Abstract
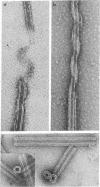
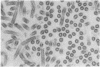
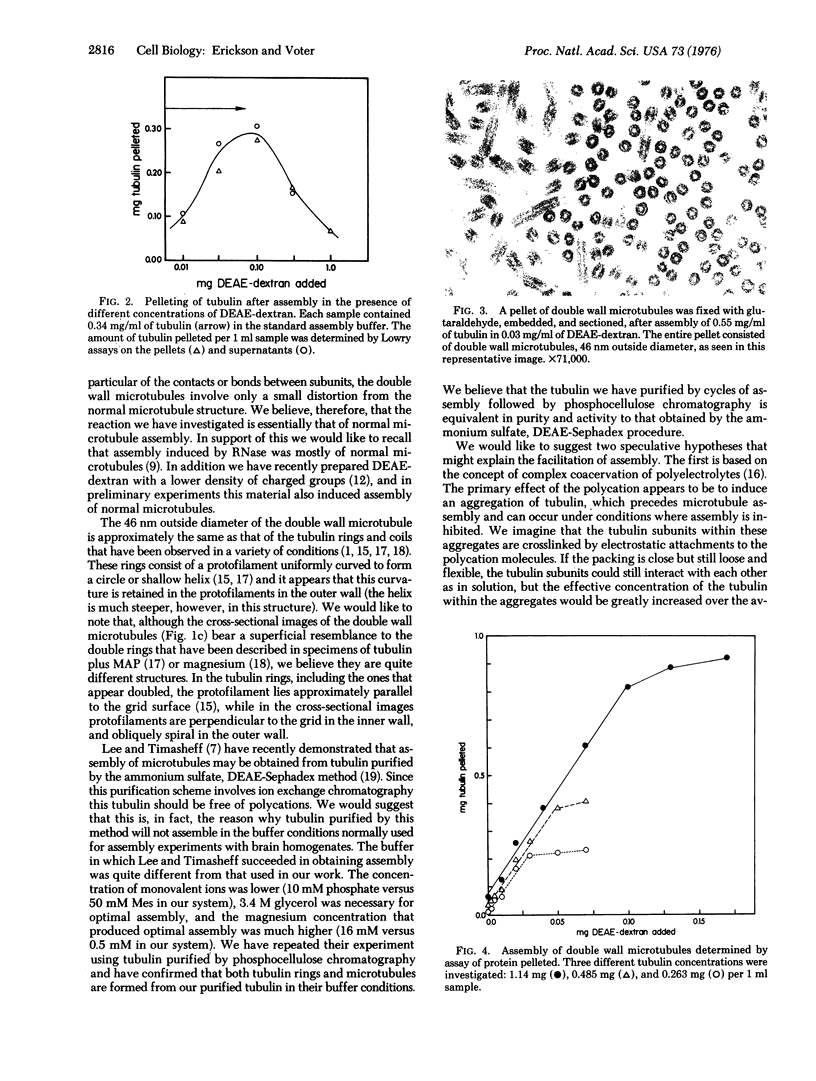
Several different polycations have been found that can substitute for the microtubule-associated proteins, or tau factor, in facilitating assembly of tubulin that has been purified by ion exchange chromatography. In low concentrations of the polycation diethylaminoethyl-dextran, 7 mg of tubulin is pelleted per 1 mg of polycation added. Under conditions favorable to microtubule assembly the entire pellet is seen by electron microscopy to consist of "double wall microtubules", which are essentially identical to normal microtubules in subunit structure and arrangement. When assembly is inhibited approximately the same amount of tubulin is pelleted, but it is in the form of clusters of curved sheets or filaments apparently related to tubulin rings. When conditions are changed to favor assembly, the tubulin within these clusters appears to reassemble to form the double wall microtubules.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borisy G. G., Marcum J. M., Olmsted J. B., Murphy D. B., Johnson K. A. Purification of tubulin and associated high molecular weight proteins from porcine brain and characterization of microtubule assembly in vitro. Ann N Y Acad Sci. 1975 Jun 30;253:107–132. doi: 10.1111/j.1749-6632.1975.tb19196.x. [DOI] [PubMed] [Google Scholar]
- Bryan J. B., Nagle B. W., Doenges K. H. Inhibition of tubulin assembly by RNA and other polyanions: evidence for a required protein. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3570–3574. doi: 10.1073/pnas.72.9.3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson H. P. Assembly of microtubules from preformed, ring-shaped protofilaments and 6-S tubulin. J Supramol Struct. 1974;2(2-4):393–411. doi: 10.1002/jss.400020228. [DOI] [PubMed] [Google Scholar]
- Erickson H. P. Microtubule surface lattice and subunit structure and observations on reassembly. J Cell Biol. 1974 Jan;60(1):153–167. doi: 10.1083/jcb.60.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigon R. P., Timasheff S. N. Magnesium-induced self-association of calf brain tubulin. II. Thermodynamics. Biochemistry. 1975 Oct 21;14(21):4567–4573. doi: 10.1021/bi00692a002. [DOI] [PubMed] [Google Scholar]
- Jacobs M., Bennett P. M., Dickens M. J. Duplex microtubule is a new form of tubulin assembly induced by polycations. Nature. 1975 Oct 23;257(5528):707–709. doi: 10.1038/257707a0. [DOI] [PubMed] [Google Scholar]
