Abstract
Cytokine responses play an important role in the pathogenesis of influenza infection. Previous studies found that cytokine expressions in patients infected with the novel A (H1N1) influenza virus (nvA (H1N1)) could reflect the severity of the disease. But the patterns of cytokine response in patients infected with seasonal influenza virus and the correlations between cytokine responses and clinical data are still unknown. Seventy-two outpatients for laboratory-confirmed seasonal influenza infection were studied: twenty-four seasonal influenza A patients and forty-eight seasonal influenza B patients. Thirty healthy volunteers were enrolled as a control group. Serum samples from influenza patients obtained on the admission day and 6 days later were measured for eight cytokines using enzyme-linked immunosorbent assay (ELISA). The clinical variables were recorded prospectively. The levels of interleukin (IL)-6, IL-33 and tumor necrosis factor (TNF)-α were significantly higher in influenza A patients than those in the control group while IL-6, IL-17A, IL-29, interferon (IFN)-γ and interferon gamma-induced protein (IP)-10 were significantly higher in influenza B patients than those in the control group. Furthermore, IL-17A, IL-29 and IP-10 were increased in seasonal influenza B patients when comparing with those in the seasonal influenza A patients. A positive correlation of IL-29 levels with fever (Spearman’s rho, P-values < 0.05) and a negative correlation of IFN-γ and IP-10 levels with lymphocyte count (Spearman’s rho, P-values < 0.05) were found in seasonal influenza infection. While a hyperactivated proinflammatory cytokine responses were found in seasonal influenza infection, a higher elevation of cytokines (IL-17A, IL-29 and IP-10) were found in seasonal influenza B infection versus influenza A. IL-29, IFN-γ and IP-10 were important hallmarks in seasonal influenza infection, which can help clinicians make timely treatment decision for severe patients.
Keywords: Adults, seasonal influenza A, seasonal influenza B, cytokine, clinical aspects, immunity
Introduction
Infections caused by seasonal influenza occur throughout the world annually and result in significant illness and great economic losses [1]. Seasonal influenza is mainly self-limited, but pregnant women, young children, elderly people and people with underlying diseases are at high risk for hospitalization and some may die from the severe complications. The mortality caused by the disease every year is estimated to be 250,000 to 500,000 cases worldwide [2]. In addition, about 11 billion dollars is spent a year in the US on the economic burden caused by seasonal influenza [3]. Early studies demonstrated an intense elevation of proinflammatory cytokine levels in patients with seasonal influenza infection [4-6]. However, the pathogenetic role and the importance of cytokines in the clinical manifestations have not been fully elucidated.
Cytokines play a significant role in the pathogenesis of the new H1N1 influenza A infection [7,8]. Kim et al and Hagau et al have demonstrated higher plasma levels of IL-6, TNF-α, IP-10 in patients with the novel influenza A (H1N1) infection and that concentrations of these cytokines correlated with disease severity [9,10]. This would be helpful because sometimes it is difficult to distinguish between severe and mild patients from the clinical manifestations. But few clinical studies were conducted in humans with seasonal influenza infection and there are limited data on cytokine responses.
Our aim was to measure serum levels of proinflammatory cytokines in adult patients with seasonal influenza infection to explore the cytokine responses and determine whether there are specific predictors associated with severity of seasonal influenza.
Materials and methods
Patients and controls
Patients included in our study represent a subset of patients enrolled in a multicenter clinical trial assessing the efficacy of zanamivir in treatment of seasonal influenza infection (NCT01459081). All the patients were outpatients recruited in the peak of the 2011/2012 influenza season, between December 2011 and April 2012, when A/H3N2 and type B were epidemic in China. Patient with 2009 pandemic influenza A infection was not included in our study. 30 healthy volunteers without chronic or acute disease were recruited as normal control group.
The study approval was obtained from the Ethics Committee for Clinical Research of Shanghai Changzheng Hospital, Second Military Medical University. Written informed consent was obtained directly from each patient or their legal representative prior to inclusion in the study and also from the healthy controls.
Inclusion criteria: Eligible patients were aged 18-65 years, presented within 48 hrs of onset of flu symptoms, including fever (oral temperature ≥37.8°C) and at least two symptoms of stuffy nose, sore throat, cough, myalgia, headache, malaise and positive by quick antigen diagnostic test kit (BinaxNow® Influenza A&B Test, America) for influenza virus antigens from nasopharyngeal swabs.
Exclusion criteria: Patients with bacterial infection, human immunodeficiency virus infection, asthma or chronic obstructive pulmonary diseases, or who were receiving steroids, immunosuppressants, antivirals, or other herbal medicines, were excluded from this study. Children under 12 years old, patients older than 65 years old and pregnant women were also excluded to avoid confusion factors during the analysis of the immune response to the virus.
All patients were assessed at enrollment and during follow-up according to the standardized data sheet. For each patient, the following data were registered: age, sex, underlying diseases (diabetes, preexisting lung disease, and preexisting cardiovascular disease), body mass index (BMI), laboratory test results (including hematological and biochemical results) and radiological findings. Symptoms were assessed by influenza patients twice daily using a 4-point scale (0, absent to 3, severe) from enrollment until Day 6. Symptoms including temperature, stuffy nose, sore throat, cough, myalgia, headache and malaise were recorded. Total symptom score for each time point was the sum of each symptom score.
