Abstract
Background: Determining factors that could accurately predict pathological features of meningiomas before histological diagnosis would help surgeons to proper balance the risk of operation and the resection grade. The aim of this study was to explore the potential risk factors for atypical (WHO Grade II) and anaplastic (WHO Grade III) meningiomas. Methods: Records of 1,239 patients between January 2009 and January 2013 were included in this research. Furthermore, immunohistochemistry with Ki67 was analysed in 368 samples. Results: The Pearson’s chi-square test showed an increased risk for male gender for atypical and anaplastic meningiomas (P < 0.001) and an increased risk for cerebral convexity for atypical and anaplastic meningiomas (P < 0.001). However, significant differences in the terms of falx/sagittal sinus and intraventricular were not found. Patients with a Ki67 index ≥ 5% were significantly more likely to have atypical and anaplastic meningiomas than those patients with a Ki67 index < 5% (P < 0.001). In addition, the percentage of patients with a Ki67 index ≥ 5% in cerebral convexity meningiomas was higher than in non-cerebral convexity location (P = 0.006). Conclusions: The results indicate that male gender, cerebral convexity are significant risk factors for atypical and anaplastic meningiomas.
Keywords: Cerebral convexity, risk factor, meningiomas
Introduction
Meningiomas are slowly growing tumors, and represent the common intracranial neoplasms in adults [1]. About 90% of meningiomas are benign (Grade I), and 5-7% are atypical (Grade II), while 1-3% are anaplastic (Grade III) [2]. Benign meningiomas have the lowest rate of recurrence following surgery with or without additional radiotherapy [3], however, due to their aggressive behavior, atypical and anaplastic meningiomas have a higher rate of recurrence, despite underwent standard therapy (surgery and radiation). At present, the pathologic examination as the gold standard for diagnosing the meningiomas, whereas, for patients with small meningiomas, gamma knife surgery is considered as the initial treatment strategy, and once if they proved to be higher-grade pathology, further treatment becomes difficult [4]. Without a pathologic diagnosis, higher-grade tumors will be missed and treated with primary gamma knife surgery or observed inappropriately [5]. So, an understanding of the probability that a lesion is higher-grade pathology before treatment initiation may help guide treatment strategies.
The aim of this study was to explore the potential risk factors for atypical and anaplastic meningiomas from a single centre. In this retrospective study, a series of 1,239 meningiomas were included. They consist of 1,048 benign meningiomas and 191 atypical/anaplastic meningiomas diagnosed by histopathology.
Methods
Patients and tissue specimens
Patients who underwent a primary operation between January 2009 and January 2013 were included in this research. Patients who underwent prior radiotherapy were excluded from our study. Patients with spinal meningiomas and multiple meningiomas were also excluded from our study. As to patients with repeated resection, only the primary operation was collected in our data analysis. The information collected included gender, age, histology of the tumor, and location of the tumor.
Pathologic review
All meningiomas samples were assessed and graded according to the 2007 WHO guidelines [6]. The tissue in these cases was fixed in 4% buffered formalin, routinely processed, and embedded in paraffin; 2-4 μm thick sections were stained with hematoxylin and eosin. For 368 (29.7%) samples, representative sections of were stained immunohistochemically for Ki-67 (1:100, MAIXIN-BiO, Mouse monoclonal, clone: MIB1) by the method of Envision. The Ki67 proliferative index was determined by counting the number of Ki67-positive cells within at least 1000 tumor cells. In addition, the Ki67 index was analysed with a cut-off value of 5%.
Tumor location
The evaluation of anatomical location was based on radiological records and/or operation notes. The locations of the meningiomas were as follows: skull-base (n = 458), cerebral convexity (n = 572), falx/sagittal sinus (n = 57), intraventricular (n = 50) and other (n = 102). In order to facilitate statistical analysis, tumors located in the skull-base were assigned in the group of skull-base tumors, and the others in the group of non-skull-base tumors. Tumors located in the cerebral convexity were assigned in the group of cerebral convexity tumors, and the others in the group of non-cerebral convexity tumors. Tumors located in the falx/sagittal sinus were assigned in the group of falx/sagittal sinus tumors, and the others in the group of non-falx/sagittal sinus tumors. Tumors located in the intraventricular were assigned in the group of intraventricular tumors, and the others in the group of non-intraventricular tumors.
