Abstract
The abnormal metabolic processes following traumatic brain injury (TBI) have been proposed to contribute to secondary injuries after TBI. Therefore, enteral nutrition (EN) support for TBI patients has received more attention. This study aimed to evaluate the complimentary effects of enteral nutrition with glutamine and hyperbaric oxygen (HBO) on the recovery of TBI. TBI model was established in SD rats, which were randomly divided into four groups: TBI, TBI + HBO, TBI + GLN, and TBI + HBO + GLN. Neuronal apoptosis in penumbra area was detected by TUNEL. Serum prealbumin level was detected by ELISA. Motor function was evaluated by beam-balance test. We found that the body weight of the rats had no significant differences in different groups before and after injury. Among the four groups, beam-balance test score was the lowest, serum prealbumin level was the highest, and neuronal apoptosis rate was the lowest in TBI + HBO + GLN group on day 3 and 7 after TBI. In conclusion, our data suggest that hyperbaric oxygen combined with enteral nutrition support with glutamine is effective in reducing neuronal apoptosis, increasing serum prealbumin concentration and improving neurological function after TBI injury.
Keywords: Hyperbaric oxygen, glutamine, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a common brain disease with the highest rate of morbidity and mortality among brain diseases. The standard treatment for TBI is to reduce intracranial pressure and maintain adequate cerebral perfusion pressure. However, TBI causes a series of secondary brain injuries. The brain is well-known to regulate a variety of metabolic activities and the abnormal metabolic processes following TBI have been proposed to contribute crucially to secondary injuries [1,2]. Therefore, enteral nutrition (EN) support for TBI patients has received more attention in recent years [3]. Hypercatabolism is a common aspect of TBI patients. In this situation, muscle proteolysis is increased and glutamine is the amino acid released most by the muscles. Consequently, glutamine consumption is accelerated to a level that exceeds its production, resulting in a negative balance. A recent study showed that glutamine (GLN) concentrations in both plasma and soleus muscle were decreases in rats subjected to TBI [4].
Other measures to reduce secondary brain injury following TBI include hyperbaric oxygen (HBO), which improves the brain tissue oxygen levels and protects the nervous system against reactive oxygen species-mediated injury [5,6]. However, no studies have evaluated the complimentary or synergistic effects of enteral nutrition with GLN and HBO on TBI. In this study, we established TBI rat models which were randomly divided them into four treatment groups, and we compared the outcomes of the rats in different treatment groups.
Experimental procedures
Animal grouping and treatment
Healthy male Sprague–Dawley rats (weight 220-250 g) were provided by Nanjing Medical University Experimental Animal Center and kept at 21 ± 1°C with free access to food and water. The rats were acclimatized to the environment for 6 days and then fasted for 12 hours before they were randomly divided into four groups (n = 15): TBI group (A); TBI + HBO group (B); TBI + GLN group (C); TBI + HBO + GLN group (D).
All rats were anesthetized by intraperitoneal injection of 1% sodium pentobarbital (30-50 mg/kg) and then their brains were positioned on stereotaxic apparatus. Under aseptic conditions craniotomy surgery was performed to create a circular window (5 mm diameter) centered at antero-posterior -2.5 mm, medio-lateral 2.5 mm with reference to the bregma. The meninges were kept intact. Next the rats were subjected to controlled cortical impact using a pneumatic impact device (Model FP302, AmScien Instruments LLC, U S A) with a 1.5 atm (1 atm = 101.325 kPa) pressure. Immediately after the cortical impact the craniotomy hole was sealed with bone wax and the scalp incision was closed with sutures. After the recovery from anesthesia, the rats were returned to their cages with postoperative care.