Samples and laboratory studies
Sample collection
Of the enrolled patients, 87.5% were male, and mean age of controls was 44 years. Peripheral venous blood samples were taken immediately at the time of recruitment (before antiviral therapy, if given), and then on day 6 for blood counts, serum chemistry and cytokine measurement. Serum samples were obtained after centrifugation (3000 g for 15 min) at 4°C and stored at -70°C until analysis.
Viral diagnosis and Haemagglutination inhibition assay (HI)
All the nasopharyngeal swabs from the patients were collected at admission and at the same time tested by a quick antigen diagnostic test kit (BinaxNow® Influenza A&B Test, America) for influenza A and B. Subsequent subtype determination of influenza virus was performed by hemagglutinin inhibition (HI) test. HI assays were performed on a 100 μl aliquot of the samples in a biosafety level-III laboratory in Shanghai Public Health Clinical Center. The sera was treated with Receptor-Destroying Enzyme (RDE) (Denka Seiken, Tokyo, Japan) by diluting one part serum with three parts enzyme and incubated overnight in a 37°C water bath. The enzyme was inactivated by a 30-minute incubation at 56°C followed by the addition of six parts 0.85% physiological saline for a final dilution of 1/10. HI assays were performed in U-bottom 96-well microtiter plates with 1.5% guinea pig erythrocytes, using inactivated influenza A /H1N1 antigens, A/H3N2 antigens, B/Yamagata antigens and B/Victoria antigens (National Institute for Biological Standards and Control, NIBSC, England). The presence of influenza virus was confirmed by the quick antigen diagnostic test and HI results.
Cytokines quantification
IL-6, IL-17A, IL-29, IL-32, IL-33, TNF-α, IFN-γ and IP-10 were evaluated with ELISA kits for quantitative determination. The detection sensitivities of IL-6, IL-17A, IL-29, IL-32, IL-33, TNF-α, IFN-γ, IP-10 detection assays were 2 pg/ml (Drkewei, China), 31.25 pg/ml (Drkewei, China), 2.0 pg/ml (eBioscience, North America), 4 pg/ml (BioLegend, America), 0.2 pg/ml (eBioscience, North America), 0.13 pg/ml (eBioscience, North America), 5 pg/ml (Drkewei, China), 1.0 pg/ml (eBioscience, North America). And the detection ranges of IL-6, IL-17A, IL-29, IL-32, IL-33, TNF-α, IFN-γ, IP-10 detection assays were 6.25-200 pg/ml, 62.5-4000 pg/ml, 15.6-1000 pg/ml, 7.8-500 pg/mL, 7.8-500 pg/mL, 0.31-20.0 pg/mL, 12.5-400 pg/ml, 3.1-200 pg/mL. These selected cytokines in our study were based on prior studies [4,5,11-14]. Normal serum reference ranges of the eight cytokines were measured from 30 healthy controls.
Statistical analysis
Data analysis was performed using SPSS version 17.0 and Graphad Prism. Data was displayed as (mean and standard deviation) for normal distributions, and as (median and interquartile range) for non-normal distributions. Comparisons between groups in oral temperature and total symptom score were performed using the Independent Samples Test. The Kruskal-Wallis test was used for comparisons of cytokine levels between groups. Correlations between cytokine concentrations and clinical or laboratory data were analyzed by calculating the Spearman correlation coefficient (r). Any value of P < 0.05 was considered statistically significant.
Results
Patient’s characteristics
Overall, 24 seasonal influenza A and 48 seasonal influenza B patients were enrolled in our study. Their demographic, underlying conditions and clinical characteristics are listed in Table 1. No significant differences were found in age, male to female ratio or clinical characteristics between the two groups. Three patients with seasonal influenza A infection and two patients with seasonal influenza B infection had underlying conditions which including diabetes, preexisting lung disease and preexisting cardiovascular disease (Table 1). Smoking was the common condition observed in our patients. Normal results of chest X-Ray was seen in all the patients (Table 1). All the patients in this study reported symptoms of acute respiratory viral infection on entry. The most frequent occurrences were: fever, myalgia, cough, malaise, sore throat, headache, stuffy nose. In addition, nine patients (37.5%) in influenza A group and twenty-three patients (46.9%) in influenza B group had the temperature over 38.5°C. The patients enrolled in this study were outpatients and showed no difficulties with respiratory function. All the patients in our study received antiviral treatment with zanamivir (20 mg/day) on admission. And the treatment was well tolerated. In consistent with the protocol, patients in this study could use acetaminophen and liquorice tablets as a rescue medication.
Table 1.
Demographic, co-morbidities and clinical characteristics of the patients
| Characteristics of patients | patients with seasonal influenza A infection (n=24) | patients with seasonal influenza B infection (n=48) | P value |
|---|---|---|---|
| Age (years) | 41 (32 to 57) | 31 (29 to 52) | 0.264 |
| Sex ratio | 10/14 | 24/24 | 0.504 |
| (male/female) | |||
| Underlying disease | |||
| Diabetes | 1/24 | 0 | |
| Preexisting lung disease | 1/24 | 2/48 | 1 |
| Preexisting cardiovascular disease | 1/24 | 0 | |
| Smoking history | 8/24 | 20/48 | 0.494 |
| Obesity (BMI > 30) | 2/24 | 5/48 | 1 |
| Presenting symptoms | |||
| Fever > 38°C | 24/24 | 48/48 | |
| Stuffy nose | 23/24 | 39/48 | 0.185 |
| Sore throat | 20/24 | 44/48 | 0.507 |
| Cough | 21/24 | 48/48 | |
| Myalgia | 24/24 | 47/48 | |
| Headache | 24/24 | 39/48 | |
| Malaise | 23/24 | 45/48 | 1 |
| Opacity in initial chest X-Ray | 0 | 0 |
Data presented as median (interquartile range), number (/) of patients. Chi-square test was used for categorical variables and Mann Whitney U test for continuous variables in differences in baseline characteristics between influenza A and influenza B patients.