- Kane R. E. Preparation and purification of polymerized actin from sea urchin egg extracts. J Cell Biol. 1975 Aug;66(2):305–315. doi: 10.1083/jcb.66.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner M. W., Williams R. C. The mechanism of microtubule assembly in vitro. J Supramol Struct. 1974;2(2-4):412–428. doi: 10.1002/jss.400020229. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee J. C., Timasheff S. N. The reconstitution of microtubules from purified calf brain tubulin. Biochemistry. 1975 Nov 18;14(23):5183–5187. doi: 10.1021/bi00694a025. [DOI] [PubMed] [Google Scholar]
- McKernan W. M., Ricketts C. R. A basic derivative of dextran and its interaction with serum albumin. Biochem J. 1960 Jul;76(1):117–120. doi: 10.1042/bj0760117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D. B., Borisy G. G. Association of high-molecular-weight proteins with microtubules and their role in microtubule assembly in vitro. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2696–2700. doi: 10.1073/pnas.72.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T. D. The role of actin in the temperature-dependent gelation and contraction of extracts of Acanthamoeba. J Cell Biol. 1976 Mar;68(3):579–601. doi: 10.1083/jcb.68.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P., Hartwig J. H. Interactions between actin, myosin, and an actin-binding protein from rabbit alveolar macrophages. Alveolar macrophage myosin Mg-2+-adenosine triphosphatase requires a cofactor for activation by actin. J Biol Chem. 1975 Jul 25;250(14):5706–5712. [PubMed] [Google Scholar]
- Tilney L. G. The polymerization of actin. III. Aggregates of nonfilamentous actin and its associated proteins: a storage form of actin. J Cell Biol. 1976 Apr;69(1):73–89. doi: 10.1083/jcb.69.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten M. D., Lockwood A. H., Hwo S. Y., Kirschner M. W. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975 May;72(5):1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenberg R. C., Borisy G. G., Taylor E. W. The colchicine-binding protein of mammalian brain and its relation to microtubules. Biochemistry. 1968 Dec;7(12):4466–4479. doi: 10.1021/bi00852a043. [DOI] [PubMed] [Google Scholar]
- Weisenberg R. C., Timasheff S. N. Aggregation of microtubule subunit protein. Effects of divalent cations, colchicine and vinblastine. Biochemistry. 1970 Oct 13;9(21):4110–4116. doi: 10.1021/bi00823a012. [DOI] [PubMed] [Google Scholar]