Abstract
We aim to detect the miRNAs that are correlated with the gastric cancer cell line SGC-7901 to provide theoretical basis for clinical application. We first analyzed miRNA expression profiles of gastric cancer patients compared with normal controls by microarray analysis and validated the results by real-time qPCR. We also determined the absolute copy numbers of these three miRNAs in normal adults. The results showed that three miRNAs (miR-150, miR-23a, and miR-130a) were identified to significantly decrease in expanded 38 gastric cancer patients compared with 90 normal controls. Molecular and statistical analysis showed that the decreased miRNAs were significant in clinical analysis. Generally speaking, this finding suggest vital roles of these miRNAs in human gastric cancer genesis and as potential biomarkers in gastric cancer diagnosis.
Keywords: microRNAs, gastric cancer, SGC-7901, diagnosis biomarker
Introduction
Gastric cancer (GC), one of the most common malignancies worldwide, is the second most frequent cause of cancer death [1,2]. The high mortality of GC is a consequence of late-stage of diagnosis, the 5-year survival rate for advanced stages is extremely poor and around 5% to 15% [3,4]. Although diagnosis and treatment of GC have improved, the survival rate has not increased substantially in couple of years. Therefore, an improved understanding of the molecular pathways involved in the progression of gastric cancer will be helpful in improving prevention, diagnosis and therapy of this disease [5].
Recently many epigenetic events are widely investigated in the cancer development [6]. Aberrant expression of miRNAs is reported in various types of cancers [7,8]. The unregulated HER-2 activates a series of signaling pathways, prevents apoptosis, and eventually leads to the cell proliferation disorders. Therefore the HER-2 fusion gene is essential for the transformed cellular phenotype in GC [9]. In addition, the interaction between the progenitor cells expressing HER-2 and the environmental matrix may provide external signals that facilitate GC development [10].
The in-depth investigations into the GC pathogenesis in recent years have led to several targeted therapeutic protocols. However, the prognosis is still poor, with a long term survival rate of only 30-40% for patients under 60 years old and 10% for patients above 60 years old [11,12]. Therefore, understanding the fundamental mechanisms of GC and developing better treatment methods remain challenging. Recent studies have shown that the abnormal expression of miRNAs is associated with a variety of tumors as evidenced by their presence at fragile site in the genome functioning as oncogenes or tumor suppressor genes [13]. Recently, many researchers are looking into the potential use of miRNAs as prognostic tools other than diagnostic application, however, this specific marker needs to correlate closely with clinical outcomes and metastatic potential. Profiling of miRNAs seems to be advantageous over mRNAs in terms of cancer phenotypes differentiation. A current report suggested a prognostic signature for gastric cancer which consist of four risk miRNAs (miR-10b, miR-21, miR-223 and miR-338; with hazard ratio > 1) and three protective miRNAs (miR-30a-5p, miR-126 and let-7a; hazard ratio < 1), and was associated with clinical outcomes [14]. Another study showed that low expressions of miR-21 and miR-181b are associated with overall survival in patients treated with S-1 and doxifluridine [15]. Low expression of miR-125a-3p is correlated with tumor size, invasion, metastasis and advanced clinical stage, and an independent prognostic marker for gastric cancer [16]. Ectopic expression of miR-125a-3p showed remarkable retardation of gastric cancer growth in vitro, illustrating the tumor suppressive property and potential clinical use of miR-125a-3p. On the other hand, the other strand of miR-125a, miR-125-5p also showed similar tumor suppressive effect in gastric cancer, and low expression was associated with poor prognosis [17]. Other study showed that HMGA2 expression was directly correlated with tumor invasiveness and prognosis, which is modulated by let-7 family [18]. A similar observation was also seen in pituitary adenomas, in which low expression of let-7 induced HGMA2 and related to tumor proliferation and invasion [19].
In GC, a growing body of evidence has implicated that miRNAs are involved in the processes of occurrence, progression and drug resistance of GC, and their associations with the specific hybrid gene could become a new therapeutic target. The present study was accordingly designed to detect cancer stem cell related miRNAs, and explore their roles in GC development and progression. We analyzed the expression profiles of the human gastric cancer cell lines SGC-7901 and the immortalized cell line GES-1 using microarray, identified the target miRNAs that displayed significant expression changes, and further investigated their functional mechanisms and potential applications in clinical diagnosis and treatment.
Materials and methods
Reagents
Restriction enzymes were purchased from TaKaRa, Biolabs, Promega and MBI; DNAse I and RNase were purchased from Invitrogen; lysozyme was purchased from Sigma; T4 DNA ligase was purchased from Promega; Taq DNA polymerase was purchased from Promega and MBI. DNA molecular weights standards: DL2000 and DL15000 were from Takara. The pBR322/Msp I was from MBI. Gel preparation reagents: acrylamide, N’N’-methylene-bis-acrylamide, urea, ammonium persulfate (AP) and SDS were from Sigma. Tris (hydroxymethyl) aminomethane (Tris) and agarose were from Gibco BRL. dATP, dGTP, dCTP, dTTP were from Boehringer Mannheim. Trizol and Lipofectamin™ 2000 were from Invitrogen. Ficoll-Paque (1.077 g/ml) was from TBD. TritonX-100 was from Fluka. SuperScript™ III reverse transcriptase kit was from Invitrogen. The transfection reagent Lipofectamine 2000 was from Invitrogen. In vitro transcription kit was from Promega. miRNA purification kit was purchased from Ambion. MACS magnetic beads kit was from Miltenyi Biotec. SYBR ® Premix Ex TaqTM extraction kit was from Takara. Miniprep kit was from Sunbio. Rapid DNA extraction and purification kit was from Bio-Dev. The tested cell line was SGC-7901 (human GC cell line).
miRNA primers in reverse transcription
miR-23a: 5’-GTC GTA TCC AGT GCA GGG TCC GAG GTA TTC GCA CTG GAT ACG ACA CGT GG-3’; miR-150: 5’-GTC GTA TCC AGT GCA GGG TCC GAG GTA TTC GCA CTG GAT ACG ACA AAC TC-3’; miR-130a: 5’-GTC GTA TCC AGT GCA GGG TCC GAG GTA TTC GCA CTG GAT ACG ACA ACC GAT-3’; U6 snRNA: 5’-AAA ATA TGG AAC GCT TCA CGA ATT TG-3’.