The results of subtype determination of seasonal influenza infection by HI was defined as a four-fold or greater increase in convalescent serum antibody titres compared with acute serum. All the patients in influenza A group were subtype H3N2, while in influenza B group thirty-six patients were B/Yamagata (75%) and twelve were B/Victoria (25%).
Cytokine responses and correlations of cytokine levels with Symptoms in Influenza Infection
Serum obtained on day 1 and day 6 were examined for IL-6, IL-17A, IL-29, IL-32, IL-33, TNF-α, IFN-γ, and IP-10. Correlation between the clinical characteristics and cytokine levels observed in the serum was investigated by subjecting the data from the seasonal influenza patients to Spearman correlation coefficient.
At entry before any antiviral treatment, serum concentrations of IL-6, IL-33 and TNF-α were higher in seasonal influenza A patients than control subjects (P < 0.05, Figure 1). And greater serum levels of IL-6, IL-17A, IL-29, IP-10 and IFN-γ were found in influenza B patients than in the control subjects (P < 0.05, Figure 1). Furthermore, the serum levels of IL-17A, IL-29 and IP-10 were higher in influenza B patients versus influenza A patients while greater IL-33 concentration was observed in influenza A patients than in influenza B patients (P < 0.05, Figure 1). Compared with control subjects, no significant difference of IL-32 levels were observed in influenza A and influenza B patients (P > 0.05, Figure 1).
Figure 1.
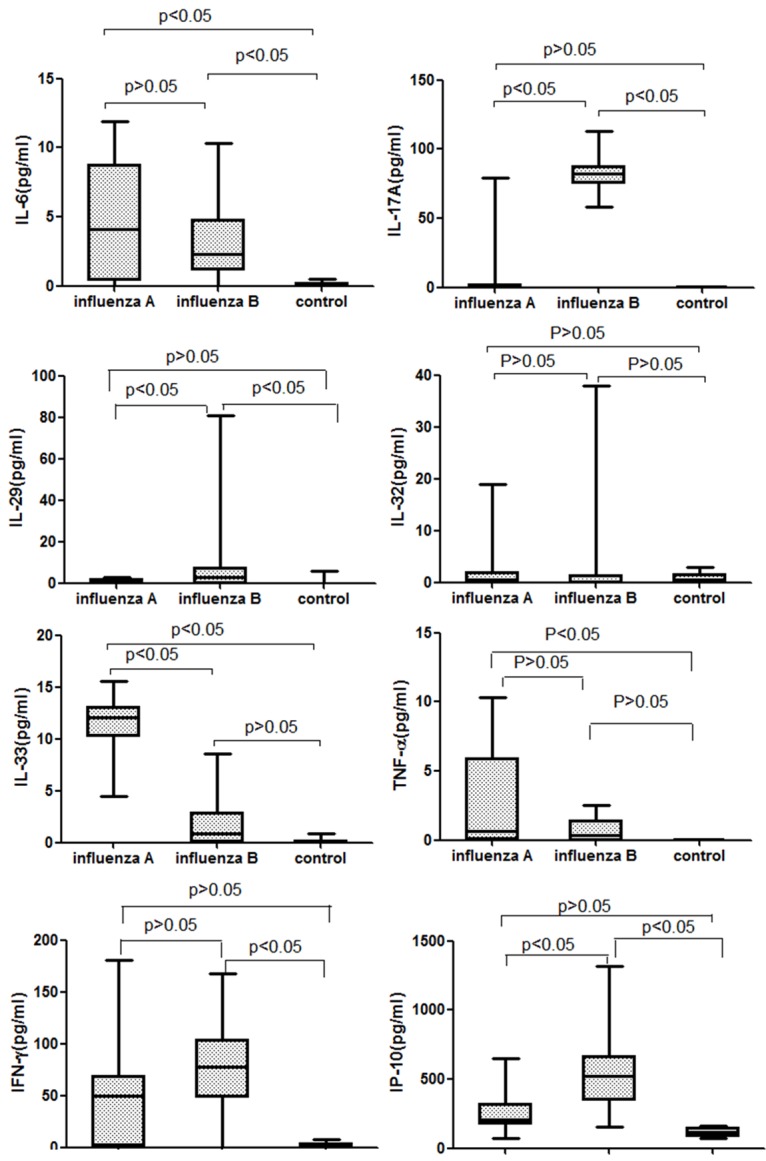
Levels of cytokines (IL-6, IL-17A, IL-29, IL-32, IL-33, TNF-α, IFN-γ and IP-10) in the three groups. The Kruskal-Wallis test was used to compare cytokine levels.
Serum cytokine levels showed a significant reduction of IL-6, IL-33 and TNF-α in patients with influenza A infection on days 6 compared with admission (P < 0.05, Table 2). Serum cytokine levels in patients with influenza B infection showed a significant reduction of IL-6, IL-17A, IL-29, IFN-γ and IP-10 on days 6 (P < 0.05, Table 2). Although there was not significant changes of IL-29 levels over time, we found serum IL-29 appeared only in 35% patients in influenza B group on day 6.
