Abstract
Helicobacter pylori (Hp) infection is known to alter levels of pepsinogens (PG) and is correlated with several disease states, including gastric and cardiovascular diseases. This study sought to assess whether Hp infection is associated with hypertension as well as to identify the value of assessing the PG I/PG II ratio in patients with hypertension. The study included 396 individuals with hypertension who were assessed for infection with Hp by colloidal gold assay. Participants’ weight, height, blood pressure, and serum lipids were measured, and participants were examined for the presence of renal or ocular damage. H. pylori infection status or PG I/PG II ratio were compared against other variables (e.g., body mass index, serum cholesterol, diastolic blood pressure) by t-test or ⇨2 test, and Pearson’s correlation analysis was used to identify associations. Consistent with other studies, the PG I/PG II ratio of patients with Hp infection was significantly lower than that of patients without Hp infection (P < 0.001). The serum total cholesterol and triglycerides of patients with Hp infection were significantly higher than those of patients without Hp infection (P < 0.001), and the PG I/PG II ratio was negatively correlated with total cholesterol (r=-0.61) and triglycerides (r=-0.56) levels. However, there was no significant difference in hypertension severity by Hp infection status or PG I/PG II ratio. Interestingly, the PG I/PG II ratio was significantly lower in patients with hypertensive nephropathy or hypertensive retinopathy than in patients without these symptoms (P < 0.05). The areas under the receiver-operating characteristic curve were 0.77 and 0.83 in the diagnosis of nephropathy and retinopathy, respectively. These findings indicate that the PG I/PG II ratio is lower in individuals with hypertensive nephropathy and hypertensive retinopathy. Thus, the detection of the PG I/PG II ratio may be valuable for diagnostic screening for hypertensive organ damage.
Keywords: Pepsinogen, Helicobacter pylori, hypertension
Introduction
Pepsinogens (PG), the precursors to the gastric protease pepsin, are categorized into 2 types based on their immunogenicity and biochemical properties. The first type (PG I) comprises subgroups 1-5, which have the same immunogenicity and are solely secreted by fundic gland. The second type (PG II) comprises subgroups 6-7, which are mainly secreted by pyloric glands and proximal duodenal mucosa. Pepsinogen is converted to pepsin through contact with hydrochloric acid or existing active pepsin in the gastric cavity. The active enzyme then decomposes proteins into fats, peptones, and a small number of peptides [1].
The serum PG level is closely correlated with the degree of infection with Helicobacter pylori (H. pylori) in the digestive tract. When H. pylori infection occurs, the serum PG I/PG II ratio decreases, but the ratio increases after the bacterium is eradicated [2-4]. H. pylori infection is also associated with liver and cardiovascular diseases [5-7], and influences blood lipid metabolism as well as contributing to peripheral vascular disease and atherosclerosis [8,9]. Similarly, pepsinogens have been associated with cardiovascular disease, and some evidence also supports a link between PG I/PG II ratio and glucose levels [10,11].
Despite these links with cardiovascular and metabolic diseases, no data exist to indicate whether H. pylori infection is associated with hypertension, a condition commonly associated with both cardiovascular disease and type 2 diabetes. Therefore, the current study explored the change in the PG I/PG II ratio in the context of H. pylori infection in hypertension patients, thus providing a theoretical basis for the screening or auxiliary examination of hypertension or complications of hypertension.
Participants and methods
Participants
For this prospective study, 396 hypertension patients who received treatment in the Second Clinical Medical College, Henan University of Traditional Chinese Medicine were recruited between December 2011 and December 2013. Of them, 213 cases were males and 183 cases were females, and the mean age was 57.96±10.054 years. Hypertension was diagnosed according to the diagnostic criteria in the 2010 Chinese Hypertension Prevention Guide. Patients with a history of radical treatment for H. pylori infection, history of gastrectomy, or with metabolic disorders, infectious diseases, or tumors were excluded.