Cell culture
The human gastric cancer cell lines SGC-7901 and the immortalized cell line GES-1 derived from normal gastric mucosa were cultured in RPMI 1640 media supplemented with 10% FBS, and maintained at 37°C in a humidified incubator with a 5% CO2 atmosphere.
Electrotransfection of SGC-7901 cells and generation of stable transfected cell lines
Twenty-four hours prior to electroporation, cells were divided and grown in appropriate medium so that they could reach the logarithmic phase during the electroporation. Cell concentration was 4×106/ml. On the day of electroporation, cells were harvested and washed in PBS twice, and then re-suspended in serum free 1640 medium at a concentration of 1.3×107/ml at room temperature; DNA (a maximum of 10 ug in 10 of 20 uL) was added to the bottom of electroporation cuvette at room temperature; 300 μL of cell suspension was added to electroporation cuvette mixed with DNA gently to avoid any bubbles and stored at room temperature; cuvette was then placed in the electroporation chamber and pulsed once with parameters of capacitor 950 μF and voltage 250 V; cuvette was removed and cells were mixed gently; cells were transferred to FBS containing medium for 48 hours and then to the selection medium.
miRNA real-time PCR
1×102--1×107 cells were harvested, washed in PBS once, and stored on ice; complete cell lysate was prepared by addition of 600 μl lysis binding buffer and vertex; 60 μl miRNA aomogenete addictive was added to the cell lysate and mixed thoroughly by inverting several times; sample was stored on ice for 10 min, followed by addition of equal volume (600 μl) of phenol: chloroform (1:1) solution; sample was mixed by inverting for 30-60 sec, and then centrifuged at 12000 g for 5 min; the supernatant was transferred to a new tube and the volume was estimated; 1/3 volume of 100% ethanol was added and mixed; the mixture was loaded to the column at room temperature and centrifuged at 10000 g for 15 sec; the flow-though was collected and the volume was then estimated; 2/3 volume of 00% ethanol was added and mixed; the mixture was loaded to column at room temperature and centrifuged at 10000 g for 15 sec; the flow-through was discarded; 700 μl miRNA wash solution was added to the column, followed by centrifugation at 10000 for 10 sec; the flow-through was discarded; 500 μl miRNA wash solution was added to the column, followed by centrifugation at 10000 for 10 sec; the flow-through was discarded; the column was transferred to a new tube and 100 μl preheated elution solution (95 degree) was added at room temperature; RNA was collected by centrifugation at 12000 g for 30 sec. miRNA PAGE and northern blotting Total RNAs were prepared using TRIZOL Reagent kit (GiBcoBRL, USA), from hematopoietic cells, induced SGC-7901 cells and non-induced SGC-7901 cells; 0.5×TBE electrotransfer buffer was freshly prepared; gel and nylon membrane was equilibrated in electrotransfer buffer for 10 min, and then a transfer sandwich (sponge-filter paper-gel-membrane-filter paper-sponge) was assembled in electrotranfective device with gel side facing the negative electrode and membrane side facing the positive; blotting buffer was added and the transfer was performed at 200 mA for 2 hours. Membrane was washed in 2×SSC for 5 min, air-dried, cross-linked under the UV, and stored at -20 degree. Fixed membrane was placed in pre-hybridization buffer (Biodev Tech) at 37 degree for 1-2 h, followed by addition of radiolabeled probe at 37 degree for 16-24 h. The blot was then rinsed twice in 0.5×SSC containing 0.1% SDS at room temperature, and once or twice (10 min each) in 1×SSC containing 0.1% SDS at 37 degree, until radioactivity signals were undetectable in the non-RNA region; Radio-autograph: blot was briefly washed in 0.1×SSC at room temperature, dried with paper towel, and sealed using plastic wrap; the radio-autograph was developed at -70 degree.
Western blot
Cells were harvested, washed twice in PBS, and lysed in lysis buffer (protease inhibitors were added immediately before use) for 30 min on ice. Lysate was centrifuged at 10000 rmp and the supernatants were collected and stored at -70 in aliquots. All procedures were carried out on ice. Protein concentration was determined using BCA assay kit (Tianlai Biotech).
Protein SDS-PAGE
Protein samples were mixed with loading buffer and boiled at 100 degree for 10 min. The samples were then centrifuged at 10000 g for 10 min at room temperature and the supernatants were transferred to new tubes. Protein samples were loaded to discontinuous SDS-PAGE. The electrophoresis was run at 80 voltages through the stacking gel and then 120 voltages though the separating gel until the blue dye reached the end of the gel. The gel was stained in Coomassie Brilliant Blue 250 or subject to further blotting analysis.
GC microarray analysis
Total RNA prepared with Trizol was precipitated in isopropanol. The concentration was determined using spectrometer and the quality was assessed using formaldehyde denaturing gel electrophoresis; 50-100.0 μg total RNA was subject to miRNA isolation with Ambion´s miRNA Isolation Kit (Cat#. 1560); the fluorescence labeled RNA sample was prepared using T4 TNA ligase. A custom microarray platform for analysis of miRNA gene expression. Labeled RNA was dissolved in 16 L hybridization solution (15% formamide; 0.2% SDS; 3×SSC; 50× Denhardt’s) and incubated with array chips overnight at 42 degree. Array chips were then washed with 2×SSC containing 0.2% SDS for 4 min at 42 degree, followed by a wash with 0.2×SSC at room temperature for 4 min. Slides were dried and scanned with LuxScan 10 K/A dual-laser scanner (CapitalBio).
Data analysis
Image data were processed using SpotData Pro software (Capitalbio). Differentially expressed genes were identified using SAM package (Significance Analysis of Microarrays, version 2.1).