Table 2.
Serum cytokine levels over time in seasonal influenza patients
| Cytokine (pg/ml) | Day 1 | Day 6 | P |
|---|---|---|---|
| IL-6 | |||
| Influenza A | 4.10 (0.40 to 8.78) | 0.34 (0.17 to 0.80) | 0.005 |
| Influenza B | 2.27 (1.18 to 4.74) | 0.17 (0.12 to 0.39) | 0.000 |
| IL-17A | |||
| Influenza A | 0.00 (0.00 to 1.74) | 0.00 (0.00 to 0.03) | 0.119 |
| Influenza B | 81.97 (75.68 to 87.51) | 0.37 (0.10 to 1.68) | 0.000 |
| IL-29 | |||
| Influenza A | 0.00 (0.00 to 1.78) | 0.00 (0.00 to 0.00) | 0.254 |
| Influenza B | 3.05 (0.00 to 7.58) | 0.00 (0.00 to 7.02) | 0.256 |
| IL-32 | |||
| Influenza A | 0.58 (0.00 to 1.89) | 0.00 (0.00 to 0.12) | 0.066 |
| Influenza B | 0.21 (0.00 to 1.35) | 0.12 (0.00 to 0.57) | 0.671 |
| IL-33 | |||
| Influenza A | 12.03 (10.28 to 13.11 ) | 0.00 (0.00 to 0.05) | 0.000 |
| Influenza B | 0.87 (0.04 to 2.90) | 0.00 (0.00 to 2.30) | 0.037 |
| TNF-α | |||
| Influenza A | 0.67 (0.04 to 5.89) | 0.00 (0.00 to 0.08) | 0.005 |
| Influenza B | 0.32 (0.00 to 1.40) | 0.00 (0.00 to 0.00) | 0.046 |
| IFN-γ | |||
| Influenza A | 49.43 (2.05 to 68.89) | 0.20 (0.11 to 1.23) | 0.005 |
| Influenza B | 77.29 (48.63 to 103.70) | 1.64 (0.49 to 5.65) | 0.000 |
| IP-10 | |||
| Influenza A | 210.40 (178.30 to321.30) | 111.30 (100.40 to204.40) | 0.010 |
| Influenza B | 522.90 (351.70 to 662.00) | 216.70 (174.80 to 263.60) | 0.000 |
Data presented as median (interquartile range). IL, interleukin; TNF, tumor necrosis factor; IFN, interferon; IP, interferon gamma-induced protein. Mann-Whitney U test was used to compare the cytokine levels.
On day 1 before any treatment, we found IL-29 levels was positively correlated with temperature values (r=0.44, P=0.008, Table 3). IFN-γ levels was negatively correlated with lymphocyte count (r=-0.39, P=0.013, Table 3). We also found IP-10 levels was negatively correlated with lymphocyte count (r=-0.44, P=0.005, Table 3).
Table 3.
Correlations between serum cytokine levels and clinical features of seasonal influenza patients on day 1
| Cytokine | Symptoms | Temperature | Lymphocyte count |
|---|---|---|---|
| IL-6 | 0.02a (0.452) | 0.16 (0.204) | -0.18 (0.158) |
| IL-17A | -0.02 (0.461) | -0.23 (0.115) | 0.21 (0.116) |
| IL-29 | 0.20 (0.146) | 0.44 (0.008) | -0.10 (0.285) |
| IL-32 | 0.01 (0.484) | 0.03 (0.439) | 0.00 (0.495) |
| IL-33 | -0.14 (0.225) | -0.13 (0.249) | 0.27 (0.061) |
| TNF-α | 0.06 (0.824) | 0.31 (0.257) | 0.31 (0.261) |
| IFN-γ | -0.17 (0.191) | 0.02 (0.456) | -0.39 (0.013) |
| IP-10 | 0.07 (0.353) | 0.25 (0.093) | -0.44 (0.005) |
NOTE. IL, interleukin; TNF, tumor necrosis factor; IFN, interferon; IP, interferon gamma-induced protein.
Spearman’s rank correlation coefficient (P).
Discussion
In this study we found elevated serum concentrations of IL-6, IL-33 and TNF-α on admission in patients with seasonal influenza A infection. In addition, markedly elevated serum concentrations of IL-6, IL-17A, IL-29, IP-10 and IFN-γ were detected in patients with seasonal influenza B infection. However, these cytokine levels in the influenza patients declined rapidly over 5 days. All the eight cytokine levels returned to normal on days 6. Serum IL-29 was positively correlated with temperature values while serum IFN-γ and IP-10 were negatively correlated with lymphocyte count. The three cytokines might be the hallmarks of disease severity. Such information may lead to timely treatment in severe patients with influenza.
In a recent study comparing cytokine response patterns in pandemic 2009 H1N1 and seasonal influenza infection among hospitalized patients, higher levels of IL-6 and CXCL10/IP-10 were found in seasonal influenza infection. And in contrast to seasonal influenza A, levels of IL-17A and IFN-r were significantly higher in seasonal influenza B infection [6]. Several in vitro and animal studies found strong induction of IL-29 and IL-33 in the lung of human infected with both seasonal and pandemic 2009 H1N1 influenza virus [15-19]. Furthermore, three previous studies showed that IL-6, TNF-α, and IP-10 levels were the risk factors for complications and admission to the intensive care unit (ICU) in seasonal influenza infection [5,20,21]. These results support the rise of IL-6, TNF-α, IL-33, IFN-γ and IP-10 found in both seasonal influenza A and influenza B patients while higher levels of IL-17A, IP-10 and IL-29 were reported in seasonal influenza B patients in our study. Of note, higher concentrations of serum IL-29 and IL-33 have been shown for the first time in patients infected with influenza virus in our study.
