Abstract
Hepatocyte growth factor (HGF) and its receptor, c-Met, are associated with invasive tumor growth. This study sought to profile the expression of HGF and c-Met in nasopharyngeal carcinoma (NPC) tissue. Of 221 patients recruited between March 2010 and September 2013, 106 were diagnosed with nasopharyngeal carcinoma; the remaining 115 had nasal inflammation (nasopharyngeal mucositis) and were used as controls. Inmmunohistochemistry was used to detect the expression of HGF and c-Met in tissue samples. HGF and c-Met were predominantly expressed in the columnar nasopharyngeal epithelium. The proportion of samples expressing HGF and c-Met was significantly higher in NPC than in controls (P < 0.001). Further, the proportion of samples expressing c-Met and HGF (P < 0.01, P < 0.05, respectively) was significantly higher in NPC stages III and IV than in stages I and II. The expression of c-Met was also significantly correlated with cervical lymph node metastasis. Thus, HGF and c-Met expression are upregulated in nasopharyngeal carcinoma. That their expression correlates with disease severity and metastasis may indicate that these proteins promote tumor invasion. Further research could determine the utility of c-Met as a biomarker for the prognosis of NPC.
Keywords: Nasopharyngeal carcinoma, hepatocyte growth factor, c-Met, immunohistochemistry
Introduction
Nasopharyngeal carcinoma is a malignant tumor that most often occurs on the top and in the side wall of the pharyngonasal cavity. It is one of the most common otolaryngological malignancies in China [1]. Nasopharyngeal carcinoma is highly malignant and is associated with early metastasis to lymph nodes and tissue invasion. Recent studies have sought to investigate the role of various oncogenes, growth factors, and their receptors in the occurrence and development of nasopharyngeal carcinoma. Hepatocyte growth factor (HGF) is a mitogen that can strongly stimulate proliferation, and its receptor, c-Met, plays an important role in tumor cell growth. Activated c-Met encourages tumorigenesis and metastasis, and can induce angiogenesis [2]. HGF plays an important role in the occurrence and evolution of many malignancies, not just in hepatocytes, and several studies have reported on the function of HGF and c-Met in promoting tumor cell invasion and metastasis [3,4]. Our study sought to investigate the role of HGF and c-Met in nasopharyngeal carcinoma.
Materials and methods
Study participants
Tissue samples were obtained from 106 nasopharyngeal carcinoma patients between March 2010 and September 2013 at The Affiliated Tumor Hospital of Harbin Medical University. Samples were evaluated by three different pathologists and classified according to the World Health Organization standard classification: 82 cases were differentiated non-keratinizing carcinoma (DNKC), namely WHO type II; 24 cases were undifferentiated non-keratinizing carcinoma (UNKC), namely WHO type III. 115 samples that were categorized as chronic inflammation of the nasopharyngeal epithelium (nasopharyngeal mucositis) during the same time period in our hospital were used as controls. Specimens were fixed in 10% formalin, embedded in paraffin, and cut into 4-μm slices. Of the nasopharyngeal carcinoma samples, 79 were obtained from males (74.5%) and 27 from females (25.5%), and patients ranged in age from 26 to 73 years (mean age 48.6 ± 9.37 years). TNM clinical stages were assigned according to the 1997 UICC criteria: stages I and II were observed in 39 cases, while stages III and IV were observed in 67 cases.
Reagents
Mouse anti-human HGF monoclonal antibody, c-Met, SP kits, and DAB chromogenic kits were purchased from Santa Cruz Biotechnology (Dallas, Texas, USA). Specimens were cut into serial sections of 4-6 μm and covered in 0.01 mol/L citrate buffer. Heat-mediated antigen retrieval was performed by microwave. Primary antibody was added and samples were incubated at 4°C in a wet box. Biotin-labeled secondary antibodies were added and samples were incubated in a wet box at 37°C for 20 minutes. SP working solution was added and samples were incubated at 37°C for 20 minutes; DAB color development and hematoxylin counterstaining was applied. Samples known to contain thyroid cancer tissue sections were used as positive controls and PBS solution was used in place of the primary antibody as a negative control.
Histology
Each tissue section was observed in 4 continuous high-power fields and the average number of cells positive for expression of HGF or c-Met was determined for every 100 cancer or control cells in each field. HGF and c-Met staining were observed in the cell membrane and cytoplasm and appeared as brown or tan granules. The percentages of HGF- and c-Met-positive cells per sample were recorded as follows: 0-20% (-), 21%-30% (+), 31%-50% (++), and > 51% (+++).