Results
Microarry detected the miRNAs that were differentially expressed between GC patients and normal human subjects
Array chips comprising 275 human mature miRNA probes identified a number of up- and down-regulated miRNAs in GC patients compared with normal subjects, among which 6 were distinctively up-regulated (such as miR-17, miR-106a, and miR-20a) and 19 were distinctively down-regulated (such as miR-23a, miR-150, and miR-130a). Hierarchical clustering analysis showed that those miRNAs were significantly differentially expressed between GC patients and normal subjects (Figure 1).
Figure 1.
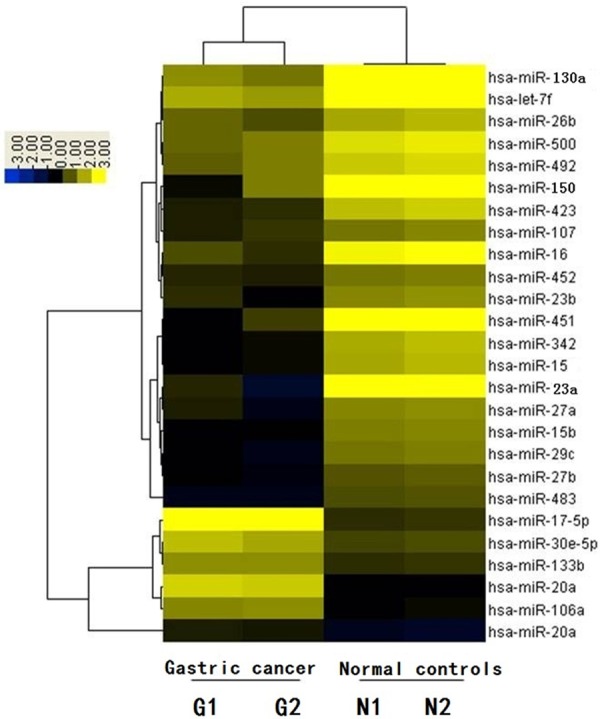
Hierarchical cluster analysis of miRNA-microarray expression data of GC and normal. Array chips comprising 275 human mature miRNA probes identified a number of up- and down-regulated miRNAs in GC patients compared with normal subjects, among which 19 were distinctively down-regulated (such as miR-23a, miR-150, and miR-130a). Hierarchical clustering analysis showed that those miRNAs were significantly differentially expressed between GC patients and normal subjects (G-1, G-2: GC patients; N-1, N-2: normal controls).
The decreased expression of miR-23a, miR-150, and miR-130a in GC
Northern blotting confirmed 3 of them: expression levels of miR-23a, miR-150 and miR-130a were distinctively lower in GC patients (Figure 2). Consequently, we selected those 3 miRNAs for further investigation of the roles they played in GC development and the potentials of their applications in GC clinical diagnosis and drug targets.
Figure 2.
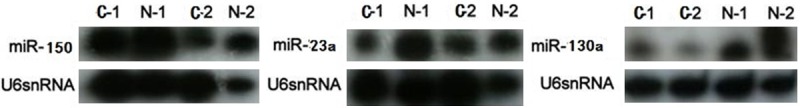
Northern blot confirmation of array results. 10 μg of total RNAs from 2 GC patients and 2 normal controls were used for hybridization. U6 snRNA was used for internal loading control. The expression levels of miR-23a, miR-150 and miR-130a were distinctively lower in GC patients confirmed by northern blot.
Real-time PCR detected that expressions of miR-23a, miR-150 and miR-130a were lower in GC patients compared with normal human subjects
We further expanded our sample size (38 GC patients and 90 normal controls, Table 1) and examined the miRNA expression levels of miR-23a, miR-150 and miR-130a (Samples information were shown in Table 2). Two groups of data (GC group and control group) were analyzed using SPSS10.0 to detect the central tendency and dispersion as shown in Figure 3A. The 3 microRNAs in GC group were significantly decreased comparing with those in the control group. The ROC curve was constructed with package SPSS10.0 to determine the accuracy of GC diagnosis based on the relative expression levels of those 3 miRNAs. Figure 3B showed that the diagnostic performances based on the areas under the curves were 0.73, 0.95, and 0.92, with P values of 0.001, 0.000 and 0.000, respectively, for miR-150, miR-23a, and miR-130a. These results suggest that the expression levels of miR-23a and miR-130a in mononuclear blood cells could be utilized in GC diagnosis in clinic, whereas the performance of miR-150 levels was relatively poor.
Table 1.
Sample information
| Gender | Age mean (SD) | ||
|---|---|---|---|
|
|
|||
| male | female | ||
| GC | 21 | 17 | 43 (18) |
| control | 55 | 35 | 41 (17) |
Table 2.