IL-6, a marker of innate immunity, was shown to induce excessive inflammation in the lung and might dys-regulate the adaptive immunity, then forming a vicious cycle in the influenza infection [22]. It has been proposed that IL-6 is an important mediator of fever and acute-phase reactions [23-25] and is responsible for much of symptom formation [26,27]. TNF-α, like IL-6, a cytokine of innate immunity, mediates systemic symptoms and induces excessive lung tissue destruction during influenza infection [10,28]. Several studies have reported higher serum levels of the two cytokines in patients with the novel H1N1 influenza infection and they also constitute the hallmarks of critical illness [6]. In our study both of the two cytokine concentrations were significantly higher in patients with seasonal influenza A infection; however, TNF-α was not significantly elevated in influenza B patients. Interpretation of the result might be that the influenza A virus was a stronger cytokine inducer than influenza B viruses [29]. In addition IL-6 and TNF-α were not correlated with clinical characters in our study. Maybe the patients recruited in our study were outpatients who were not severe ones; thus, the cytokine activation was lower in our patients and we did not find the relationship of IL-6 and TNF-α levels to clinical characters.
There was a strong induction of adaptive-immunity related cytokine (e.g. IL-17A, IFN-γ and IP-10) in seasonal influenza B patients in our study. IL-17A, a key cytokine of Th17 cells, which was previously known as IL-17, has been related with numerous immune and inflammatory responses by inducing expression of various inflammatory mediators, such as adhesion molecules, proinflammatory and neutrophil-mobilizing cytokines [25,30]. There is emerging evidence that Th17 adaptive immunity is effective in clearing pathogens including influenza virus [5,10,25,31]. Lee et al have reported that higher serum levels of IL-17A was found in severe non critical group of pandemic 2009 H1N1 influenza and seasonal influenza B patients, which could show a beneficial role of IL-17A in host defense [5]. In the present study elevated IL-17A was shown in seasonal influenza B patients but IL-17A was not the cytokine responsible for symptom production. IFN-γ plays a role in virus control by orchestrating Th1 differentiated from T cells in influenza infection [32]. Like IP-10, high levels of IFN-γ were detected in seasonal influenza B infection and correlated with lymphocyte count in our study. IP-10, another cytokine of the adaptive Th1/Th17-immunity, has shown a higher increase in seasonal influenza than pandemic 2009 H1N1 influenza [9]. Recent studies have reported that in severe pandemic 2009 H1N1 influenza infection, T-cell functions were injured and the cytokine response were downregulated [33]. In the present study, IP-10 levels were higher in seasonal influenza B patients versus the control subjects and negatively correlated with lymphocyte count.
IL-29, a newly described cytokine, has a variety of bioactivity, such as anti-virus, inducing target cell death, regulating immune modulating function and modulating CD4+ T-cell function [35]. IL-29 has been demonstrated to exert a direct antiviral effect in response to influenza infection through activation of antiviral genes Myxovirus 1 (MX1), Oligoadenylate synthetase 1 (OAS1), and interferon-stimulated genes56 (ISG56) [16]. Studies have also suggested that the release of IL-29 can be induced in influenza infection and allergic inflammation in the airway [16,35]. In some reports, human macrophages and monocyte were shown to react to IL-29 through producing IL-6, IL-8 and IL-10, meaning that IL-29 is important in regulating the innate immune response in viral infection [36]. An additional view is that IL-29 can increase mRNA levels of MIG and IP-10 in human peripheral blood mononuclear cells (PBMC) and pretreatment with IL-29 can decrease the viral titer [37]. It is notable that the cytopathic and cytokine response were different in different influenza virus strain infection. So in the present study, serum IL-29 levels were found to be elevated in seasonal influenza B patients for the first time and IL-29 was positively correlated with temperature value.
IL-32, a key regulator in innate and adaptive immune responses, has been reported to induce the production of IL-1β, IL-6, TNF-α and chemokines through the signal pathway of nuclear factor-κB (NF-κB) and p38 mitogen-activated protein kinases (MAPKs) [38,39]. IL-32 also plays an important role in various autoimmune diseases, bacterial and viral infections [34,40,41]. It has been reported that IL-32 expression can be activated by influenza virus through the NF-kB and CREB pathways as well as site-specific demethylation of CRE in the IL-32 promoter [12]. In the present study, IL-32 concentration was not elevated in patients with seasonal influenza infection because there was a weaker cytokine responses induced by seasonal influenza virus than the novel H1N1 influenza virus [29]. Wang et al has shown that cytokine responses depends on viral replication and the high viral titer and prolonged viral replication in the novel H1N1 influenza results in a robust cytokine responses, far stronger than seasonal influenza [16]. In addition, cytokine responses in seasonal influenza are soon downregulated, shorter than the novel H1N1 influenza.
IL-33, the latest IL-1 family member, presents dual functionality. Among the functions that IL-33 exert in the lung tissue, IL-33 was demonstrated to play a role in the induction of mucosal immunity against the novel H1N1 influenza A virus, which suggested a potent role of IL-33 as crucial amplifier of the immune responses in viral infection [42]. Several mice in vivo and in vitro studies have shown the profound expression of IL-33 in the new H1N1 influenza virus infected lung tissue and bronchoalveolar lavages [17,18]. In the present study, we found a significant elevation of IL-33 in seasonal influenza A patients, in agreement with recent animal model studies.