Participants’ heights and weights were recorded for determination of body mass index (BMI). Blood pressure was also recorded. Venous blood was collected from all fasting participants in the morning for analysis of blood chemistry and pepsinogens. Participants were also clinically examined for hypertensive renal disease, judged as present damage to kidney function, urinary albumin excretion rate > 20 μg/min, or renal insufficiency. To assess participants for retinopathy, all participants underwent mydriasis via eucatropine, with subsequent direct ophthalmoscopy and slit lamp microscopy. Ocular fundus lesions caused by factors involving metabolic disorders or diseases of eyes themselves were ruled out. All participants were examined by the same experienced ophthalmologist. This study was approved by the Hospital Ethics Committee, and informed consent was obtained from each participant.
Blood chemistry
Peripheral blood (5 mL) was drawn from each subject. Three mL of peripheral blood were centrifuged at 1300 × g for 10 minutes at 4°C. Enzymatic kits (Sigma-Aldrich, St. Louis, MO, USA) were used to assess serum triglycerides, total cholesterol, and high-density lipoprotein. A latex-enhanced immunoturbidimetry kit to measure pepsinogen I and pepsinogen II was purchased from Sangon Biotech (Shanghai, China). An Olympus AU5400 fully automatic biochemical analyzer (Japan) was used according to the manufacturer’s instructions.
Diagnosis of Helcobacter pylori infection
Colloidal gold, a solid-phase indirect immune chromatographic technique, was used to diagnose H. pylori infection in serum samples. The products were manufactured by ACON Biotech (Hangzhou) Co. Ltd. When infection is present, the H. pylori antigens combine with the antibodies of the tested sample to form an antigen-antibody complex, which is exposed to colloidal gold-labeled anti-human IgG for detection. There are A, B and C bands on the products. Band A serves as a control line; the appearance of band B indicates H. pylori infection, and the presence of protein antibodies as current infection markers (CIMs) indicates an active infection; the appearance of band C indicates that the blood contained IgG antibodies.
Statistical analysis
Data entry was performed using EpiData version 3.1 to create a data bank, and logic checks were then performed. SAS 9.2 (SAS Institute, Cary, NC, USA) was used to analyze data by t-test, analysis of variance, chi-square test, and Pearson’s correlation analysis. Receiver-operating characteristic (ROC) curve was used to analyze the test efficiency of the PG I/PG II ratio in diagnosing ocular fundus lesions and renal damage. P <0.05 was considered to indicate a difference was statistically significant.
Results
H. pylori infection and characteristics of hypertension patients
To determine whether individuals with hypertension have different characteristics based on the presence of H. pylori infection, participants with hypertension were assessed for clinical characteristics and grouped by H. pylori status (Table 1). The levels of both total cholesterol and triglycerides were significantly higher in the sera of Hp-infected patients than in non-infected patients (each P value <0.001). Additionally, the PG I/PG II ratio was significantly lower in Hp-infected patients than in non-infected patients (P <0.001). In contrast, the differences in age, sex, BMI, systolic blood pressure, diastolic blood pressure, and high-density lipoprotein between Hp-infected and non-infected participants were not statistically significant (each P value > 0.05).
Table 1.
The relationship between H. pylori (Hp) infection and hypertension-related variables [n (%) or (X̅±s)]
| Variables | Hp+ (n=211) | Hp- (n=185) | ⇨2/t | P |
|---|---|---|---|---|
| Sex | 0.011 | 0.918 | ||
| Male | 114 (54.03) | 99 (53.51) | ||
| Female | 97 (45.97) | 86 (46.49) | ||
| Age (years) | 56.90±9.03 | 58.21±10.02 | -1.368 | 0.172 |
| BMI (kg/m2) | 23.02±4.31 | 22.85±4.11 | 0.400 | 1.311 |
| Diastolic blood pressure (mm Hg) | 89±15 | 88±14 | 0.683 | 1.505 |
| Systolic blood pressure (mm Hg) | 173±25 | 167±22 | 2.519 | 1.988 |
| Serum total cholesterol (mg/dL) | 259±40 | 200±36* | 15.341 | <0.001 |
| Triglycerides (mg/dL) | 192±80 | 143±66* | 6.593 | <0.001 |
| High-density lipoprotein (mg/dL) | 28±12 | 30±13 | -1.591 | 0.112 |
| PG I/II | 7.98±2.61 | 12.63±2.87* | -16.883 | <0.001 |
PG I/PG II ratio and blood lipid chemistry
A Pearson’s correlation analysis showed that the PG I/PG II ratio in patients was significantly negatively correlated with levels of total cholesterol (r=-0.61) and triglycerides (r=-0.56) (each P value <0.05) (Table 2). In contrast, PG I/PG II was not correlated with high-density lipoprotein (P > 0.05), as was seen for H. pylori infection status.