Statistical analysis
SAS 9.2 (SAS Institute, Inc., Cary, North Carolina, USA) was used to analyse data. χ2 and nonparametric rank sum tests were applied for identifying differences between groups. A p value of < 0.05 was considered statistically significant.
Results
HGF and c-Met are more commonly expressed in NPC tissues
In NPC tissues, HGF protein was predominantly expressed in the tumor stroma (Figure 1), while c-Met was expressed in the cytoplasm or membrane of the cells (Figure 2). In control samples, HGF and c-Met expression were limited to columnar epithelial cell membranes or cytoplasm. c-Met protein was more commonly expressed in NPC than in control tissues with nasopharyngeal mucositis (P < 0.001). HGF was also more commonly expressed in NPC than in control samples (P < 0.001) (Table 1).
Figure 1.
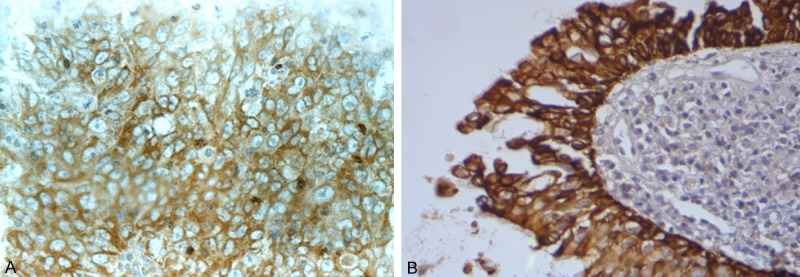
c-Met expression in the membrane and/or cytoplasm of NPC cells (A) and controls (B), stained brown (400×).
Figure 2.
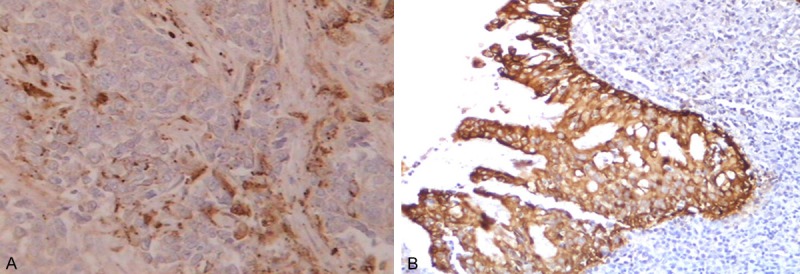
HGF expression in the membrane and/or cytoplasm of NPC cells (A) and the normal interstitial tissue (B) surrounding the tumor, stained brown. A few scattered spots were seen in the NPC cells (400×).
Table 1.
Expression of HGF and c-Met protein detected by immunohistochemistry in nasopharyngeal carcinoma (NPC) and control tissues
| Group | c-Met expression | Percentage of positive samples | HGF expression | Percentage of positive samples | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| - | + | ++ | +++ | - | + | ++ | +++ | |||
| Control (n = 115) | 92 | 23 | 0 | 0 | 20.00 | 45 | 39 | 31 | 0 | 60.9 |
| NPC (n = 106) | 32 | 12 | 29 | 33 | 69.8 | 11 | 35 | 23 | 37 | 89.6 |
| χ2 | 53.00 | 63.00 | ||||||||
| P | < 0.001 | < 0.001 | ||||||||
The expression of HGF and its receptor, c-Met, increases with NPC severity
To determine whether HGF and/or c-Met expression correlated with any characteristics of NPC, NPC samples were categorized as either not expressing (-) or expressing (+, ++, and +++ designations combined) these proteins and analyzed by clinical and pathological features. Both HGF and c-Met expression in NPC were more commonly detected in stage III and IV tissue samples than in stages I and II (P < 0.05 and P < 0.01, respectively). c-Met was also more commonly expressed among NPC patients with cervical lymph node metastasis than those without metastasis (P < 0.01). However, HGF and c-Met protein expression showed no difference among patients of different age, gender, and tissue typing (P > 0.05) (Table 2).
Table 2.