The rats in TBI group received only brain injury, whereas the rats in the other groups received the following treatments after TBI. The rats in TBI + GLN group received intra-gastric gavage of 200 mg glutamine particles (Yaoyou Pharmaceutical Co, Ltd, Chongqing, China). The rats in TBI + HBO group received hyperbaric oxygen treatment as follows: The chamber was flushed with pure oxygen and then the pressure was increased at a rate of 0.15 MPa per 20 min. The oxygen concentration in the chamber was maintained at 95%, and treatment last for 1 h. Decompression was performed at a uniform rate of 0.01 MPa/min. The rats in TBI + HBO + GLN group received both intra-gastric gavage of 200 mg glutamine particles and hyperbaric oxygen treatment.
Measurement of body weight
Body weight of the rats was monitored before injury and on day 1, 3 and 7 after injury, using a CS2000 Compact Digital Scale (Ohaus, Parsippany, NJ, U S A).
Beam-balance test
Gross motor function of the rats was evaluated by beam-balance test as described previously [7]. Briefly, the rats were placed on a suspended narrow wooden beam and the time they remained on the beam was measured. The beam was 1.5 cm wide and 80 cm long. The rating of beam-balance performance was from 1 (balances with steady posture) to 6 (no attempt to balance). The animal was placed on the beam 3 times and the mean of 3 trials was used. Beam-balance assay was performed on day 1, 3 and 7 after injury.
Measurement of serum prealbumin level
Serum prealbumin levels of the rats were measured by ELISA before injury and on day 1, 3 and 7 after injury.
Apoptosis assay
The apoptosis of neurons was detected by terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling (TUNEL) on rat brain tissue sections. TUNEL staining was performed using the DeadEnd™ Fluorometric TUNEL System (Promega, WI, USA) following the manufacturer’s protocols. The numbers of apoptotic cells were counted from three sections in the injured penumbra of cerebral cortex.
Statistical analysis
Data were expressed as mean ± standard deviation and analyzed using the SPSS version 18.0 statistical analysis package (SPSS Inc., Chicago, IL, USA). The comparisons were performed by using the t-test and ANOVA. P < 0.05 was accepted as statistically significant.
Results
Body weight of the rats in each group
Before TBI, the body weight was not significantly different among the four groups (P > 0.05). On day 1, 3 and 7 after TBI, the body weight was still not significantly different among the four groups (P > 0.05, Table 1).
Table 1.
Body weight of the rats in 4 groups after TBI (g)
| Group | 0 day | 1 day | 3 day | 7 day |
|---|---|---|---|---|
| TBI | 270.5 ± 13.5 | 266.7 ± 11.9 | 263.4 ± 12.9 | 265.4 ± 10.7 |
| TBI + HBO | 265.8 ± 14.0 | 260.5 ± 12.9 | 258.5 ± 12.4 | 269.5 ± 12.5 |
| TBI + GLN | 272.4 ± 12.4 | 268.0 ± 13.3 | 263.4 ± 15.0 | 270.4 ± 13.2 |
| TBI + HBO + GLN | 273.4 ± 10.9 | 268.4 ± 12.8 | 263.6 ± 12.5 | 274.3 ± 12.3 |
P > 0.05, not statistically significant.
Beam-balance performance of the rats in each group
As shownin Table 2, beam-balance performance of the rats was damaged after TBI. On day 1 after TBI, beam-balance performance score was not significantly different among the four groups (P > 0.05). However, on day 3 after TBI, beam-balance performance score was significantly decreased in TBI + HBO + GLN group, compared to TBI group (P < 0.05) and TBI + GLN group (P < 0.01). Furthermore, on day 7 after TBI, beam-balance performance scores were decreased in all groups compared to those on day 1 and 3, indicating the recovery of motor function. However, beam-balance performance score was significantly decreased in TBI + HBO + GLN group, compared to TBI group (P < 0.05) and TBI + GLN group (P < 0.01). These data suggest that the combination of hyperbaric oxygen treatment and enteral nutrition support with glutamine improves motor function recovery after TBI in the rats.
Table 2.