Table 2.
The correlation between PG I/PG II and selected variables of hypertension
| Variable | PG I/PG II | |
|---|---|---|
|
| ||
| r | P | |
| Serum total cholesterol (mg/dL) | -0.61 | 0.021 |
| Triglycerides (mg/dL) | -0.56 | 0.014 |
| High-density lipoprotein (mg/dL) | -0.47 | 0.130 |
Correlation between H. pylori infection and hypertension severity and complications
To determine whether H. pylori infection is associated with hypertension severity or complications of hypertension, participants were divided by hypertension severity or presence or absence of hypertensive nephropathy and hypertensive retinopathy and compared by infection status (Table 3). H. pylori status was not significantly different in patients with different grades of hypertension. However, H. pylori infection was significantly more common in hypertension patients with renal disease and retinopathy (P <0.05).
Table 3.
The relationship between Hp infection and features of hypertension [n (%)]
| Variable | Hp+ (n=211) | Hp- (n=185) | ⇨2 | P |
|---|---|---|---|---|
| Grading of hypertension | 0.356 | 0.837 | ||
| Hypertension I | 101 (53.16) | 89 (46.84) | ||
| Hypertension II | 72 (54.96) | 59 (45.04) | ||
| Hypertension III | 38 (50.67) | 37 (49.33) | ||
| Hypertensive nephropathy | 5.592 | 0.018 | ||
| No | 163 (50.46) | 160 (49.54) | ||
| Yes | 48 (65.75) | 25 (34.25) | ||
| Hypertensive retinopathy | 9.796 | 0.002 | ||
| No | 98 (46.01) | 115 (53.99) | ||
| Yes | 113 (61.75) | 70 (38.25) |
Correlation between PG I/PG II ratio and hypertension severity and complications
Similar to the findings for H. pylori status, PG I/PG II ratio did not significantly differ among patients with different hypertension severity. Interestingly, in patients with hypertensive nephropathy or retinopathy, independent of H. pylori status, the PG I/PG II ratios were significantly lower than those in patients without the complications (each P value <0.05, Table 4).
Table 4.
The relationship between PG I/PG II and features of hypertension (X̅±s)
| Variable | Hp+ (n=211) | Hp- (n=185) | Total (n=396) |
|---|---|---|---|
| Grading of hypertension | |||
| Hypertension I | 8.05±2.46 | 11.78±2.83 | 9.73±2.59 |
| Hypertension II | 8.39±2.49 | 11.86±2.89 | 10.26±2.68 |
| Hypertension III | 8.18±2.31 | 10.07±2.54 | 10.14±2.41 |
| Hypertensive nephropathy | |||
| No | 9.12±2.24 | 12.70±2.19 | 11.02±2.20 |
| Yes | 5.16±1.59* | 8.83±1.95* | 6.37±1.79* |
| Hypertensive retinopathy | |||
| No | 9.95±2.29 | 13.41±2.58 | 11.98±2.47 |
| Yes | 6.37±1.88# | 8.87±2.19# | 7.25±2.04# |
P <0.01 vs non-hypertensive nephropathy;
P < 0.01 vs non-retinopathy.