Relationship between HGF/c-Met expression and clinical and pathological parameters of NPC
| Parameter | c-Met expression Percentage of positive samples | χ2, P | HGF expression Percentage of positive samples | χ2, P | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| -- | +~+++ | (%) | -- | +~+++ | (%) | |||
| Sex | 0.81, 0.37 | 2.58, 0.11 | ||||||
| Male (n = 79) | 22 | 57 | 72.2 | 6 | 73 | 92.4 | ||
| Female (n = 27) | 10 | 17 | 63.0 | 5 | 22 | 81.5 | ||
| Age | 0.01, 0.90 | 3.62, 0.06 | ||||||
| < 50 (n = 72) | 22 | 50 | 69.4 | 7 | 65 | 90.3 | ||
| ≥ 50 (n = 34) | 10 | 24 | 70.6 | 4 | 30 | 88.2 | ||
| Histologic grade | 0.40, 0.53 | 1.32, 0.25 | ||||||
| DNKC (n = 82) | 26 | 56 | 68.3 | 7 | 75 | 91.5 | ||
| UNKC (n = 24) | 6 | 18 | 75.0 | 4 | 20 | 83.3 | ||
| Clinical stage | 13.03, < 0.01 | < 0.01* | ||||||
| I, II (n = 39) | 20 | 19 | 48.7 | 11 | 28 | 71.8 | ||
| III, IV (n = 67) | 12 | 55 | 82.1 | 0 | 67 | 100.0 | ||
| Cervical lymph node metastasis | 17.10, < 0.01 | 1.55, 0.21 | ||||||
| Positive (n = 83) | 17 | 66 | 79.5 | 7 | 76 | 91.6 | ||
| Negative (n = 23) | 15 | 8 | 34.8 | 4 | 19 | 82.6 | ||
Fisher’s exact test.
Discussion
HGF has a strong mitogen effect on liver cells and can rapidly form new tissues, promote fission, and induce epithelial cell migration and angiogenesis. Stromal fibroblasts are a major source of HGF, while its receptor, c-Met, is present in vascular endothelial cells and tumor cells, but not stromal cells [5]. HGF and c-Met have been shown to be over-expressed in many types of tumor tissues, suggesting a role for HGF-c-Met signaling in the development of solid tumors [4,6].
Our study showed that, in NPC tissues, HGF protein was mainly expressed in the tumor stroma, while slight positive expression was also observed in cancer cells. The expression of c-Met protein in NPC was located in the cytoplasm or cell membranes of cancer cells, consistent with previous findings [6,7]. We also showed that HGF and c-Met protein were expressed in nasopharyngeal mucositis, mainly confined to columnar epithelial cell membranes or cytoplasm. The expression of c-Met protein in NPC was significantly higher than that in controls. The expression of HGF protein in NPC stroma was also higher than that in the controls, suggesting that HGF overexpression may be associated with metastasis and invasion of nasopharyngeal carcinoma.
c-Met is a tyrosine kinase protein usually found on the surface of epithelial cells. We observed low expression of c-Met in the nasopharyngeal mucosa of patients without cancer, similar to findings reported by Qian et al. [8]. Tissue samples from NPC patients, however, showed significantly higher levels of c-Met expression suggesting that overexpression of c-Met is correlated with benign or malignant lesions of the nasopharynx.
Taken together, these results indicate that HGF and c-Met are overexpressed in NPC tissues and may play a role in promoting differentiation, proliferation, and malignant transformation among nasopharyngeal mucosal cells. Most cases of NPC derive from columnar epithelial cells of the nasopharynx, while HGF is produced by mesenchymal cells and can activate c-Met on cancer cells and promote tumor cell proliferation [9,10]. The HGF/c-Met system transmits signals mainly through the MPAK and PI3K pathways, resulting in a variety of cellular responses including proliferation, differentiation, and apoptosis [11]. In addition to HGF, cytokines and growth factors such as IL-1, IL-6, and epidermal growth factor can also induce up-regulation of c-Met expression [12]. Future studies will seek to determine the effects of cytokines on nasopharyngeal carcinoma tissues and their relationship to HGF.
Our results showed that nasopharyngeal carcinoma c-Met expression was significantly higher in samples taken from patients with lymph node metastasis compared to those without lymph node metastasis, and that the expression of c-Met increased with clinical severity, consistent with the findings by Sun et al. [13]. c-Met expression was not different among samples from patients of different ages, gender, or tissue typing, consistent with the findings by Li Yun-cheng et al [14].
In summary, our results indicate that HGF and c-Met are overexpressed in nasopharyngeal carcinoma cells. It may be possible that HGF induces c-Met expression via paracrine signaling, and that an increase in expression of HGF/c-Met may play a role in metastasis and the evolution of nasopharyngeal carcinoma cells. We also found that c-Met expression was related to the invasion and metastasis of NPC, suggesting that it could have the potential to serve as a marker for determining NPC prognosis.