Beam-balance test score of the rats in 4 groups after TB
| Group | 1 day | 3 day | 7 day |
|---|---|---|---|
| TBI | 3.7 ± 0.41 | 3.2 ± 0.22 | 1.8 ± 0.20 |
| TBI + HBO | 3.6 ± 0.35 | 3.0 ± 0.32 | 1.2 ± 0.26* |
| TBI + GLN | 3.6 ± 0.37 | 3.3 ± 0.26 | 1.5 ± 0.38 |
| TBI + HBO + GLN | 3.7 ± 0.16 | 2.8 ± 0.30*,Δ | 1.0 ± 0.16*,Δ |
Compared with TBI group:
P < 0.05;
Compared with TBI + GLN group:
P < 0.05.
Serum prealbumin levels of the rats in each group
As shown in Table 3, serum prealbumin levels were not significantly different among the four groups before TBI (P > 0.05). Serum prealbumin levels were decreased on day 1 and 3 after TBI, but they still did not show significant differences among the four groups. However, on day 7 after TBI, serum prealbumin levels were recovered to baseline levels in TBI + HBO + GLN and TBI + GLN groups, but not in TBI and TBI + HBO groups. These data suggest that enteral nutrition support with glutamine improves serum prealbumin levels after TBI in the rats.
Table 3.
Serum prealbumin levels of the rats in 4 groups after TBI (g/L)
| Group | 0 day | 1 day | 3 day | 7 day |
|---|---|---|---|---|
| TBI | 270.5 ± 32.5 | 247.8 ± 30.8 | 178.5 ± 25.5 | 210.5 ± 25.6 |
| TBI + HBO | 265.4 ± 35.3 | 239.4 ± 25.6 | 185.4 ± 22.6 | 238.4 ± 30.3 |
| TBI + GLN | 256.4 ± 30.0 | 229.4 ± 18.4 | 182.6 ± 27.3 | 255.6 ± 22.6* |
| TBI + HBO + GLN | 269.4 ± 24.1 | 231.5 ± 23.7 | 195.3 ± 26.7 | 260.6 ± 28.5* |
Compared with TBI group:
P < 0.05.
Apoptosis of neurons in the rats in each group
By TUNEL assay we detected apoptotic neurons in penumbra of cerebral cortex of the rats. On day 1 after TBI, the numbers of apoptotic cells were significantly higher in TBI group (A) and TBI + GLN group (C) than in TBI + HBO group (B) and TBI + HBO + GLN group (D) (Figure 1). On day 3 and 7 after TBI, the numbers of apoptotic neurons were gradually decreased in all groups compared to those on day 1, indicating the self-repair of neurons. However, on day 3 after TBI, the numbers of apoptotic neurons were significantly lower in TBI + HBO + GLN group and TBI + HBO group than in TBI group and TBI + GLN group (P < 0.05, Table 4). These data suggest that hyperbaric oxygen treatment help enhance the self-repair ability of neurons against apoptosis after TBI in the rats.
Figure 1.
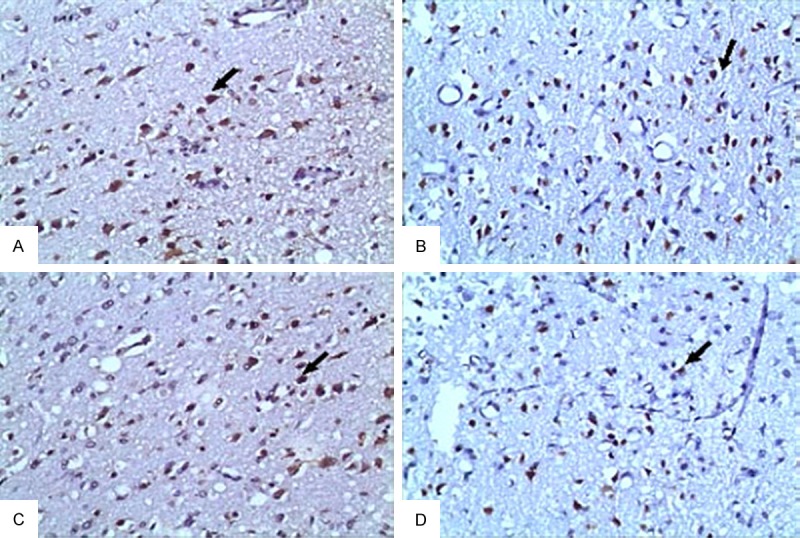
TUNEL staining of apoptotic neurons in penumbra area of the rats on day 1 after TBI. Shown were representative images. A. TBI group. B. TBI + HBO group. C. TBI + GLN group. D. TBI + HBO + GLN group. Arrow indicated apoptotic neurons.