ROC curve analysis
An ROC curve was used to validate the findings of this study; the larger the area under the ROC curve, the better the diagnostic result. When the area fell within the range of 0.5-0.7, the results have low accuracy; when within the range of 0.7-0.9, the results have some accuracy; when above 0.9, the results are highly accurate. Generally, area > 0.75 was considered to be of great diagnostic value. In hypertension patients with kidney damage, the area of 0.77 was considered to indicate a good test efficiency of the PG I/PG II ratio (Figure 1A); in those with ocular fundus lesions, an area of 0.83 was considered to indicate a good test efficiency of the PG I/PG II ratio (Figure 1B).
Figure 1.
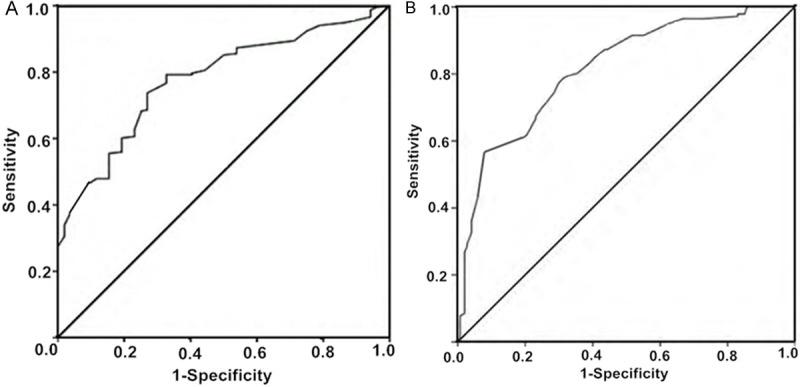
ROC curve of PG I/PG II (A) for nephropathy diagnosis; (B) for retinopathy diagnosis.
Discussion
Helicobacter pylori infection is closely correlated with the PG I/PG II ratio; however, to date, studies on PG or PG I/PG II have mostly focused on digestive tract diseases, and fewer reports have addressed PG and cardiovascular diseases [12-14]. This study was aimed at exploring the correlation between the PG I/PG II ratio and hypertension or its complications, thereby providing a theoretical basis for the clinical role of PG detection in hypertension.
The results of our analyses showed that differences in systolic blood pressure, diastolic blood pressure, and high-density lipoprotein by H. pylori status were not statistically significant. Nor were differences in PG I/PG II ratios by different grades of hypertension statistically significant. Thus, H. pylori infection and PG I/PG II ratio are not correlated with blood pressure values in hypertension. The main finding of this study, therefore, was the correlation between PG I/PG II ratio and complications of hypertension, specifically ocular fundus lesions and hypertensive kidney damage. In total participants, and participants divided by Hp-infection status, the PG I/PG II ratios were significantly lower in participants with hypertensive renal disease and/or ocular fundus lesions than those in patients with no renal disease or no ocular fundus lesions. Analysis of areas under the ROC curves indicated that the PG I/PG II ratio had a high efficiency for diagnosing these complications of hypertension. The results suggests that when hypertension develops to such an extent that organ lesions appear, the PG I/PG II ratio will drop, making the detection of this ratio valuable for detecting complications of hypertension.
H. pylori infection can decrease the PG I/PG II ratio, inhibit the gastric mucosa from secreting vitamin C, and damage gastric epithelial cells [15]. Such changes can influence the metabolism of lipids in patients [16]. A drop in the PG I/PG II ratio can cause patients to present with metabolic disorders including altered serum total cholesterol and triglycerides levels [17,18]. Similarly, this study showed that the PG I/PG II ratio showed a negative correlation with total cholesterol and triglycerides levels in sera. H. pylori infection can also influence the total cholesterol and triglycerides in serums of patients with cardiovascular disease, thereby aggravating their condition [19-21]. The causes of organ damage in hypertension patients may also stem from the damage H. pylori infection causes to gastric epithelial cells, which leads to inflammatory responses that can damage the blood vessel endothelium and thereby result in vascular lesions.
