Abstract
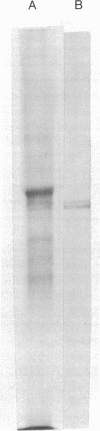
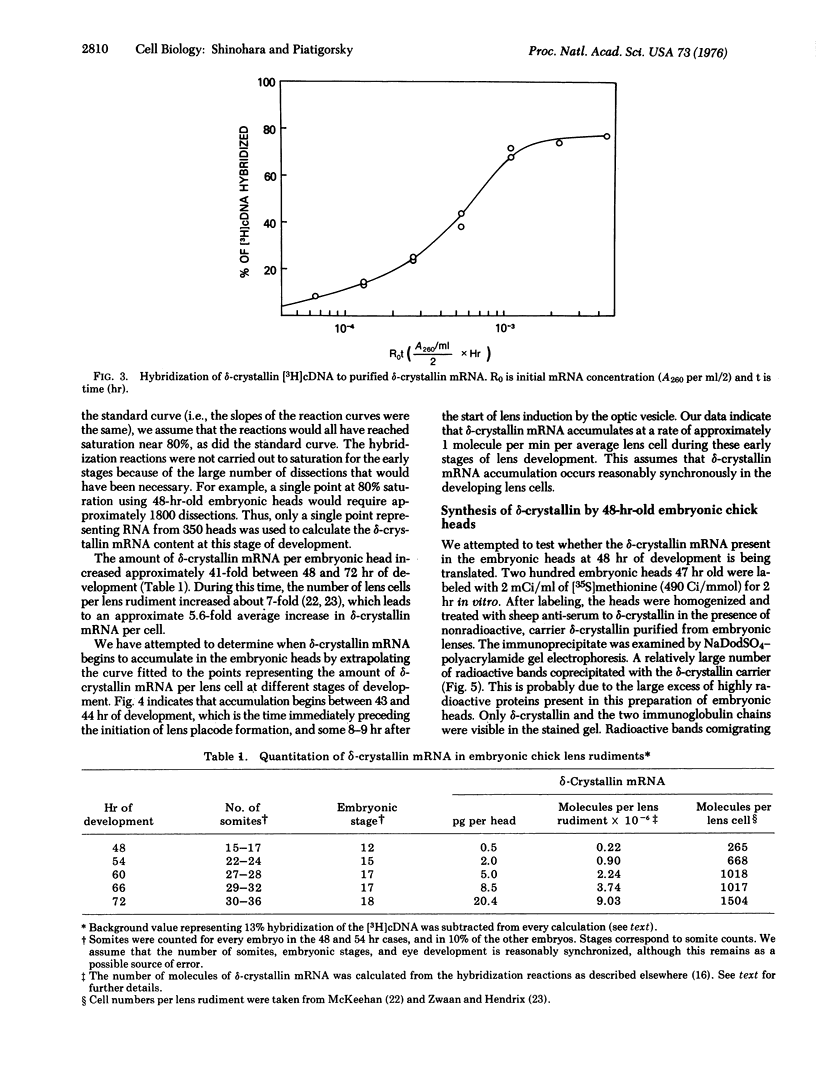
Embryonic chick cells of the presumptive lens ectoderm are induced to differentiate into a lens by the optic vesicle, an outgrowth from the developing brain. This lens induction involves formation of the lens placode by cell elongation between about 44 and 50 hr of development, followed by invagination of the placode between about 50 and 55 hr of development. The amount of delta-crystallin messenger RNA (mRNA) in heads of embryos between 48 and 72 hr of development was determined by molecular hybridization with [3H]DNA complementary to purified delta-crystallin mRNA. An average of approximately 0.5 pg of delta-crystallin mRNA was found per embryonic head at 48 hr of development. This leads to approximately 265 molecules of delta-crystallin mRNA per lens cell, if one assumes that the mRNA is confined to the lens rudiment, as is indicated by immunofluorescence studies of delta-crystallin performed by other investigators. Pulse-labeling experiments with [35S]methionine indicated that the delta-crystallin mRNA is being translated already at this time. delta-Crystallin mRNA accumulated at an average rate of 1 molecule per cell per minute between 48 and 72 hr of development. Thus, by extrapolation, the initiation of delta-crystallin mRNA accumulation coincides with the initiation of lens placode formation at approximately 43-44 hr of development, which is some 8-9 hr after the initiation of lens induction by the optic vesicle. These data suggest that delta-crystallin synthesis is regulated by the accumulation of delta-crystallin mRNA during lens induction in chick embryos.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brawerman G. Eukaryotic messenger RNA. Annu Rev Biochem. 1974;43(0):621–642. doi: 10.1146/annurev.bi.43.070174.003201. [DOI] [PubMed] [Google Scholar]
- Craig J. P., Piatigorsky J. Cell elongation and -crystallin synthesis without RNA synthesis in cultured early embryonic chick lens epithelia. Biochim Biophys Acta. 1973 Apr 11;299(4):642–653. doi: 10.1016/0005-2787(73)90237-2. [DOI] [PubMed] [Google Scholar]
- Genis-Galvez J. M., Castro J. M., Battaner E. Lens soluble proteins: correlation with the cytological differentiation in the young adult organ of the chick. Nature. 1968 Feb 17;217(5129):652–654. doi: 10.1038/217652a0. [DOI] [PubMed] [Google Scholar]
- Groudine M., Holtzer H., Scherrer K., Therwath A. Lineage-dependent transcription of globin genes. Cell. 1974 Nov;3(3):243–247. doi: 10.1016/0092-8674(74)90138-x. [DOI] [PubMed] [Google Scholar]
- HAM R. G. An improved nutrient solution for diploid Chinese hamster and human cell lines. Exp Cell Res. 1963 Feb;29:515–526. doi: 10.1016/s0014-4827(63)80014-2. [DOI] [PubMed] [Google Scholar]
- Hendrix R. W., Zwaan J. Changes in the glycoprotein concentration of the extracellular matrix between lens and optic vesicle associated with early lens differentiation. Differentiation. 1974 Dec;2(6):357–362. doi: 10.1111/j.1432-0436.1974.tb00371.x. [DOI] [PubMed] [Google Scholar]
- Humphries S., Windass J., Williamson R. Mouse globin gene expression in erythroid and non-erythroid tissues. Cell. 1976 Feb;7(2):267–277. doi: 10.1016/0092-8674(76)90026-x. [DOI] [PubMed] [Google Scholar]
- Ikeda A., Zwaan J. Immunofluorescence studies on induction and differentiation of the chicken eye lens. Invest Ophthalmol. 1966 Aug;5(4):402–412. [PubMed] [Google Scholar]