Table 4.
The percentage of apoptotic neurons in penumbra surrounding cortex in 4 groups after TBI (%)
| Group | 1 day | 3 day | 7 day |
|---|---|---|---|
| TBI | 23.30 ± 2.16 | 13.34 ± 2.53 | 4.03 ± 1.12 |
| TBI + HBO | 18.33 ± 1.64* | 8.06 ± 1.22# | 2.52 ± 1.28 |
| TBI + GLN | 21.39 ± 2.90 | 10.35 ± 1.56 | 3.16 ± 1.50 |
| TBI + HBO + GLN | 15.28 ± 2.98*,Δ | 6.25 ± 1.52*,Δ | 2.10 ± 1.49* |
Compared with TBI group:
P < 0.05;
Compared with TBI + GLN group:
P < 0.05.
Discussion
Great advances have been made in hyperbaric oxygen medicine in recent years. Currently there is still controversy on the efficacy of HBO treatment on TBI. The differences in the treatment outcomes may depdent on the the time and presssure of exposure to HBO [8,9]. In this study we found that beam-balance performance was significantly improved in TBI rats receiving HBO treatment, indicating that HBO help the recovery of motor function. Futhermore, we found that the apoptosis of neurons was at the maximum on day 1 after TBI and HBO treatment significantly recduced neuron apoptosis after TBI, consistent with previous study [10]. These results suggest that the efficacy of HBO to improve brain funciton after TBI is correlated with the inhibition of neuronal apoptosis.
Currently, nutritional support is focused on providing sufficient nutrients of the protein, vitamins and trace elements without having to meet calorie needs. The body is in hyperglycemic state under TBI and the body’s energy needs increase. However, there is not enough evidence to prove that proving TBI patients with adequate nutrition could reduce the mortality [11]. Previous studies have shown that early nutritional support on patients with moderate TBI could enhance immunity, reduce infection, help the recovery of neurological function and reduce the mortality [12]. TBI is known to induce the expression of inflammatory cytokines such as TNF-α, IL-1 and IL-6, and increase the levels of hormones such as cortisol, glucagon, catecholamines, resulting in high metabolic state in the body. Glutamine is the most abundant free amino acids in the human body, and its consumption is increased under trauma, inflammation and other stress conditions. Nutirent support with glutamine could promote protein synthesis, maintain nitrogen balance, and improve the prognosis in TBI patients [13]. In this study, our results showed that serum prealbumin levels were significantly imporved in glutamine nutrition group than non-glutamine group. These results suggest that glutamine nutrition can improve immune function and promote the functional recovery of patients after TBI.
In summary, this is the first study that evaluated the combination of hyperbaric oxygen treatment with nutritional therapy on the outcomes of traumatic brain injury in a rat model. Our results suggest that hyperbaric oxygen combined with enteral nutrition support with glutamine may be effective in reducing neuronal apoptosis, increasing serum prealbumin concentration and improving neurological function of TBI patients.
Acknowledgements
This work was supported by the Social Development Foundation of Jiangsu, China (No. BS2007037), Jiangsu Province Key Talent Foundation (No. RC2007029), and the Science and Technology Development Foundation of Huaian, China (No. HAS07025 & No. HG201213).
