncr2820570711>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 13.Kirchhoff C. Molecular characterization of epididymal proteins. Rev Reprod. 1998;3:86–95. doi: 10.1530/ror.0.0030086. [DOI] [PubMed] [Google Scholar]
- 14.Kirchhoff C, Habben I, Ivell R, Krull N. A major human epididymis-specific cDNA encodes a protein with sequence homology to extracellular proteinase inhibitors. Biol Reprod. 1991;45:350–357. doi: 10.1095/biolreprod45.2.350. [DOI] [PubMed] [Google Scholar]
- 15.Wiedow O, Schroder JM, Gregory H, Young JA, Christophers E. Elafin: an elastase-specific inhibitor of human skin. Purification, characterization, and complete amino acid sequence. J Biol Chem. 1990;265:14791–14795. [PubMed] [Google Scholar]
- 16.Thompson RC, Ohlsson K. Isolation, properties, and complete amino acid sequence of human secretory leukocyte protease inhibitor, a potent inhibitor of leukocyte elastase. Proc Natl Acad Sci U S A. 1986;83:6692–6696. doi: 10.1073/pnas.83.18.6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anastasi E, Granato T, Marchei GG, Viggiani V, Colaprisca B, Comploj S, Reale MG, Frati L, Midulla C. Ovarian tumor marker HE4 is differently expressed during the phases of the menstrual cycle in healthy young women. Tumour Biol. 2010;31:411–415. doi: 10.1007/s13277-010-0049-1. [DOI] [PubMed] [Google Scholar]
- 18.Hellstrom I, Raycraft J, Hayden-Ledbetter M, Ledbetter JA, Schummer M, McIntosh M, Drescher C, Urban N, Hellstrom KE. The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma. Cancer Res. 2003;63:3695–3700. [PubMed] [Google Scholar]
- 19.Schummer M, Ng WV, Bumgarner RE, Nelson PS, Schummer B, Bednarski DW, Hassell L, Baldwin RL, Karlan BY, Hood L. Comparative hybridization of an array of 21,500 ovarian cDNAs for the discovery of genes overexpressed in ovarian carcinomas. Gene. 1999;238:375–385. doi: 10.1016/s0378-1119(99)00342-x. [DOI] [PubMed] [Google Scholar]
- 20.Moore RG, Brown AK, Miller MC, Skates S, Allard WJ, Verch T, Steinhoff M, Messerlian G, DiSilvestro P, Granai CO, Bast RC Jr. The use of multiple novel tumor biomarkers for the detection of ovarian carcinoma in patients with a pelvic mass. Gynecol Oncol. 2008;108:402–408. doi: 10.1016/j.ygyno.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 21.Huhtinen K, Suvitie P, Hiissa J, Junnila J, Huvila J, Kujari H, Setala M, Harkki P, Jalkanen J, Fraser J, Makinen J, Auranen A, Poutanen M, Perheentupa A. Serum HE4 concentration differentiates malignant ovarian tumours from ovarian endometriotic cysts. Br J Cancer. 2009;100:1315–1319. doi: 10.1038/sj.bjc.6605011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andersen MR, Goff BA, Lowe KA, Scholler N, Bergan L, Drescher CW, Paley P, Urban N. Use of a Symptom Index, CA125, and HE4 to predict ovarian cancer. Gynecol Oncol. 2010;116:378–383. doi: 10.1016/j.ygyno.2009.10.087. [DOI] [PMC free article] [PubMed] [Google Scholar]


