Abstract
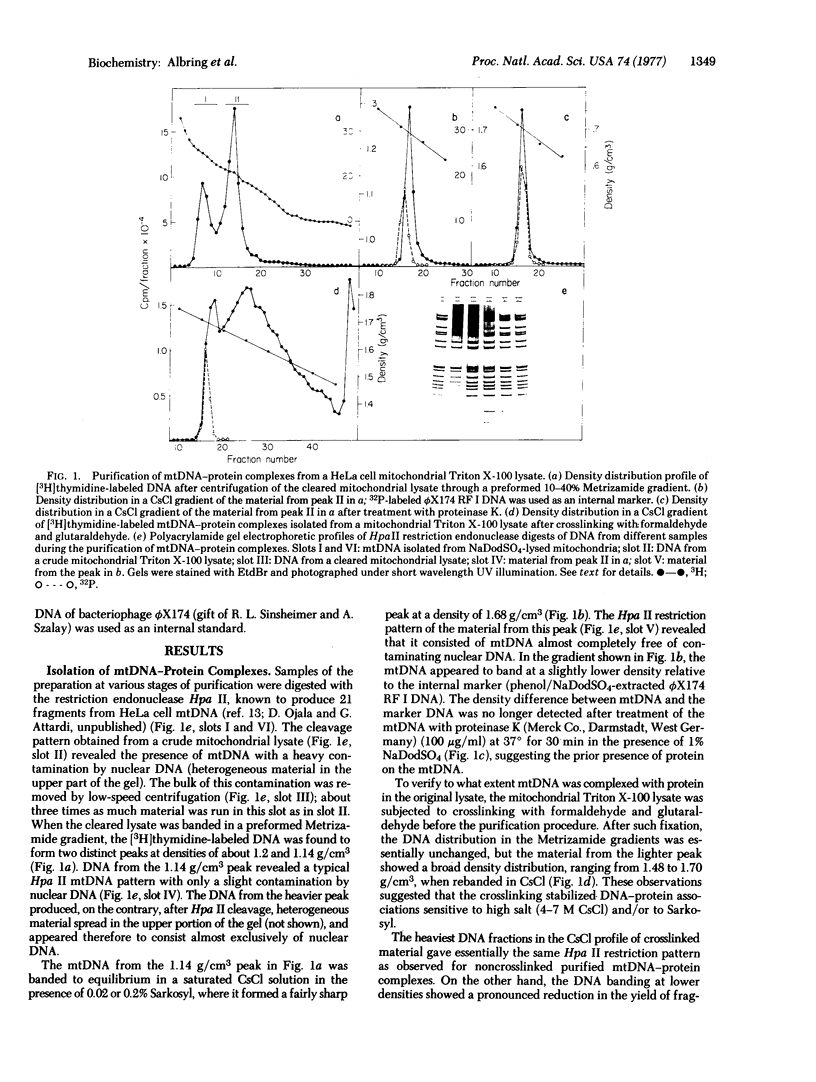
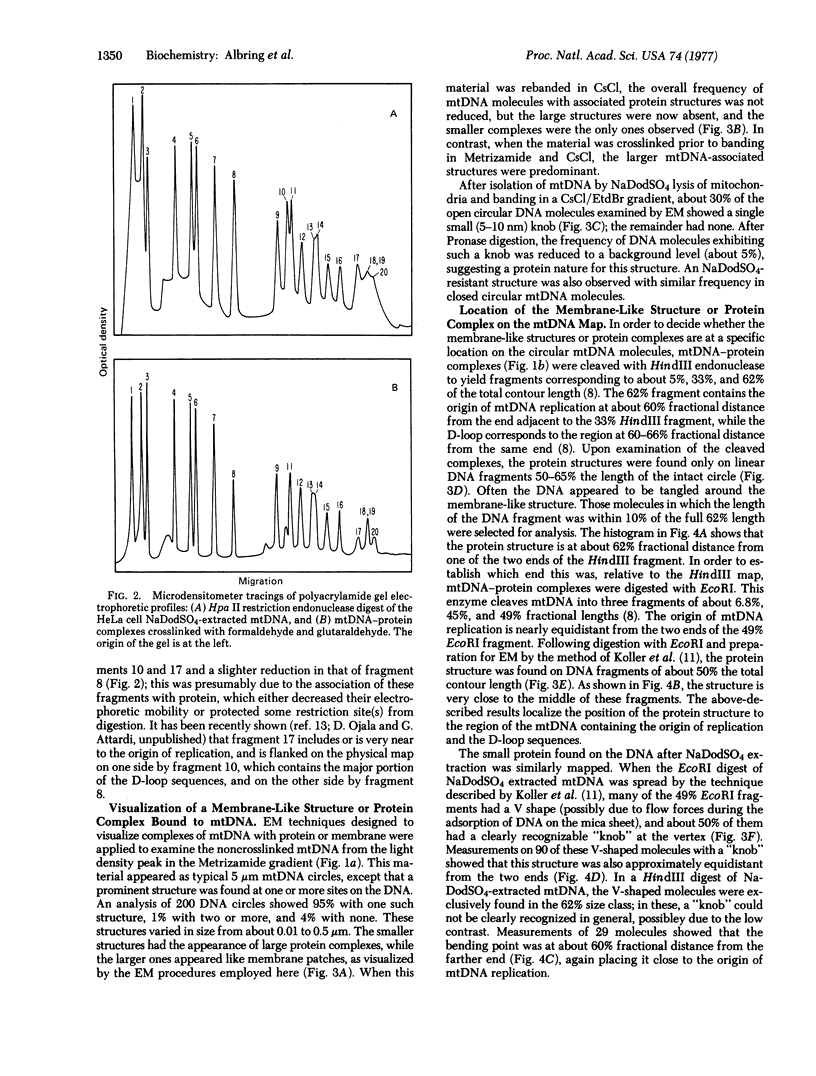
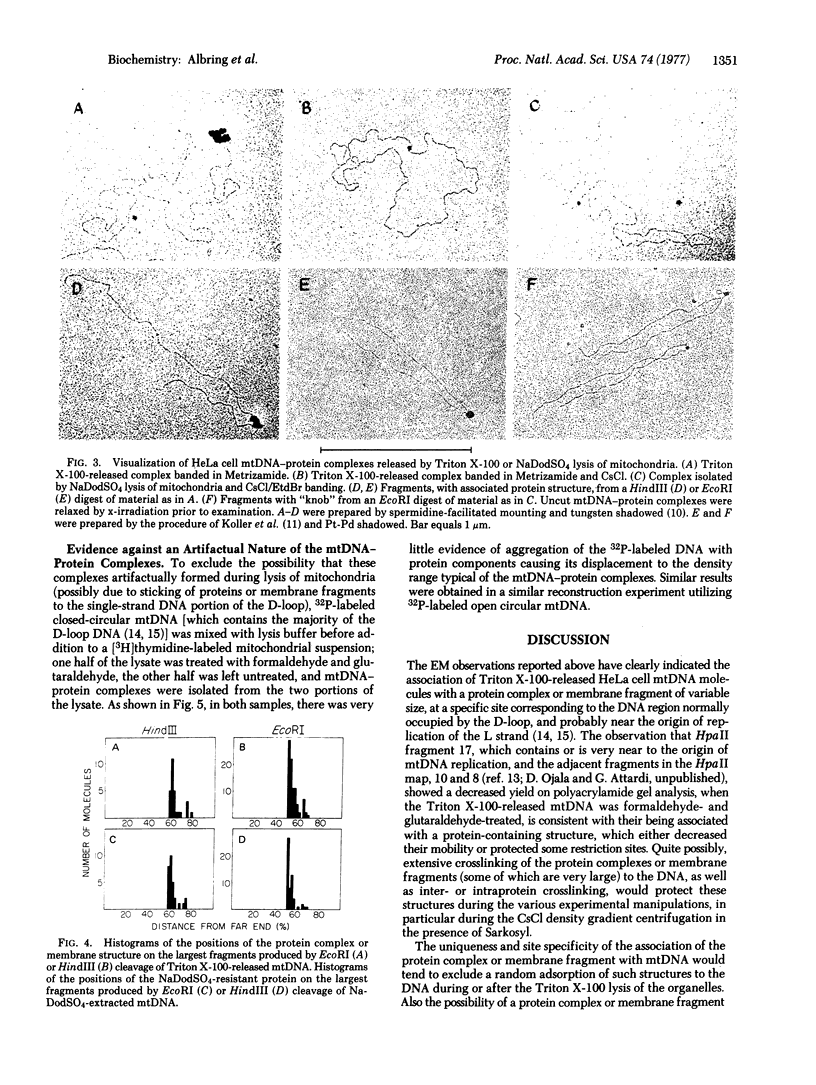
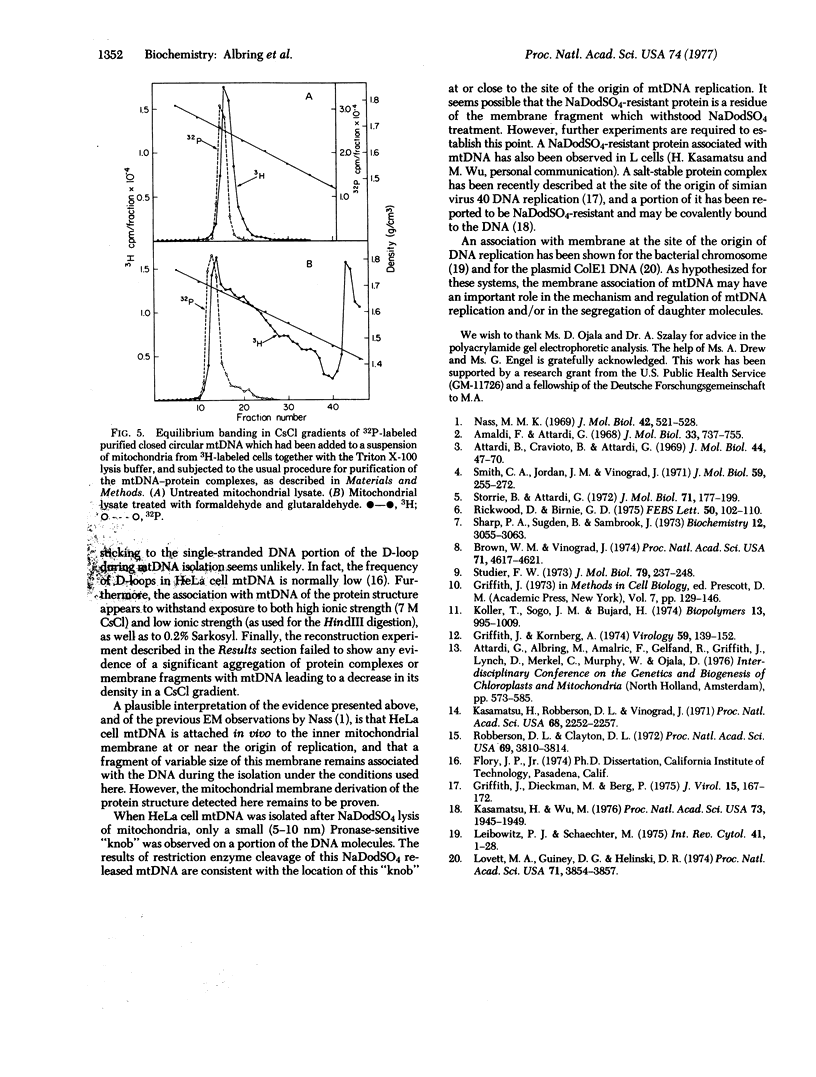
Almost all (about 95%) of the mitochondrial DNA molecules released by Triton X-100 lysis of HeLa cell mitochondria in the presence of 0.15 M salt are associated with a single protein-containing structure varying in appearance between a 10-20 nm knob and a 100-500 nm membrane-like patch. Analysis by high resolution electron microscopy and by polyacrylamide gel electrophoresis after cleavage of mitochondrial DNA with the endonucleases EcoRI, HindIII, and Hpa II has shown that the protein structure is attached to the DNA in the region of the D-loop, and probably near the origin of mitochondrial DNA replication. The data strongly suggest that HeLa cell mitochondrial DNA is attached in vivo to the inner mitochondrial membrane at or near the origin of replication, and that a membrane fragment of variable size remains associated with the DNA during the isolation. After sodium dodecyl sulfate extraction of mitochondrial DNA, a small 5-10 nm protein is found at the same site on a fraction of the mitochondrial DNA molecules.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amaldi F., Attardi G. Partial sequence analysis of ribosomal RNA from HeLa cells. I. Oligonucleotide pattern of 28 s and 18 s RNA after pancreatic ribonuclease digestion. J Mol Biol. 1968 May 14;33(3):737–755. doi: 10.1016/0022-2836(68)90317-3. [DOI] [PubMed] [Google Scholar]