Some limitations of our study should be considered. First, patients in our study were enrolled at different stages of the disease meaning that serum collected from patients was not at the same time point. Second, children and elderly people can’t inhale zanamivir orally in a correct way and in consequence, the exclusion of children and elderly people is another limitation. However, children and elderly people are important groups in seasonal influenza season. Third, recruiting outpatients placed constraints on our blood sampling frame because it was impractical to collect blood samples daily during the first 6 days; thus, the time-sensitive cytokine responses in the disease did not be explored.
Acknowledgements
The authors would like to thank all of the nurses and residents for their assistance in the sample collection and the care to the patients with seasonal influenza infection.
Disclosure of conflict of interest
None.
References
- 1.World Health Organization: (WHO) Influenza (Seasonal) Available: http//www.Who.int/mediacentre/factsheets/fs211/en/index.html. Accessed 19 Oct 2011.
- 2.World Health Organization. Influenza. Available: http://www.who.int/topics/influenza/en. Accessed 1 May 2011.
- 3.Weycker D, Edelsberg J, Halloran ME, Longini IM Jr, Nizam A, Ciuryla V, Oster G. Population-wide benefits of routine vaccination of children against influenza. Vaccine. 2005;23:1284–1293. doi: 10.1016/j.vaccine.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 4.Tsou TP, Shao PL, Lu CY, Chang LY, Kao CL, Lee PI, Yang PC, Lee CY, Huang LM. Viral load and clinical features in children infected with seasonal influenza B in 2006/2007. J Formos Med Assoc. 2012;111:83–7. doi: 10.1016/j.jfma.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Lee N, Wong CK, Chan PK, Chan MC, Wong RY, Lun SW, Ngai KL, Lui GC, Wong BC, Lee SK, Choi KW, Hui DS. Cytokine response patterns in severe pandemic 2009 h1n1 and seasonal influenza among hospitalized adults. PLoS One. 2011;6:e26050. doi: 10.1371/journal.pone.0026050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayden FG, Treanor JJ, Fritz RS, Lobo M, Betts RF, Miller M, Kinnersley N, Mills RG, Ward P, Straus SE. Use of the oral neuraminidase inhibitor oseltamivir in experimental human influenza: randomized controlled trials for prevention and treatment. JAMA. 1999;282:1240–6. doi: 10.1001/jama.282.13.1240. [DOI] [PubMed] [Google Scholar]
- 7.Wang J, Nikrad MP, Travanty EA, Zhou B, Phang T, Gao B, Alford T, Ito Y, Nahreini P, Hartshorn K, Wentworth D, Dinarello CA, Mason RJ. Innate immune response of human alveolar macrophages during influenza A infection. PLoS One. 2012;7:e29879. doi: 10.1371/journal.pone.0029879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu S, Metcalf JP, Wu W. Innate immune response to influenza virus. Curr Opin Infect Dis. 2011;24:235–240. doi: 10.1097/QCO.0b013e328344c0e3. [DOI] [PubMed] [Google Scholar]
- 9.Kim YH, Kim JE, Hyun MC. Cytokine response in pediatric patients with pandemic influenza H1N1 2009 virus infection and pneumonia: comparison with pediatric pneumonia without H1N1 2009 infection. Pediatr Pulmonol. 2011;46:1233–1239. doi: 10.1002/ppul.21496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hagau N, Slavcovici A, Gonganau DN, Oltean S, Dirzu DS, Brezoszki ES, Maxim M, Ciuce C, Mlesnite M, Gavrus RL, Laslo C, Hagau R, Petrescu M, Studnicska DM. Clinical aspects and cytokine response in severe H1N1 influenza A virus infection. Crit Care. 2010;14:R203. doi: 10.1186/cc9324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayden FG, Osterhaus AD, Treanor JJ, Fleming DM, Aoki FY, Nicholson KG, Bohnen AM, Hirst HM, Keene O, Wightman K. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenza virus infections. N Engl J Med. 1997;337:874–880. doi: 10.1056/NEJM199709253371302. [DOI] [PubMed] [Google Scholar]
- 12.Li W, Sun W, Liu L, Yang F, Li Y, Chen Y, Fang J, Zhang W, Wu J, Zhu Y. IL-32: a host proinflammatory factor against influenza viral replication is upregulated by aberrant epigenetic modifications during influenza A virus infection. J Immunol. 2010;185:5056–5065. doi: 10.4049/jimmunol.0902667. [DOI] [PubMed] [Google Scholar]
- 13.Mäkelä MJ, Pauksens K, Rostila T, Fleming DM, Man CY, Keene ON, Webster A. Clinical efficacy and safety of the orally inhaled neuraminidase inhibitor zanamivir in the treatment of influenza: a randomized, double-blind, placebo-controlled european study. J Infec. 2000;40:42–48. doi: 10.1053/jinf.1999.0602. [DOI] [PubMed] [Google Scholar]