Disclosure of conflict of interest
None.
References
- 1.Cook AM, Peppard A, Magnuson B. Nutrition considerations in traumatic brain injury. Nutr Clin Pract. 2008;23:608–620. doi: 10.1177/0884533608326060. [DOI] [PubMed] [Google Scholar]
- 2.Vizzini A, Aranda-Michel J. Nutritional support in head injury. Nutrition. 2011;27:129–132. doi: 10.1016/j.nut.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Bistrian BR, Askew W, Erdman JW, Oria MP. Nutrition and traumatic brain injury: a perspective from the Institute of Medicine report. JPEN J Parenter Enteral Nutr. 2011;35:556–559. doi: 10.1177/0148607111416122. [DOI] [PubMed] [Google Scholar]
- 4.Moinard C, Delpierre E, Loï C, Neveux N, Butel MJ, Cynober L, Charrueau C. An oligomeric diet limits the response to injury in traumatic brain-injured rats. J Neurotrauma. 2013;30:975–80. doi: 10.1089/neu.2012.2707. [DOI] [PubMed] [Google Scholar]
- 5.Huang L, Obenaus A. Hyperbaric oxygen therapy for traumatic brain injury. Med Gas Res. 2011;1:21. doi: 10.1186/2045-9912-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei XE, Li YH, Zhao H, Li MH, Fu M, Li WB. Quantitative evaluation of hyperbaric oxygen efficacy in experimental traumatic brain injury: an MRI study. Neurol Sci. 2014;35:295–302. doi: 10.1007/s10072-013-1514-6. [DOI] [PubMed] [Google Scholar]
- 7.Hamm RJ, White-Gbadebo DM, Lyeth BG, Jenkins LW, Hayes RL. The effect of age on motor and cognitive deficits after traumatic brain injury in rats. Neurosurgery. 1992;31:1072–7. doi: 10.1227/00006123-199212000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Rockswold SB, Rockswold GL, Zaun DA, Zhang X, Cerra CE, Bergman TA, Liu J. A prospective, randimized clinical trial to compare the effect of hyperbaric to normobaric hyperoxia on cerebral metabolism, intracranial pressure, and oxygen toxicity in severe traumatic brain injury. J Neurosurg. 2010;112:1080–94. doi: 10.3171/2009.7.JNS09363. [DOI] [PubMed] [Google Scholar]
- 9.Prakash A, Parelkar SV, Oak SN, Gupta RK, Sanghvi BV, Bachani M, Patil R. Role of hyperbaric oxygen therapy in severe head injury in children. J Pediatr Neurosci. 2012;7:4–8. doi: 10.4103/1817-1745.97610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang L, Obenaus A. Hyperbaric oxygen therapy for traumatic brain injury. Med Gas Res. 2011;1:21. doi: 10.1186/2045-9912-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bochicchio GV, Bochicchio K, Nehman S, Casey C, Andrews P, Scalea TM. Tolerance and efficacy of enteral nutrition in traumatic brain-injured patients induced into barbiturate coma. JPEN J Parenter Enteral Nutr. 2006;30:503–6. doi: 10.1177/0148607106030006503. [DOI] [PubMed] [Google Scholar]
- 12.Chourdakis M, Kraus MM, Tzellos T, Sardeli C, Peftoulidou M, Vassilakos D, Kouvelas D. Effect of early compared with delayed enteral nutrition on endocrine function in patients with traumatic brain injury: an open-labeled randomized trial. JPEN J Parenter Enteral Nutr. 2012;36:108–16. doi: 10.1177/0148607110397878. [DOI] [PubMed] [Google Scholar]
- 13.Wernerman J. Role of glutamine supplementation in critically patients. Curr Opin Anaesthesiol. 2008;21:155–9. doi: 10.1097/ACO.0b013e3282f54fd6. [DOI] [PubMed] [Google Scholar]