Statistical analysis
SPSS software (version 21.0, IBM) was used for statistical analysis. A p-value greater than 0.05 was treated as no statistical significance.
Results
A total of 1,239 patients were included in our study. The information of these patients was summarized in the Table 1. The results of the Pearson’s chi-square test are listed in the Table 2.
Table 1.
Clinical information of patients (n = 1,239)
| Characteristic | Number | Value (%) |
|---|---|---|
| Gender | ||
| Male | 355 | 28.7 |
| Female | 884 | 71.3 |
| Age (years) | ||
| < 65 | 1,033 | 83.4 |
| ≥ 65 | 206 | 16.6 |
| Tumor location | ||
| Skull base | 458 | 37.0 |
| Cerebral convexity | 572 | 46.2 |
| Falx/sagittal sinus | 57 | 4.6 |
| Intraventricular | 50 | 4.0 |
| Other | 102 | 8.2 |
| Histology | ||
| Benign | 1,048 | 84.6 |
| Atypical and anaplastic | 191 | 15.4 |
Table 2.
The Pearson’s chi-square test of potential risk factors for atypical and anaplastic meningiomas
| Characteristic | No. of atypical and anaplastic | P value |
|---|---|---|
| Gender | ||
| Male | 77 | < 0.001 |
| Female | 114 | |
| Age (years) | ||
| < 65 | 161 | 0.711 |
| ≥ 65 | 30 | |
| Tumor location | ||
| Skull base | 36 | < 0.001 |
| Non-skull base | 155 | |
| Cerebral convexity | 122 | < 0.001 |
| Non-cerebral convexity | 69 | |
| Falx/sagittal sinus | 9 | 0.936 |
| Non-falx/sagittal sinus | 182 | |
| Intraventricular | 8 | 0.907 |
| Non-intraventricular | 183 |
There were 884 females and 355 males (female-male ratio, 2.5) included in this study. There were 77 males had atypical and anaplastic meningiomas, while 114 females had atypical and anaplastic meningiomas. The Pearson’s chi-square test showed an increased risk for male gender for atypical and anaplastic meningiomas (21.7% vs. 12.9%, P < 0.001).
The mean age of the patients was 51.76 years old, ranging from 0.4 to 85 years old. Patients with benign meningiomas and atypical/anaplastic meningiomas had about the same mean age (51.8 vs. 51.3 years; P = 0.064, t-test). The Pearson’s chi-square test showed that there was no statistical significance between age < 65 years and age ≥ 65 years for atypical and anaplastic meningiomas (15.6% vs. 14.6%, P = 0.711).
The most common site of atypical and anaplastic meningiomas was the cerebral convexity (n = 122, 63.9%), followed by the skull base (n = 36, 18.9%), other (n = 16, 8.4%), the falx/sagittal sinus region (n = 9, 4.7%) and the intraventricular region (n = 8, 4.2%). The Pearson’s chi-square test showed a decreased risk for skull base for atypical and anaplastic meningiomas (7.9% vs. 19.8%, P < 0.001). And an increased risk for cerebral convexity for atypical and anaplastic meningiomas (21.3% vs. 10.3%, P < 0.001). There was no statistical significance between the falx/sagittal sinus region and the non-falx/sagittal sinus region for atypical and anaplastic meningiomas (15.8% vs. 15.4%, P = 0.936, the Pearson’s chi-square test). And there was no statistical significance between the intraventricular region and the non-intraventricular region for atypical and anaplastic meningiomas (16.0% vs. 15.4%, P = 0.907, the Pearson’s chi-square test). The Ki67 index was obtainable in 368 (29.7%) cases. When analysed with a cut-off value of 5%, 203 (55.2%) cases were < 5% and 165 (44.8%) were ≥ 5%. Patients with a Ki67 index ≥ 5% were significantly more likely to have atypical and anaplastic meningiomas than those patients with a Ki67 index < 5% (49.7% vs. 6.9%, P < 0.001, the Pearson’s chi-square test). In addition, the percentage of patients with a Ki67 index ≥ 5% in cerebral convexity meningiomas was higher than in non-cerebral convexity location (52.8% vs. 38.5%, P = 0.006, the Pearson’s chi-square test).