- 14.Puhakka T, Lehti H, Vainionpää R, Jormanainen V, Pulkkinen M, Sharp S, Kerr C, Dempsey M, Ring CJ, Ward C, Tisdale M. Zanamivir: a Significant Reduction in Viral Load During Treatment in Military Conscripts with Influenza. Scand J Infect Dis. 2003;35:52–58. doi: 10.1080/0036554021000026981. [DOI] [PubMed] [Google Scholar]
- 15.Lee SM, Chan RW, Gardy JL, Lo CK, Sihoe AD, Kang SS, Cheung TK, Guan YI, Chan MC, Hancock RE, Peiris MJ. Systems-level comparison of host responses induced by pandemic and seasonal influenza A H1N1 viruses in primary human type I-like alveolar epithelial cells in vitro. Respir Res. 2010;11:147. doi: 10.1186/1465-9921-11-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J, Oberley-Deegan R, Wang S, Nikrad M, Funk CJ, Hartshorn KL, Mason RJ. Differentiated human alveolar type II cells secrete antiviral IL-29 (IFN-lambda 1) in response to influenza A infection. J Immunol. 2009;182:1296–304. doi: 10.4049/jimmunol.182.3.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Goffic R, Arshad MI, Rauch M, L’Helgoualc’h A, Delmas B, Piquet-Pellorce C, Samson M. Infection with influenza virus induces IL-33 in murine lungs. Am J Respir Cell Mol Biol. 2011;45:1125–32. doi: 10.1165/rcmb.2010-0516OC. [DOI] [PubMed] [Google Scholar]
- 18.Chang YJ, Kim HY, Albacker LA, Baumgarth N, McKenzie AN, Smith DE, Dekruyff RH, Umetsu DT. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of daptive immunity. Nat Immunol. 2011;12:631–638. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kayamuro H, Yoshioka Y, Abe Y, Arita S, Katayama K, Nomura T, Yoshikawa T, Kubota-Koketsu R, Ikuta K, Okamoto S, Mori Y, Kunisawa J, Kiyono H, Itoh N, Nagano K, Kamada H, Tsutsumi Y, Tsunoda S. Interleukin-1 family cytokines as mucosal vaccine adjuvants for induction of protective immunity against influenza virus. J Virol. 2010;84:12703–12. doi: 10.1128/JVI.01182-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang YM, Song BM, Lee JS, Kim HS, Seo SH. Pandemic H1N1 influenza virus causes a stronger inflammatory response than seasonal H1N1 influenza virus in ferrets. Arch Virol. 2011;156:759–767. doi: 10.1007/s00705-010-0914-7. [DOI] [PubMed] [Google Scholar]
- 21.Guan X, Yang W, Sun X, Wang L, Ma B, Li H, Zhou J. Association of influenza virus infection and inflammatory cytokines with acute myocardial infarction. Inflamm Res. 2012;61:591–8. doi: 10.1007/s00011-012-0449-3. [DOI] [PubMed] [Google Scholar]
- 22.Bermejo-Martin JF, Martin-Loeches I, Rello J, Antón A, Almansa R, Xu L, Lopez-Campos G, Pumarola T, Ran L, Ramirez P, Banner D, Ng DC, Socias L, Loza A, Andaluz D, Maravi E, Gómez- Sánchez MJ, Gordón M, Gallegos MC, Fernandez V, Aldunate S, León C, Merino P, Blanco J, Martin-Sanchez F, Rico L, Varillas D, Iglesias V, Marcos MÁ, Gandía F, Bobillo F, Nogueira B, Rojo S, Resino S, Castro C, Ortiz de Lejarazu R, Kelvin D. Host adaptive immunity deficiency in severe pandemic influenza. Crit Care. 2010;14:R167. doi: 10.1186/cc9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee N, Chan PK, Wong CK, Wong KT, Choi KW, Joynt GM, Lam P, Chan MC, Wong BC, Lui GC, Sin WW, Wong RY, Lam WY, Yeung AC, Leung TF, So HY, Yu AW, Sung JJ, Hui DS. Viral clearance and inflammatory response patterns in adults hospitalized for pandemic 2009 influenza A (H1N1) virus pneumonia. Antivir Ther. 2011;16:237–247. doi: 10.3851/IMP1722. [DOI] [PubMed] [Google Scholar]
- 24.Wu W, Booth JL, Duggan ES, Wu S, Patel KB, Coggeshall KM, Metcalf JP. Innate immune response to H3N2 and H1N1 influenza virus infection in a human lung organ culture model. Virology. 2010;396:178–188. doi: 10.1016/j.virol.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakajima N, Van Tin N, Sato Y, Thach HN, Katano H, Diep PH, Kumasaka T, Thuy NT, Hasegawa H, San LT, Kawachi S, Liem NT, Suzuki K, Sata T. Pathological study of archival lung tissues from five fatal cases of avian H5N1 influenza in Vietnam. Mod Pathol. 2013;26:357–69. doi: 10.1038/modpathol.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Svitek N, Rudd PA, Obojes K, Pillet S, von Messling V. Severe seasonal influenza in ferrets correlates with reduced interferon and increased IL-6 induction. Virology. 2008;376:53–59. doi: 10.1016/j.virol.2008.02.035. [DOI] [PubMed] [Google Scholar]