- Attardi B., Cravioto B., Attardi G. Membrane-bound ribosomes in HeLa cells. I. Their proportion to total cell ribosomes and their association with messenger RNA. J Mol Biol. 1969 Aug 28;44(1):47–70. doi: 10.1016/0022-2836(69)90404-5. [DOI] [PubMed] [Google Scholar]
- Brown W. M., Vinograd J. Restriction endonuclease cleavage maps of animal mitochondrial DNAs. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4617–4621. doi: 10.1073/pnas.71.11.4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith J. D. Electron microscopic visualization of DNA in association with cellular components. Methods Cell Biol. 1973;7:129–146. doi: 10.1016/s0091-679x(08)61774-4. [DOI] [PubMed] [Google Scholar]
- Griffith J., Dieckmann M., Berg P. Electron microscope localization of a protein bound near the origin of simian virus 40 DNA replication. J Virol. 1975 Jan;15(1):167–172. doi: 10.1128/jvi.15.1.167-172.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith J., Kornberg A. Mini M13 bacteriophage: circular fragments of M13 DNA are replicated and packaged during normal infections. Virology. 1974 May;59(1):139–152. doi: 10.1016/0042-6822(74)90211-6. [DOI] [PubMed] [Google Scholar]
- Kasamatsu H., Robberson D. L., Vinograd J. A novel closed-circular mitochondrial DNA with properties of a replicating intermediate. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2252–2257. doi: 10.1073/pnas.68.9.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasamatsu H., Wu M. Structure of a nicked DNA-protein complex isolated from simian virus 40: covalent attachment of the protein to DNA and nick specificity. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1945–1949. doi: 10.1073/pnas.73.6.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller T., Sogo J. M., Bujard H. An electron microscopic method for studying nucleic acid-protein complexes. Visualization of RNA polymerase bound to the DNA of bacteriophages T7 and T3. Biopolymers. 1974 May;13(5):995–1009. doi: 10.1002/bip.1974.360130514. [DOI] [PubMed] [Google Scholar]
- Leibowitz P. J., Schaechter M. The attachment of the bacterial chromosome to the cell membrane. Int Rev Cytol. 1975;41:1–28. doi: 10.1016/s0074-7696(08)60964-x. [DOI] [PubMed] [Google Scholar]
- Lovett M. A., Guiney D. G., Helinski D. R. Relaxation complexes of plasmids ColE1 and ColE2: unique site of the nick in the open circular DNA of the relaxed complexes. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3854–3857. doi: 10.1073/pnas.71.10.