Discussion
Determining factors that could accurately predict pathological features of meningiomas before histological diagnosis would help surgeons to proper balance the risk of operation and the resection grade. This is also critical for a non-invasive treatment modality. In this study, we stratified the case according to anatomical locations. The results indicate that male gender and cerebral convexity are significant risk factors for atypical and anaplastic meningiomas. Although female gender has an overall higher incidence of meningiomas [7,8], our results showed an increased risk for male gender for higher-grade meningiomas which was according to the previous studies [4,5,9-15]. The possible reasons for the male gender association with higher-grade meningiomas are still unclear. The chromosome abnormalities, hormone receptor status and hormone levels might affect the trend for higher grade tumors [4].
In our study, patients with benign meningiomas and atypical/anaplastic meningiomas had about the same mean age (51.8 vs. 51.3 years; P = 0.064, t-test). This is in line with previous findings [16]. In contrast, Wang et al. [15] reported that the mean age of patients with higher-grade meningiomas was significantly lower than those with benign meningiomas. Based on prior studies [5,10], we did not find that patients ≥ 65 years correlated positively with atypical and anaplastic meningiomas. Therefore, the relationship of age and meningioma grade is still controversial.
The observations that non-skull base meningiomas are more likely to be the higher-grade meningiomas is supported by many studies [4,5,10,14]. This was also confirmed by our present study. Based on the further detailed anatomical stratification of non-skull base location, we found that the most common site of atypical and anaplastic meningiomas was the cerebral convexity. Based on the Pearson’s chi-square test, we found that meningiomas with cerebral convexity location have an increased risk of atypical and anaplastic meningiomas. However, significant differences in the terms of falx/sagittal sinus and intraventricular were not found. Furthermore, we found the Ki67 index significant increased with the grade of meningiomas, this is in line with previous study [17]. Additionally, the percentage of patients with a Ki67 index ≥ 5% in cerebral convexity meningiomas was significantly higher than in non-cerebral convexity location. This result corroborates our conclusion that cerebral convexity location is a risk factor for atypical and anaplastic meningiomas. Hasseleid et al. [18] reported that patients with meningiomas underwent Simpson Grade I resection have a lower recurrence rate than those underwent Simpson resection Grades II and III. Therefore, they believe that convexity meningiomas should reach the goal of Simpson Grade I resection. This is in line with our clinical practice (Figure 1).
Figure 1.
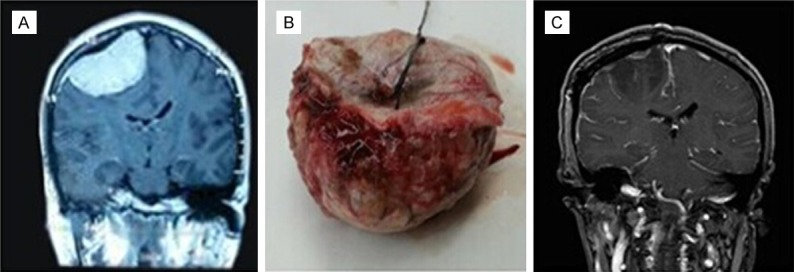
The patient with meningioma underwent Simpson Grade I resection.
In this large series, we found that cerebral convexity, male gender are preoperative risk factors for atypical and anaplastic meningiomas. Significant differences in the terms of falx/sagittal sinus and intraventricular were not found. However, the limitations of this research should be acknowledged. First, this is only a retrospective study. Second, the possible association between the anatomical locations and the survival time of patients with meningiomas was not performed. Because the duration of follow-up in this study was so short, that it could not be statistically analyzed. This remained to be carried out in future research.
Disclosure of conflict of interest
None.