Detailed information on GC patients
| Sample ID | Age | Gender | WBC (109/L)1 | DC (%)2 | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| PN | BN | SN | M | L | ||||
| G-1 | 23 | male | 65.9 | 3 | - | 6 | 86 | 8 |
| G-2 | 34 | female | 53.1 | 51 | 12 | 19 | - | 7 |
| 1 | 27 | male | 3.9 | 31 | 22 | 29 | - | 13 |
| 2 | 45 | male | 7.3 | 32 | 11 | 9 | 15 | 2 |
| 3 | 69 | female | 54.9 | 12 | - | - | 11 | 6 |
| 4 | 31 | male | 28.7 | 34 | 23 | 7 | 8 | - |
| 5 | 34 | female | 16.5 | 54 | 12 | 12 | 10 | 8 |
| 6 | 54 | female | 1.7 | 91 | - | 2 | - | 2 |
| 7 | 47 | male | 20.1 | 20 | 10 | 40 | 6 | 24 |
| 8 | 49 | female | 17.4 | 27 | - | 58 | - | 15 |
| 9 | 31 | male | 6.9 | 98 | - | 1 | - | 1 |
| 10 | 29 | male | 14.9 | 61 | - | 1 | - | 38 |
| 11 | 51 | male | 15.4 | 92 | - | 3 | - | 5 |
| 12 | 31 | female | 48.2 | 20 | 10 | 40 | 6 | 24 |
| 13 | 71 | male | 27.0 | - | - | 3 | 92 | 5 |
| 14 | 45 | female | 5.5 | - | - | 40 | 40 | 20 |
| 15 | 54 | female | 32.6 | 71 | - | 11 | 2 | 16 |
| 16 | 27 | female | 5.6 | 90 | - | 2 | - | 8 |
| 17 | 72 | female | 7.4 | 50 | - | 25 | - | 25 |
| 18 | 25 | male | 22.5 | 80 | - | 5 | - | 15 |
| 19 | 36 | male | 15.7 | - | - | - | 87 | 3 |
| 20 | 19 | male | 4.3 | 91 | 1 | 3 | - | 2 |
| 21 | 39 | female | 9.8 | 75 | - | 20 | - | 10 |
| 22 | 71 | female | 2.9 | 17 | 10 | 30 | 13 | 30 |
| 23 | 39 | male | 23.1 | 89 | - | 8 | - | 3 |
| 24 | 53 | female | 19.1 | 45 | 1 | 3 | - | 8 |
| 25 | 67 | male | 35.3 | 72 | - | 22 | - | 6 |
| 26 | 34 | female | 28.3 | 74 | - | 5 | 3 | 2 |
| 27 | 29 | female | 19.3 | - | - | 2 | 93 | 5 |
| 28 | 67 | female | 8.9 | 41 | 19 | 32 | - | 5 |
| 29 | 54 | female | 18.1 | 43 | - | - | - | 10 |
| 30 | 33 | male | 5.2 | - | - | 10 | 79 | 11 |
| 31 | 44 | male | 11.7 | 13 | - | 20 | 54 | 13 |
| 32 | 50 | male | 34.0 | 38 | - | - | 18 | 35 |
| 33 | 44 | female | 2.7 | 61 | - | 7 | 18 | 14 |
| 34 | 43 | male | 3.1 | 65 | 3 | 10 | 7 | 15 |
| 35 | 22 | male | 2.5 | 87 | - | 2 | 3 | 4 |
| 36 | 44 | male | 3.8 | 67 | 2 | - | - | 38 |
| 37 | 29 | male | 3.0 | 89 | - | 2 | - | - |
| 38 | 75 | male | 11.3 | 19 | 8 | - | 9 | 18 |
WBC represents the total number of white cells in peripheral blood.
DC represents.
PN, progranulocyte; BN, band neutrophil; SN, segmented neutrophil; M, monocyte, L, lymphocyte.
Figure 3.
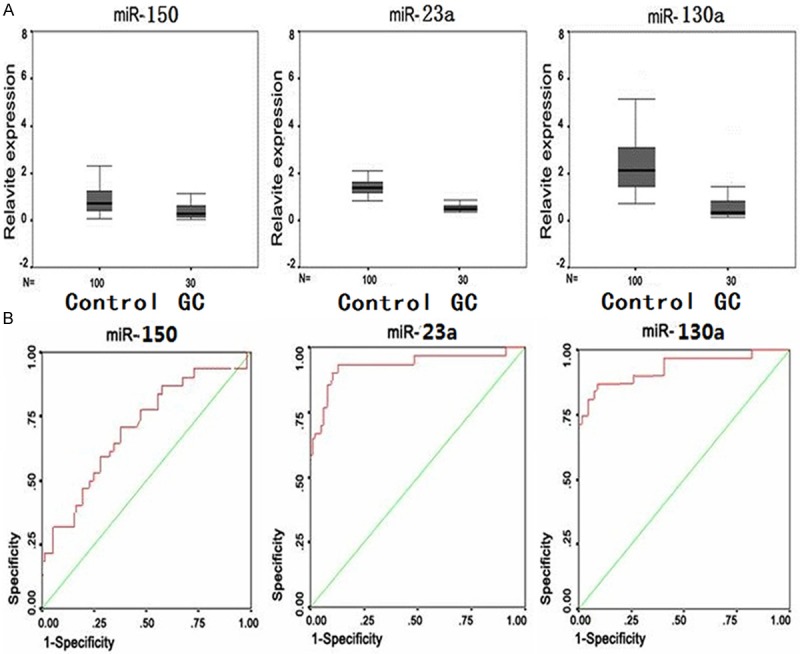
The scatterplot of expression levels of miR-150, miR-23a, and miR-130a in GC patients and normal human subjects. A. Quantitative real time RT-PCR analysis of expression of miR-150, miR-23a, and miR-130a in 38 GC patients and 90 controls. U6 snRNA was used as internal control. The relative expression was normalized against the same control sample (miRNA prep from mononuclear cells of a normal blood donor). Comparative miRNA real-time PCR was performed in triplicated and expression level was normalized to U6 snRNA. B. ROC curve analysis using miR-150, miR-23a, and miR-130a expression as diagnositic markers in GC patients.
Statistical analysis of GC diagnosis based on the expression levels of miR-23a, miR-150 and miR-130a
We evaluated the GC screening results from 128 tested subjects (38 GC patients and 90 normal control subjects) that were based on the expression levels of miR-23a, miR-130a and miR-150. Table 3 showed the results. Consistent with the results in the previous section, miR-23a and miR-130a, individually or in combination, displayed high diagnostic values with satisfactory stability, whereas the performance of miR-150 was relatively low. These findings suggested that expression levels of miR-23a and miR-130a in mononuclear blood cells could be applied in clinical diagnosis of GC.
Table 3.