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nass M. M. Mitochondrial DNA. I. Intramitochondrial distribution and structural relations of single- and double-length circular DNA. J Mol Biol. 1969 Jun 28;42(3):521–528. doi: 10.1016/0022-2836(69)90240-x. [DOI] [PubMed] [Google Scholar]
- Rickwood D., Birnie G. D. Metrizamide, a new density-gradient medium. FEBS Lett. 1975 Feb 1;50(2):102–110. doi: 10.1016/0014-5793(75)80467-4. [DOI] [PubMed] [Google Scholar]
- Robberson D. L., Clayton D. A. Replication of mitochondrial DNA in mouse L cells and their thymidine kinase - derivatives: displacement replication on a covalently-closed circular template. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3810–3814. doi: 10.1073/pnas.69.12.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Jordan J. M., Vinograd J. In vivo effects of intercalating drugs on the superhelix density of mitochondrial DNA isolated from human and mouse cells in culture. J Mol Biol. 1971 Jul 28;59(2):255–272. doi: 10.1016/0022-2836(71)90050-7. [DOI] [PubMed] [Google Scholar]
- Storrie B., Attardi G. Expression of the mitochondrial genome in HeLa cells. 13. Effect of selective inhibition of cytoplasmic or mitochondrial protein synthesis on mitochondrial nucleic acid synthesis. J Mol Biol. 1972 Nov 14;71(2):177–199. doi: 10.1016/0022-2836(72)90345-2. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]