References
- 1.Deltour I, Johansen C, Auvinen A, Feychting M, Klaeboe L, Schüz J. Time trends in brain tumor incidence rates in Denmark, Finland, Norway, and Sweden, 1974-2003. J Natl Cancer Inst. 2009;10:1721–1724. doi: 10.1093/jnci/djp415. [DOI] [PubMed] [Google Scholar]
- 2.Whittle IR, Smith C, Navoo P, Collie D. Meningiomas. Lancet. 2004;363:1535–1543. doi: 10.1016/S0140-6736(04)16153-9. [DOI] [PubMed] [Google Scholar]
- 3.Sherman WJ, Raizer JJ. Medical management of meningiomas. CNS Oncol. 2013;2:161–170. doi: 10.2217/cns.13.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou P, Ma W, Yin S, Li Y, Jiang S. Three risk factors for WHO grade II and III meningiomas: A study of 1737 cases from a single center. Neurol India. 2013;61:40–44. doi: 10.4103/0028-3886.107928. [DOI] [PubMed] [Google Scholar]
- 5.Kane AJ, Sughrue ME, Rutkowski MJ, Shangari G, Fang S, McDermott MW, Berger MS, Parsa AT. Anatomic location is a risk factor for atypical and malignant meningiomas. Cancer. 2011;117:1272–1278. doi: 10.1002/cncr.25591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. WHO Classification of tumours of the central nervous system. Lyon: IARC Press; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bondy M, Ligon BL. Epidemiology and etiology of intracranial meningiomas: a review. J Neurooncol. 1996;29:197–205. doi: 10.1007/BF00165649. [DOI] [PubMed] [Google Scholar]
- 8.Rohringer M, Sutherland GR, Louw DF, Sima AA. Incidence and clinicopathological features of meningioma. J Neurosurg. 1989;71:665–672. doi: 10.3171/jns.1989.71.5.0665. [DOI] [PubMed] [Google Scholar]
- 9.Alvarez F, Roda JM, Perez Romero M, Morales C, Sarmiento MA, Blázquez MG. Malignant and atypical meningiomas: a reappraisal of clinical, histological, and computed tomographic features. Neurosurgery. 1987;205:688–694. doi: 10.1227/00006123-198705000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Cornelius JF, Slotty PJ, Steiger HJ, Hänggi D, Polivka M, George B. Malignant potential of skull base versus non-skull base meningiomas: clinical series of 1,663 cases. Acta Neurochir. 2013;155:407–413. doi: 10.1007/s00701-012-1611-y. [DOI] [PubMed] [Google Scholar]
- 11.Mahmood A, Caccamo DV, Tomecek FJ, Malik GM. Atypical and malignant meningiomas: a clinicopathological review. Neurosurgery. 1993;33:955–963. doi: 10.1227/00006123-199312000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Mahmood A, Qureshi NH, Malik GM. Intracranial meningiomas: analysis of recurrence after surgical treatment. Acta Neurochir. 1994;126:53–58. doi: 10.1007/BF01476410. [DOI] [PubMed] [Google Scholar]
- 13.Marosi C, Hassler M, Roessler K, Reni M, Sant M, Mazza E, Vecht C. Meningioma. Crit Rev Oncol Hematol. 2008;67:153–171. doi: 10.1016/j.critrevonc.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Pasquier D, Bijmolt S, Veninga T, Rezvoy N, Villa S, Krengli M, Weber DC, Baumert BG, Canyilmaz E, Yalman D, Szutowicz E, Tzuk-Shina T, Mirimanoff RO Rare Cancer Network. Atypical and malignant meningioma: outcome and prognostic factors in 119 irradiated patients. A multicenter, retrospective study of the Rare Cancer Network. Int J Radiat Oncol Biol Phys. 2008;71:1388–1393. doi: 10.1016/j.ijrobp.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 15.Wang DJ, Xie Q, Gong Y, Mao Y, Wang Y, Cheng HX, Zhong P, Che XM, Jiang CC, Huang FP, Zheng K, Li SQ, Gu YX, Bao WM, Yang BJ, Wu JS, Xie LQ, Zheng MZ, Tang HL, Zhu HD, Chen XC, Zhou LF. Histopathological classification and location of consecutively operated meningiomas at a single institution in China from 2001 to 2010. Chin Med J (Engl) 2013;126:488–493. [PubMed] [Google Scholar]
- 16.Jaaskelainen J, Haltia M, Servo A. Atypical and anaplastic meningiomas: radiology, surgery, radiotherapy, and outcome. Surg Neurol. 1986;25:233–242. doi: 10.1016/0090-3019(86)90233-8. [DOI] [PubMed] [Google Scholar]
- 17.Roser F, Samii M, Ostertag H, Bellinzona M. The Ki-67 proliferation antigen in meningiomas. Experience in 600 cases. Acta Neurochir (Wien) 2004;146:37–44. doi: 10.1007/s00701-003-0173-4. [DOI] [PubMed] [Google Scholar]
- 18.Hasseleid BF, Meling TR, Ronning P, Scheie D, Helseth E. Surgery for convexity meningioma: Simpson Grade I resection as the goal: clinical article. J Neurosurg. 2012;117:999–1006. doi: 10.3171/2012.9.JNS12294. [DOI] [PubMed] [Google Scholar]