Statistic analysis of screening results of GC
| MiRNA | sensitivity (%) | False negative (%) | specificity (%) | false positive (%) | accuracy (%) | positive likelihood ratio (+LR) | negative likelihood ratio (-LR) | consistency (%) | positive predictive value (%) | negative predictive value (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| miR-150 | 23.3 | 76.7 | 94 | 6 | 17.3 | 3.88 | 0.82 | 77.7 | 53.8 | 80.3 |
| miR-23a | 70 | 30 | 95 | 5 | 65 | 14 | 0.32 | 89.2 | 80.8 | 91.3 |
| miR-130a | 76.7 | 23.3 | 94 | 6 | 70.7 | 12.8 | 0.25 | 90.8 | 82.1 | 93.1 |
| miR-23a and miR-130a | 70 | 30 | 95 | 5 | 65 | 14 | 0.32 | 89.2 | 80.8 | 91.3 |
| miR-23a or miR-130a | 76.7 | 23.3 | 96 | 4 | 82.7 | 19.2 | 0.24 | 91.5 | 85.2 | 93.2 |
Quantitative analysis of the absolute expression levels of miR-23a, miR-150 and miR-130a
From the perspective of convenient application, we developed the absolute method using quantitative PCR (qPCR) to determine the copy number of a specific miRNA in. We used standard curve of real-time PCR (RT-PCR) to perform the absolute quantification analysis (Figure 4). Standard curve was generated by amplifying a specific RNA species in the standard sample with the known copy number, that is, the log copy number of the specific RNA was linearly related to the PCR Ct number (the cycle numbers required for the fluorescent signal to cross the threshold in PCR reaction). The Ct number from an unknown sample could then be used to calculate the quantity of the corresponding target RNA. Since 2 μg of total RNA was used in all the samples, the miRNA copy number per unit mass of total RNA (copy number/total RNA) could therefore be obtained. Standard samples were the purified plasmid DNA containing the target sequences that were amplified using the specific upstream and downstream primers and then cloned into pGEMT vector. Its copy number was determined with 260 nm absorbance. A serial dilution of the standard samples was made to obtain the following concentrations: 1×1010, 1×109, 1×108, 1×107, 1×106, 1× 105, 1×104, 1×103, 1×102, and 1×101 copy number/μL, and 1 μL of these diluted standards were used as templates in qRT-PCR reactions.
Figure 4.
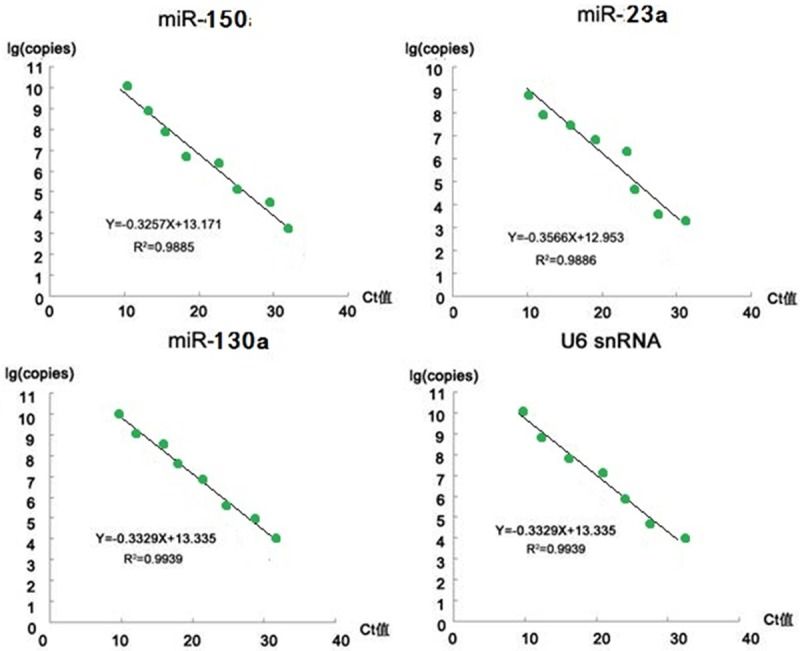
Standard curve in the absolute method of RT-PCR quantification of miRNA. We used standard curve of real-time PCR (RT-PCR) to perform the absolute quantification analysis. Data analysis showed a skewed distribution and the reference values (p=0.05) were determined using percentile method. The ranges of the reference values (copy number /μg total RNA) were as follows: miR-150: 1.5×105~6.9×106; miR-23a: 2.7×108~1.8×109; miR-130a: 2.2×105~1.8×106. In an absolute method, the accuracy was assured by the consistent sample loading (consistent U6 snRNA copy number). From these results, the ranges of ratio of miRNA copy number to U6snRNA copy number in normal human subject blood were also derived: miR-150: 4.71~115.88×10-6; miR-23a: 9.29~56.78×10-3; miR-130a: 7.01~45.72×10-6. Below the normal reference ratio range lower limit would be considered as a reference diagnostic criteria for GC.
Data analysis showed a skewed distribution and the reference values (P=0.05) were determined using percentile method. The ranges of the reference values (copy number/μg total RNA) were as follows: miR-150: 1.5×105~6.9× 106; miR-23a: 2.7×108~1.8×109; miR-130a: 2.2×105~1.8×106. In an absolute method, the accuracy was assured by the consistent sample loading (consistent U6 snRNA copy number). We therefore tested the copy number of U6 snRNA per unit mass of the total RNA in each sample, which was (2.90±0.12) ×1010 (copy number/μg total RNA). From these results, the ranges of ratio of miRNA copy number to U6snRNA copy number in normal human subject blood were also derived: miR-150: 4.71~115.88×10-6; miR-23a: 9.29~56.78× 10-3; miR-130a: 7.01~45.72×10-6. Below the normal reference ratio range lower limit would be considered as a reference diagnostic criteria for GC.
Discussion
Although the incidence of gastric cancer has steadily declined in past decades, gastric cancer is the second leading cause of death from cancer worldwide [20,21]. Evidence has indicated that environmental factors such as Helicobacter pylori (H. pylori) colonization, cigarette smoking, and diet may play an important role in gastric carcinogenesis [22,23]. Metastasis is the most terrible aspects of cancer and has been studied for more than 100 years [24]. In gastric cancer, the high mortality mainly attributes to delayed diagnosis because of the lack of specific symptoms in early stage. And metastasis is responsible for the gastric cancer-related mortality [25]. Migration and invasion of cancer cells are essential processes during cancer metastatic procession which consists of a series of interrelated steps, including proliferation, detachment, circulation, transport, arrest in organs, adherence to vessel wall, extravasation, establishment of a microenvironment, and proliferation in distant organs. In gastric cancer, cells invasion into the surrounding tissue is a crucial early step [26]. However, the mechani- sms of gastric cancer cells migration, invasion and metastasis have not been fully understood.