- 27.McGill J, Heusel JW, Legge KL. Innate immune control and regulation of influenza virus infections. J Leukoc Biol. 2009;86:803–812. doi: 10.1189/jlb.0509368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsumoto Y, Kawamura Y, Nakai H, Sugata K, Yoshikawa A, Ihira M, Ohashi M, Kato T, Yoshikawa T. Cytokine and chemokine responses in pediatric patients with severe pneumonia associated with pandemic A/H1N1/2009 influenza virus. Microbiol Immunol. 2012;56:651–5. doi: 10.1111/j.1348-0421.2012.00489.x. [DOI] [PubMed] [Google Scholar]
- 29.Geiler J, Michaelis M, Sithisarn P, Cinatl J Jr. Comparison of proinflammatory cytokine expression and cellular signal transduction in human macrophages infected with different influenza A viruses. Med Microbiol Immunol. 2011;200:53–60. doi: 10.1007/s00430-010-0173-y. [DOI] [PubMed] [Google Scholar]
- 30.Kim SR, Lee KS, Park SJ, Min KH, Lee KY, Choe YH, Lee YR, Kim JS, Hong SJ, Lee YC. PTEN down-regulates IL-17 expression in a murine model of toluene diisocyanate-induced airway disease. J Immunol. 2007;179:6820–6829. doi: 10.4049/jimmunol.179.10.6820. [DOI] [PubMed] [Google Scholar]
- 31.Arankalle VA, Lole KS, Arya RP, Tripathy AS, Ramdasi AY, Chadha MS, Sangle SA, Kadam DB. Role of host immune response and viral load in the differential outcome of pandemic H1N1 (2009) influenza virus infection in Indian patients. PLoS One. 2010;5:e13099. doi: 10.1371/journal.pone.0013099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Augustine JJ, Hricik DE. T-cell immune monitoring by the ELISPOT assay for interferon gamma. Clin Chim Acta. 2012;413:1359–63. doi: 10.1016/j.cca.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 33.Agrati C, Gioia C, Lalle E, Cimini E, Castilletti C, Armignacco O, Lauria FN, Ferraro F, Antonini M, Ippolito G, Capobianchi MR, Martini F. Association of profoundly impaired immune competence in H1N1v-infected patients with a severe or fatal clinical course. J Infect Dis. 2010;202:681–689. doi: 10.1086/655469. [DOI] [PubMed] [Google Scholar]
- 34.Nold MF, Nold-Petry CA, Pott GB, Zepp JA, Saavedra MT, Kim SH, Dinarello CA. Endogenous IL-32 controls cytokine and HIV-1 production. J Immunol. 2008;181:557–565. doi: 10.4049/jimmunol.181.1.557. [DOI] [PubMed] [Google Scholar]
- 35.He S, Li T, Chen H, Ma W, Yao Q, Yang H, Wang H, Wang F, Zhao C, Yang P. CD14+ cell-derived IL-29 modulates proinflammatory cytokine production in patients with allergic airway inflammation. Allergy. 2011;66:238–246. doi: 10.1111/j.1398-9995.2010.02455.x. [DOI] [PubMed] [Google Scholar]
- 36.Jordan WJ, Eskdale J, Boniotto M, Rodia M, Kellner D, Gallagher G. Modulation of the human cytokine response by interferon λ-1 (IFN-λ1/IL-29) Genes Immun. 2007;8:13–20. doi: 10.1038/sj.gene.6364348. [DOI] [PubMed] [Google Scholar]
- 37.Pekarek V, Srinivas S, Eskdale J, Gallagher G. Interferon λ-1 (IFN-λ1/IL-29) induces ELR- CXC chemokine mRNA in human peripheral blood mononuclear cells, in an IFN-γ-independent manner. Genes Immun. 2007;8:177–180. doi: 10.1038/sj.gene.6364372. [DOI] [PubMed] [Google Scholar]
- 38.Kim SH, Han SY, Azam T, Yoon DY, Dinarello CA. Interleukin-32: a cytokine and inducer of TNF alpha. Immunity. 2005;22:131–142. doi: 10.1016/j.immuni.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 39.Netea MG, Azam T, Ferwerda G, Girardin SE, Walsh M, Park JS, Abraham E, Kim JM, Yoon DY, Dinarello CA, Kim SH. IL-32 synergizes with nucleotide oligomerization domain (NOD)1 and NOD2 ligands for IL-1beta and IL-6 production through a caspase 1-dependent mechanism. Proc Natl Acad Sci U S A. 2005;102:16309–16314. doi: 10.1073/pnas.0508237102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li W, Liu Y, Mukhtar MM, Gong R, Pan Y, Rasool ST, Gao Y, Kang L, Hao Q, Peng G, Chen Y, Chen X, Wu J, Zhu Y. Activation of interleukin-32 proinflammatory pathway in response to influenza A virus infection. PLoS One. 2008;3:e1985. doi: 10.1371/journal.pone.0001985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li W, Yang F, Liu Y, Gong R, Liu L, Feng Y, Hu P, Sun W, Hao Q, Kang L, Wu J, Zhu Y. Negative feedback regulation of IL-32 production by iNOS activation in response to dsRNA or influenza virus infection. Eur J Immunol. 2009;39:1019–1024. doi: 10.1002/eji.200838885. [DOI] [PubMed] [Google Scholar]
- 42.Kayamuro H, Yoshioka Y, Abe Y, Arita S, Katayama K, Nomura T, Yoshikawa T, Kubota-Koketsu R, Ikuta K, Okamoto S, Mori Y, Kunisawa J, Kiyono H, Itoh N, Nagano K, Kamada H, Tsutsumi Y, Tsunoda S. Interleukin-1 family cytokines as mucosal vaccine adjuvants for in duction of protective immunity against influenza virus. J Virol. 2010;84:12703–12712. doi: 10.1128/JVI.01182-10. [DOI] [PMC free article] [PubMed] [Google Scholar]