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G. Targeting MET in cancer: rationale and progress. Nat Rev Cancer. 2012;12:89–103. doi: 10.1038/nrc3205. [DOI] [PubMed] [Google Scholar]
- 3.Liu X, Newton RC, Scherle PA. Developing c-MET pathway inhibitors for cancer therapy: progress and challenges. Trends Mol Med. 2010;16:37–45. doi: 10.1016/j.molmed.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Toiyama Y, Yasuda H, Saigusa S, Matushita K, Fujikawa H, Tanaka K, Mohri Y, Inoue Y, Goel A, Kusunoki M. Co-expression of hepatocyte growth factor and c-Met predicts peritoneal dissemination established by autocrine hepatocyte growth factor/c-Met signaling in gastric cancer. Int J Cancer. 2012;130:2912–2921. doi: 10.1002/ijc.26330. [DOI] [PubMed] [Google Scholar]
- 5.Shojaei F, Lee JH, Simmons BH, Wong A, Esparza CO, Plumlee PA, Feng J, Stewart AE, Hu-Lowe DD, Christensen JG. HGF/c-Met acts as an alternative angiogenic pathway in sunitinib-resistant tumors. Cancer Res. 2010;70:10090–10100. doi: 10.1158/0008-5472.CAN-10-0489. [DOI] [PubMed] [Google Scholar]
- 6.Knowles LM, Stabile LP, Egloff AM, Rothstein ME, Thomas SM, Gubish CT, Lerner EC, Seethala RR, Suzuki S, Quesnelle KM, Morgan S, Ferris RL, Grandis JR, Siegfried JM. HGF and c-Met participate in paracrine tumorigenic pathways in head and neck squamous cell cancer. Clin Cancer Res. 2009;15:3740–3750. doi: 10.1158/1078-0432.CCR-08-3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie LQ, Bian LJ, Li Z, Li Y, Liang YJ. Co-elevated expression of hepatocyte growth factor and Interleukin-8 contributes to poor prognosis of patients with primary nasopharyngeal carcinoma. Oncol Rep. 2010;23:141. [PubMed] [Google Scholar]
- 8.Qian CN, Guo X, Cao B, Kort EJ, Lee CC, Chen J, Wang LM, Mai WY, Min HQ, Hong MH, Vande Woude GF, Resau JH, Teh BT. Met protein expression level correlates with survival in patients with late-stage nasopharyngeal carcinoma. Cancer Res. 2002;62:589–596. [PubMed] [Google Scholar]
- 9.Xie L, Bian L, Li Z, Li Y, Li ZX, Li B. Altered expression of E-cadherin by hepatocyte growth factor and effect on the prognosis of nasopharyngeal carcinoma. Ann Surg Oncol. 2010;17:1927–1936. doi: 10.1245/s10434-010-0922-6. [DOI] [PubMed] [Google Scholar]
- 10.Lijuan B, Bing L, Zhi L, Yang L, Yingjie L. Hepatocyte growth factor suppresses tumor cell apoptosis in nasopharyngeal carcinoma by upregulating Bcl-2 protein expression. Pathol Res Pract. 2009;205:828–837. doi: 10.1016/j.prp.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 11.Whittaker S, Marais R, Zhu AX. The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene. 2010;29:4989–5005. doi: 10.1038/onc.2010.236. [DOI] [PubMed] [Google Scholar]
- 12.Hov H, Tian E, Holien T, Holt RU, Vatsveen TK, Fagerli UM, Waage A, Børset M, Sundan A. c-Met signaling promotes IL-6-induced myeloma cell proliferation. Eur J Haematol. 2009;82:277–287. doi: 10.1111/j.1600-0609.2009.01212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun R, Zhang Q, Guo L, Chen MY, Sun Y, Cao B, Sun J. HGF stimulates proliferation through the HGF/c-Met pathway in nasopharyngeal carcinoma cells. Oncol Lett. 2012;3:1124–1128. doi: 10.3892/ol.2012.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Zhang S, Tang Z, Chen J, Kong W. Silencing of c-Met by RNA interference inhibits the survival, proliferation, and invasion of nasopharyngeal carcinoma cells. Tumour Biol. 2011;32:1217–1224. doi: 10.1007/s13277-011-0225-y. [DOI] [PubMed] [Google Scholar]
- 15.Davis IJ, McFadden AW, Zhang Y, Coxon A, Burgess TL, Wagner AJ, Fisher DE. Identification of the receptor tyrosine kinase c-Met and its ligand, hepatocyte growth factor, as therapeutic targets in clear cell sarcoma. Cancer Res. 2010;70:639–645. doi: 10.1158/0008-5472.CAN-09-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]