MiRNA regulates multiple processes including growth, differentiation and apoptosis, in tissues and cells [27,28]. Their abnormal expression altered many of those processes, specifically resulting in the cancer development. Many miRNAs with known function are involved in cell proliferation, apoptosis, differentiation, and migration, which are commonly implicated in cancer development [29]. The increasing amounts of data have shown that miRNAs can act as oncogenes or tumor suppressors and participate in cancer development and progression [30].
Studies on miRNA and tumors started with large-scale screening of miRNA microarray to detect the differentially expressed miRNAs between tumor tissues and normal tissues aiming at developing new strategies in tumor diagnosis and treatment in clinic [31,32]. For instance, in human acute lymphocytic leukemia, the expression levels of miR-15a and miR-16-1 are decreased [33]; in lung cancer, let-7 is down-regulated whereas miR-17-92 is up-regulated [34]; in Burkitt’s lymphoma, expression level of miR-155 is increased; in esophageal cancer, miR-103 and miR-107 expression levels are increased [35]; in liver cancer, miR-21 expression level is increased [23,36]. The important roles of those miRNAs with altered expression have further been revealed, which forms the basis of their applications in clinical tumor treatments [37].
MiRNAs have their own characteristics and advantages in tumor diagnosis and treatment [32-34]. They are relatively small, and present in low amounts with higher detection sensitivities compared with the traditional protein assays, therefore suitable for establishing rapid and convenient clinical test methods [35,36]. In treatment, the replacement of missing miRNA using gene therapy has achieved promising results in mouse model. Meanwhile, injection of anti-sense oligonucleotides targeting the abnormally activated miRNA has also been found to be significantly effective [32]. Currently, the interaction between miRNA and tumor is a hot topic in tumor research, representing a completely new research field [37]; however, our understanding about it is still poor. Further in-depth investigations about the regulatory mechanisms of miRNA in tumor development and progression can provide novel strategies and approaches for tumor diagnosis and treatments in clinic, which will greatly contribute to the prevention of tumor occurrence [38-40].
In view of all these advances, we first utilized the miRNA microarray screening to identify a number of up- or down-regulated miRNA molecules relevant to human GC (normal human subjects as control); the northern blotting further confirmed a group of miRNAs with altered expression in GC patients, which included 6 miRNA with significantly increased expression (such as miR-17, miR-106a and miR-20a) and 19 miRNAs with significantly decreased expression (such as miR-23a, miR-150, miR-130a and let-7). miR-17 and miR-20a belong to the same miRNA family, which has been implicated in cancer development as oncogenes and displayed elevated levels of expression in variable types of tumors. Similar mechanisms might occur to GC. A loss of q arm of chromosome 7 is common in GC patients and the molecular effects of this loss are unclear. Therefore, it stands to reason that the decreased expression levels of miRNAs mapped to this region might be one of the causes that lead to leukemia. Let-7 family is consisting of many members, including let-7a, let-7b, let-7c, let-7d, and let-7f, all of which displayed decreased expression. It is been confirmed that the common target gene of let-7 family is RAS, which has been shown to be involved in many malignant tumors, including GC. The down-regulated let-7 in GC suggested its possible roles. The predicted target genes of miR-23a and miR-130a included many GC relevant oncogenes including SALL4, RAS and HOXA9, further indicating their importance in GC.
Then we focused on 3 miRNAs: miR-23a, miR-150 and miR-130a, with further exploration of their functioning mechanisms and potential as GC clinical diagnostic markers and drug targets. We performed qRT-PCR analysis and found that their expression levels were significantly decreased in 38 GC patients from our pre-diagnosis compared with normal controls. The ROC curves showed that the expression levels of miR-23a and miR-130a could serve as diagnostic markers with high accuracy in clinic and the results were statistically significant (P < 0.01). We also determined the miRNA levels in normal human subject using absolute method and the values could be used as references in clinical diagnosis.
All these results indicated that those 3 miRNAs could act as tumor suppressor and get involved in GC development, and their decreased expression might be one of the causes of leukemia. Accordingly, clarifying their regulatory mechanisms might delineate their potentials as drug targets of gene therapy for GC.
Disclosure of conflict of interest
None.