In summary, the results of this study showed that the complications of hypertension, specifically retinopathy and nephropathy, are correlated with a lower PG I/PG II ratio. Thus, the detection of PG I/PG II may offer a valuable diagnostic tool for identifying organ damage in hypertension.
Disclosure of conflict of interest
None.
References
- 1.Chu S, Schubert ML. Gastric secretion. Curr Opin Gastroenterol. 2012;28:587–593. doi: 10.1097/MOG.0b013e328358e5cc. [DOI] [PubMed] [Google Scholar]
- 2.Zhang X, Xue L, Xing L, Wang J, Cui J, Mi J, Xing X, Wang J, Du Z, Misumi J, Tian Q, Qang L. Low serum pepsinogen I andpepsinogen I/II ratio and Helicobacter pylori infection are associated with increased risk of gastric Cancer: 14-year follow up result in arural Chinese community. Int J Cancer. 2012;130:1614–1619. doi: 10.1002/ijc.26172. [DOI] [PubMed] [Google Scholar]
- 3.Yanaoka K, Oka M, Ohata H, Yoshimura N, Deguchi H, Mukoubayashi C, Enomoto S, Inoue I, Iguchi M, Maekita T, Ueda K, Utsunomiya H, Tamai H, Fujishiro M, Iwane M, Takeshita T, Mohara O, Ichinose M. Eradication of Helicobacter pylori prevents cancer development in subjects with mild gastric atrophy identified by serum pepsinogen levels. Int J Cancer. 2009;125:2697–2703. doi: 10.1002/ijc.24591. [DOI] [PubMed] [Google Scholar]
- 4.Haneda M, Kato M, Ishigaki S, Suzuki M, Takahashi M, Nakagawa M, Ono S, Mori Y, Mabe K, Nakagawa S, Kudo T, Shimizu Y, Asaka M. Identification of a high risk gastric cancer group using serum pepsinogen after successful eradication of Helicobacter pylori. J Gastroenterol Hepatol. 2013;28:78–83. doi: 10.1111/j.1440-1746.2012.07285.x. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Chen J, Yan S. Correlation between Helicobacter pylori infection and coronary heart disease. Chinese Journal of Difficult and Complicated Cases. 2008;7:27–28. [Google Scholar]
- 6.Chen JJ, Changchien CS, Tai DI, Chiou SS, Lee CM, Kuo CH. Role of Helicobacter pylori in cirrhotic patients with peptic ulcer. Dig Dis Sci. 1994;39:1565–1568. doi: 10.1007/BF02088065. [DOI] [PubMed] [Google Scholar]
- 7.Rogha M, Nikvarz M, Pourmoghaddas Z, Shirneshan K, Dadkhah D, Pourmoghaddas M. Is helicobacter pylori infection a risk factor for coronary heart disease? ARYA Atheroscler. 2012;8:5–8. [PMC free article] [PubMed] [Google Scholar]
- 8.Xiang Z, Chen YP, Ye YF, Ma KF, Chen SH, Zheng L, Yang YD, Jin X. Helicobacter pylori and Crohn’s disease: a retrospective single-center study from China. World J Gastroenterol. 2013;19:4576–4581. doi: 10.3748/wjg.v19.i28.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christodoulou DK, Milionis HJ, Pappa P, Katsano KH, Sigounas D, Florentin M, Elisaf M, Tsianos EV. Association of Helicobacter pylori infection with cardiovascular disease-Is it just a myth? Eur J Intern Med. 2011;22:191–194. doi: 10.1016/j.ejim.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 10.van Oijen MG, Sipponen P, Laheij RJ, Verheugt FW, Jansen JB. Gastric status and vitamin B12 levels in cardiovascular patients. Dig Dis Sci. 2007;52:2186–2189. doi: 10.1007/s10620-006-9260-8. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka M, Fukui M, Kuroda M, Yamazaki M, Hasegawa G, Oda Y, Naito Y, Toda H, Yoshikawa T, Nakamura N. Pepsinogen I/II ratio is related to glucose, triacylglycerol, and uric acid levels. Nutrition. 2012;28:418–421. doi: 10.1016/j.nut.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Lomba-Viana R, Dinis-Ribeiro M, Fonseca F, Vieira AS, Bento MJ, Lomba-Viana H. Serum pepsinogen test for early detection of gastric cancer in a European country. Eur J Gastroenterol Hepatol. 2012;24:37–41. doi: 10.1097/MEG.0b013e32834d0a0a. [DOI] [PubMed] [Google Scholar]