- Katoh A., Yashida K. Delta crystallin synthesis during chick lens differentiation. Exp Eye Res. 1973 Mar;15(3):353–360. doi: 10.1016/0014-4835(73)90150-4. [DOI] [PubMed] [Google Scholar]
- Milstone L. M., Piatigorsky J. Rates of protein synthesis in explanted embryonic chick lens epithelia: differential stimulation of delta-crystallin synthesis. Dev Biol. 1975 Mar;43(1):91–100. doi: 10.1016/0012-1606(75)90133-5. [DOI] [PubMed] [Google Scholar]
- Milstone L. M., Zelenka P., Piatigorsky J. Delta-crystallin mRNA in chick lens cells: mRNA accumulates during differential stimulation of delta-crystallin synthesis in cultured cells. Dev Biol. 1976 Feb;48(2):197–204. doi: 10.1016/0012-1606(76)90084-1. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W., Means A. R. Female steroid hormones and target cell nuclei. Science. 1974 Feb 15;183(4125):610–620. doi: 10.1126/science.183.4125.610. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. Quantitation of parameters that determine the rate of ovalbumin synthesis. Cell. 1975 Mar;4(3):189–189. doi: 10.1016/0092-8674(75)90167-1. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J., Webster H. D., Craig S. P. Protein synthesis and ultrastructure during the formation of embryonic chick lens fibers in vivo and in vitro. Dev Biol. 1972 Feb;27(2):176–189. doi: 10.1016/0012-1606(72)90096-6. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J., Zelenka P., Simpson R. T. Molecular weight and subunit structure of delta-crystallin from embryonic chick lens fibers. Exp Eye Res. 1974 May;18(5):435–446. doi: 10.1016/0014-4835(74)90080-3. [DOI] [PubMed] [Google Scholar]
- RABAEY M. Electrophoretic and immunoelectrophoretic studies on the soluble proteins in the developing lens of birds. Exp Eye Res. 1962 Jun;1:310–316. doi: 10.1016/s0014-4835(62)80017-7. [DOI] [PubMed] [Google Scholar]
- Ramirez F., Gambino R., Maniatis G. M., Rifkind R. A., Marks P. A., Bank A. Changes in globin messenger RNA content during erythroid cell differentiation. J Biol Chem. 1975 Aug 10;250(15):6054–6058. [PubMed] [Google Scholar]
- Ross J., Gielen J., Packman S., Ikawa Y., Leder P. Globin gene expression in cultured erythroleukemic cells. J Mol Biol. 1974 Aug 25;87(4):697–714. doi: 10.1016/0022-2836(74)90079-5. [DOI] [PubMed] [Google Scholar]
- Sutton W. D. A crude nuclease preparation suitable for use in DNA reassociation experiments. Biochim Biophys Acta. 1971 Jul 29;240(4):522–531. doi: 10.1016/0005-2787(71)90709-x. [DOI] [PubMed] [Google Scholar]
- Yoshida K., Katoh A. Crystallin synthesis by chicken lens. I. Analysis of radioactive crystallins by cellulose acetate membrane electrophoresis. Exp Eye Res. 1971 Jan;11(1):122–131. doi: 10.1016/s0014-4835(71)80074-x. [DOI] [PubMed] [Google Scholar]
- Zelenka P., Piatigorsky J. Isolation and in vitro translation of delta-crystallin mRNA from embryonic chick lens fibers. Proc Natl Acad Sci U S A. 1974 May;71(5):1896–1900. doi: 10.1073/pnas.71.5.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelenka P., Piatigorsky J. Molecular weight and sequence complexity of delta-crystallin mRNA. Exp Eye Res. 1976 Feb;22(2):115–124. doi: 10.1016/0014-4835(76)90038-5. [DOI] [PubMed] [Google Scholar]
- Zwaan J., Ikeda A. Macromolecular events during differentiation of the chicken lens. Exp Eye Res. 1968 Apr;7(2):301–311. doi: 10.1016/s0014-4835(68)80081-8. [DOI] [PubMed] [Google Scholar]
- van de Kamp M., Zwann J. Intracellular localization of lens antigens in the developing eye of the mouse embryo. J Exp Zool. 1973 Oct;186(1):23–32. doi: 10.1002/jez.1401860105. [DOI] [PubMed] [Google Scholar]