References
- 1.Kim HP, Cho GA, Han SW, Shin JY, Jeong EG, Song SH, Lee WC, Lee KH, Bang D, Seo JS, Kim JI, Kim TY. Novel fusion transcripts in human gastric cancer revealed by transcriptome analysis. Oncogene. 2013;33:5434–41. doi: 10.1038/onc.2013.490. [DOI] [PubMed] [Google Scholar]
- 2.Marquitz AR, Mathur A, Chugh PE, Dittmer DP, Raab-Traub N. Expression profile of microRNAs in Epstein-Barr virus-infected AGS gastric carcinoma cells. J Virol. 2014;88:1389–93. doi: 10.1128/JVI.02662-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim JY, Yoon SO, Seol SY, Hong SW, Kim JW, Choi SH, Lee JS, Cho JY. Overexpression of miR-196b and HOXA10 characterize a poor-prognosis gastric cancer subtype. World J Gastroenterol. 2013;19:7078–88. doi: 10.3748/wjg.v19.i41.7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X, Li X, Tan Z, Liu X, Yang C, Ding X, Hu X, Zhou J, Xiang S, Zhou C, Zhang J. MicroRNA-373 is upregulated and targets TNFAIP1 in human gastric cancer, contributing to tumorigenesis. Oncol Lett. 2013;6:1427–1434. doi: 10.3892/ol.2013.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eto K, Iwatsuki M, Watanabe M, Ida S, Ishimoto T, Iwagami S, Baba Y, Sakamoto Y, Miyamoto Y, Yoshida N, Baba H. The microRNA-21/PTEN pathway regulates the sensitivity of HER2-positive gastric cancer cells to trastuzumab. Ann Surg Oncol. 2014;21:343–50. doi: 10.1245/s10434-013-3325-7. [DOI] [PubMed] [Google Scholar]
- 6.Kim O, Yoon JH, Choi WS, Ashktorab H, Smoot DT, Nam SW, Lee JY, Park WS. GKN2 contributes to the homeostasis of gastric mucosa by inhibiting GKN1 activity. J Cell Physiol. 2014;229:762–71. doi: 10.1002/jcp.24496. [DOI] [PubMed] [Google Scholar]
- 7.Guo SL, Ye H, Teng Y, Wang YL, Yang G, Li XB, Zhang C, Yang X, Yang ZZ, Yang X. Akt-p53-miR-365-cyclin D1/cdc25A axis contributes to gastric tumorigenesis induced by PTEN deficiency. Nat Commun. 2013;4:2544. doi: 10.1038/ncomms3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fei B, Wu H. MiR-378 inhibits progression of human gastric cancer MGC-803 cells by targeting MAPK1 in vitro. Oncol Res. 2012;20:557–64. doi: 10.3727/096504013X13775486749254. [DOI] [PubMed] [Google Scholar]
- 9.Xu X, Yang X, Xing C, Zhang S, Cao J. miRNA: The nemesis of gastric cancer (Review) Oncol Lett. 2013;6:631–641. doi: 10.3892/ol.2013.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noto JM, Piazuelo MB, Chaturvedi R, Bartel CA, Thatcher EJ, Delgado A, Romero-Gallo J, Wilson KT, Correa P, Patton JG, Peek RM Jr. Strain-specific suppression of microRNA-320 by carcinogenic Helicobacter pylori promotes expression of the antiapoptotic protein Mcl-1. Am J Physiol Gastrointest Liver Physiol. 2013;305:G786–96. doi: 10.1152/ajpgi.00279.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong J, Li J, Wang Y, Liu C, Jia H, Jiang C, Wang Y, Luo M, Zhao H, Dong L, Song W, Wang F, Wang W, Zhang J, Yu J. Characterization of microRNA-29 family expression and investigation of their mechanistic roles in gastric cancer. Carcinogenesis. 2014;35:497–506. doi: 10.1093/carcin/bgt337. [DOI] [PubMed] [Google Scholar]
- 12.Qi YJ, Cui S, Yang YZ, Han JQ, Cai BJ, Sheng CF, Ma Y, Wuren T, Ge RL. Excision repair cross-complementation group 1 codon 118 polymorphism, micro ribonucleic acid and protein expression, clinical outcome of the advanced gastric cancer response to first-line FOLFOX-4 in Qinghai-Tibetan plateau population. J Cancer Res Ther. 2013;9:410–5. doi: 10.4103/0973-1482.119319. [DOI] [PubMed] [Google Scholar]
- 13.Tong F, Cao P, Yin Y, Xia S, Lai R, Liu S. MicroRNAs in gastric cancer: from benchtop to bedside. Dig Dis Sci. 2014;59:24–30. doi: 10.1007/s10620-013-2887-3. [DOI] [PubMed] [Google Scholar]
- 14.Fang Y, Shen H, Li H, Cao Y, Qin R, Long L, Zhu X, Xie C, Xu W. miR-106a confers cisplatin resistance by regulating PTEN/Akt pathway in gastric cancer cells. Acta Biochim Biophys Sin (Shanghai) 2013;45:963–72. doi: 10.1093/abbs/gmt106. [DOI] [PubMed] [Google Scholar]
- 15.Wu XJ, Mi YY, Yang H, Hu AK, Li C, Li XD, Zhang QG. Association of the hsa-mir-499 (rs3746444) polymorphisms with gastric cancer risk in the Chinese population. Onkologie. 2013;36:573–6. doi: 10.1159/000355518. [DOI] [PubMed] [Google Scholar]
- 16.Shiotani A, Murao T, Kimura Y, Matsumoto H, Kamada T, Kusunoki H, Inoue K, Uedo N, Iishi H, Haruma K. Identification of serum miRNAs as novel non-invasive biomarkers for detection of high risk for early gastric cancer. Br J Cancer. 2013;109:2323–30. doi: 10.1038/bjc.2013.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoon JH, Choi YJ, Choi WS, Nam SW, Lee JY, Park WS. Functional analysis of the NH2-terminal hydrophobic region and BRICHOS domain of GKN1. Biochem Biophys Res Commun. 2013;440:689–95. doi: 10.1016/j.bbrc.2013.09.123. [DOI] [PubMed] [Google Scholar]
- 18.Iwaya T, Fukagawa T, Suzuki Y, Takahashi Y, Sawada G, Ishibashi M, Kurashige J, Sudo T, Tanaka F, Shibata K, Endo F, Katagiri H, Ishida K, Kume K, Nishizuka S, Iinuma H, Wakabayashi G, Mori M, Sasako M, Mimori K. Contrasting expression patterns of histone mRNA and microRNA 760 in patients with gastric cancer. Clin Cancer Res. 2013;19:6438–49. doi: 10.1158/1078-0432.CCR-12-3186. [DOI] [PubMed] [Google Scholar]
- 19.Zhao H, Wang Y, Yang L, Jiang R, Li W. MiR-25 promotes gastric cancer cells growth and motility by targeting RECK. Mol Cell Biochem. 2014;385:207–13. doi: 10.1007/s11010-013-1829-x. [DOI] [PubMed] [Google Scholar]