- 13.Haj-sheykholeslami A, Rakhshani N, Amirzargar A, Rafiee R, Shahidi SM, Nikbin B, Khosravi F, Massarrat S. Serum pepsinogen I, pepsinogen II, and gastrin 17 in relatives of gastric cancer patients: comparative study with type and severity of gastritis. Clin Gastroenterol Hepatol. 2008;6:174–179. doi: 10.1016/j.cgh.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 14.Cavaleiro-Pinto M, Peleteiro B, Lunet N, Barros H. Helicobacter pylori infection and gastric cardia cancer: systematic review and meta-analysis. Cancer Causes Control. 2011;22:375–387. doi: 10.1007/s10552-010-9707-2. [DOI] [PubMed] [Google Scholar]
- 15.Onoda N, Katsuragi K, Maeda K, Sawada T, Wakasa K, Hirakawa K. Helicobacter pylori infection, but not mucosal atrophy, significantly affects serum pepsinogen level after gastric cancer surgery. Hepatogastroenterology. 2006;53:619–623. [PubMed] [Google Scholar]
- 16.Gen R, Demir M, Ataseven H. Effect of Helicobacter pylori eradication on insulin resistance, serum lipids and low-grad inflammation. South Med J. 2010;103:190–196. doi: 10.1097/SMJ.0b013e3181cf373f. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka M, Fukui M, Kuroda M, Yamazaki M, Hasegawa G, Oda Y, Naito Y, Toda H, Yoshikawa T, Nakamura N. Pepsinogen I/II ratio is related to glucose, triacylglycerol, and uric acid levels. Nutrition. 2012;28:418–421. doi: 10.1016/j.nut.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Isomoto H, Ueno H, Saenko VA, Mondal MS, Nishi Y, Kawano N, Ohnita K, Mizuta Y, Ohtsuru A, Yamashita S, Nakazato M, Kohno S. Impact of Helicobacter pylori infection on gastric and plasma ghrelin dynamics in humans. Am J Gastroenterol. 2005;100:1711–1720. doi: 10.1111/j.1572-0241.2005.41492.x. [DOI] [PubMed] [Google Scholar]
- 19.Mayr M, Kiechl S, Willeit J, Wick G, Xu Q. Infections, immunity, and atherosclerosis associations of antibodies to Chlamydia pneumoniae, Helicobacter pylori, and cytomegalovirus with immune reactions to heat-shock protein 60 and carotid or femoral atherosclerosis. Circulation. 2000;102:833–839. doi: 10.1161/01.cir.102.8.833. [DOI] [PubMed] [Google Scholar]
- 20.Hoffmeister A, Rothenbacher D, Bode G, Persson K, März W, Nauck MA, Brenner H, Hombach V, Koenig W. Current infection with Helicobacter pylori, but not seropositivity to Chlamydia pneumoniae or cytomegalovirus, is associated with an atherogenic, modified lipid profile. Arterioscler Thromb Vasc Biol. 2001;21:427–432. doi: 10.1161/01.atv.21.3.427. [DOI] [PubMed] [Google Scholar]
- 21.Oshima T, Ozono R, Yano Y, Oishi Y, Teragawa H, Higashi Yoshizumi M, Kambe M. Association of Helicobacter pylori infection with systemic inflammation and endothelial dysfunction in healthy male subjects. J Am Coll Cardiol. 2005;45:1219–1222. doi: 10.1016/j.jacc.2005.01.019. [DOI] [PubMed] [Google Scholar]