- 20.Cao W, Yang W, Fan R, Li H, Jiang J, Geng M, Jin Y, Wu Y. miR-34a regulates cisplatin-induce gastric cancer cell death by modulating PI3K/AKT/survivin pathway. Tumour Biol. 2014;35:1287–95. doi: 10.1007/s13277-013-1171-7. [DOI] [PubMed] [Google Scholar]
- 21.Shen J, Niu W, Zhou M, Zhang H, Ma J, Wang L, Zhang H. MicroRNA-410 Suppresses Migration and Invasion by Targeting MDM2 in Gastric Cancer. PLoS One. 2014;9:e104510. doi: 10.1371/journal.pone.0104510. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.McLean MH, El-Omar EM. Genetics of gastric cancer. Nat Rev Gastroenterol Hepatol. 2014 doi: 10.1038/nrgastro.2014.143. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Shin VY, Chu KM. MiRNA as potential biomarkers and therapeutic targets for gastric cancer. World J Gastroenterol. 2014;20:10432–10439. doi: 10.3748/wjg.v20.i30.10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu Z, Qian F, Yang X, Jiang H, Chen Y, Liu S. Circulating miR-222 in plasma and its potential diagnostic and prognostic value in gastric cancer. Med Oncol. 2014;31:164. doi: 10.1007/s12032-014-0164-8. [DOI] [PubMed] [Google Scholar]
- 25.Toiyama Y, Okugawa Y, Goel A. DNA methylation and microRNA biomarkers for noninvasive detection of gastric and colorectal cancer. Biochem Biophys Res Commun. 2014 doi: 10.1016/j.bbrc.2014.08.001. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xia T, Liao Q, Jiang X, Shao Y, Xiao B, Xi Y, Guo J. Long noncoding RNA associated-competing endogenous RNAs in gastric cancer. Sci Rep. 2014;4:6088. doi: 10.1038/srep06088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhai R, Kan X, Wang B, Du H, Long Y, Wu H, Tao K, Wang G, Bao L, Li F, Zhang W. miR-152 suppresses gastric cancer cell proliferation and motility by targeting CD151. Tumour Biol. 2014;35:11367–73. doi: 10.1007/s13277-014-2471-2. [DOI] [PubMed] [Google Scholar]
- 28.Zhu M, Zhang N, He S. Similarly up-regulated microRNA-106a in matched formalin-fixed paraffin-embedded and fresh frozen samples and the dynamic changes during gastric carcinogenesis and development. Pathol Res Pract. 2014;210:909–15. doi: 10.1016/j.prp.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 29.Du Y, Wang D, Luo L, Guo J. miR-129-1-3p Promote BGC-823 Cell Proliferation by Targeting PDCD2. Anat Rec (Hoboken) 2014;297:2273–9. doi: 10.1002/ar.23003. [DOI] [PubMed] [Google Scholar]
- 30.Shang H, Wang T, Shang F, Huang KM, Li YQ. A germline mutation in the miR-125a coding region reduces miR-125a expression and is associated with human gastric cancer. Mol Med Rep. 2014;10:1839–44. doi: 10.3892/mmr.2014.2441. [DOI] [PubMed] [Google Scholar]
- 31.Duan JH, Fang L. MicroRNA-92 promotes gastric cancer cell proliferation and invasion through targeting FXR. Tumour Biol. 2014;35:11013–9. doi: 10.1007/s13277-014-2342-x. [DOI] [PubMed] [Google Scholar]
- 32.Wu K, Yang L, Li C, Zhu CH, Wang X, Yao Y, Jia YJ. MicroRNA-146a enhances helicobacter pylori induced cell apoptosis in human gastric cancer epithelial cells. Asian Pac J Cancer Prev. 2014;15:5583–6. doi: 10.7314/apjcp.2014.15.14.5583. [DOI] [PubMed] [Google Scholar]
- 33.Zhao X, He L, Li T, Lu Y, Miao Y, Liang S, Guo H, Bai M, Xie H, Luo G, Zhou L, Shen G, Guo C, Bai F, Sun S, Wu K, Nie Y, Fan D. SRF expedites metastasis and modulates the epithelial to mesenchymal transition by regulating miR-199a-5p expression in human gastric cancer. Cell Death Differ. 2014;21:1900–13. doi: 10.1038/cdd.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang HB, Yang TJ, Lu P, Ma YJ. Gene expression profiling of gastric cancer. Eur Rev Med Pharmacol Sci. 2014;18:2109–15. [PubMed] [Google Scholar]
- 35.Li R, Yuan W, Mei W, Yang K, Chen Z. MicroRNA 520d-3p inhibits gastric cancer cell proliferation, migration, and invasion by downregulating EphA2 expression. Mol Cell Biochem. 2014;396:295–305. doi: 10.1007/s11010-014-2164-6. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Z, Li Z, Li Y, Zang A. MicroRNA and signaling pathways in gastric cancer. Cancer Gene Ther. 2014;21:305–16. doi: 10.1038/cgt.2014.37. [DOI] [PubMed] [Google Scholar]
- 37.Xu Y, Jin J, Liu Y, Huang Z, Deng Y, You T, Zhou T, Si J, Zhuo W. Snail-Regulated MiR-375 Inhibits Migration and Invasion of Gastric Cancer Cells by Targeting JAK2. PLoS One. 2014;9:e99516. doi: 10.1371/journal.pone.0099516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naito Y, Yasuno K, Tagawa H, Sakamoto N, Oue N, Yashiro M, Sentani K, Goto K, Shinmei S, Oo HZ, Yanagihara K, Hirakawa K, Yasui W. MicroRNA-145 is a potential prognostic factor of scirrhous type gastric cancer. Oncol Rep. 2014;32:1720–6. doi: 10.3892/or.2014.3333. [DOI] [PubMed] [Google Scholar]
- 39.Park D, Lee SC, Park JW, Cho SY, Kim HK. Overexpression of miR-17 in gastric cancer is correlated with proliferation-associated oncogene amplification. Pathol Int. 2014;64:309–14. doi: 10.1111/pin.12178. [DOI] [PubMed] [Google Scholar]
- 40.Li BS, Zuo QF, Zhao YL, Xiao B, Zhuang Y, Mao XH, Wu C, Yang SM, Zeng H, Zou QM, Guo G. MicroRNA-25 promotes gastric cancer migration, invasion and proliferation by directly targeting transducer of ERBB2, 1 and correlates with poor survival. Oncogene. 2014 doi: 10.1038/onc.2014.214. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


