Abstract
The IRF (Interferon Regulatory Factor) family member IRF-8 participates in transcriptional activation of ISRE (Interferon Stimulated Response Element) or GAS (Gamma interferon Activation Site) elements containing gene promotors, in response to IFN-γ. To test the role of IRF-8 in host defenses against tuberculosis, BXH-2 mice which bear a defective IRF-8R294C allele, were challenged with low-doses of virulent Mycobacterium tuberculosis via the intravenous and aerosol routes. BXH-2 mice were found to be extremely susceptible to M. tuberculosis, as demonstrated by rapid and uncontrolled microbial replication in spleen, liver and lungs leading to very early death. The BXH-2 defect was expressed very early (10 days post-infection) as uncontrolled intracellular pathogen replication in Nos2 expressing lung macrophages, impaired granuloma formation, rapid dissemination of the infection to distant sites, and rapid necrosis of infected tissues. There was complete absence of IL-12p40 induction, severely reduced IFN-γ production, and impaired T cell priming in the lungs of infected BXH-2, highlighting the critical role of IRF-8 in this process. Collectively, these results identify IRF-8 as a critical regulator of host defenses against TB.
Keywords: rodent, bacterial, monocytes/macrophages, gene regulation, lung
Introduction
Pulmonary tuberculosis (TB) remains a global health problem of enormous proportions. It is estimated that one third of the world population has been exposed to, or carry the pathogen (1), with approximately 8 million new cases of active disease per year (2) with 1-1.5 million death annually. The encounter of Mycobacterium tuberculosis with its human host may have different outcomes, ranging from asymptomatic infection, to severe progressive pulmonary or extra-pulmonary TB. Humans can remain healthy carriers for years but may develop TB, following a process known as reactivation. The relatively low fraction of individuals exposed to M. tuberculosis that go on to develop active TB suggest that humans possess robust innate and immune host defenses against this pathogen. However, the nature of such protective immune defenses and the reason why their failure leads to active TB or long-term persistence of M. tuberculosis in mononuclear phagocytes remain poorly understood. It has been established that both virulence factors of M. tuberculosis, and genetic determinants of the host contribute to the onset, progression and ultimate outcome of disease (3).
Briefly, studies in humans and in animal models suggest that M. tuberculosis transmitted via the aerosol route, is taken up by lung mononuclear phagocytes where it survives intracellularly by inhibiting phagosome maturation (4). Infected phagocytes (macrophages, monocytes, dendritic cells) migrate to peripheral lymph nodes to prime T-cells, which then migrate to the lungs to initiate the formation of characteristic multicellular structures within infected-lung tissue called granulomas, which are composed of macrophages, lymphocytes and epithelial cells. Macrophages secrete IL-12 and IL-23 (heterodimeric cytokines composed of a common p40 subunit and unique p35 and p19 subunits, respectively) to activate T lymphocyte response (polarization of Th1 phenotype). CD4+ and CD8+ T cells recognize bacterial antigens presented by phagocytes in association with Class II and Class I MHC molecules, respectively, and secrete type I cytokines, most importantly IFN-γ and TNF-α that result in macrophage activation, and enhanced bactericidal activity (5). The formation and maintenance of granulomas, and the production of protective type I cytokines play a central role in containing M. tuberculosis infection. The critical role of IL-12 and IFN-γ in protective host response is highlighted by the observation that mutant mice deficient in the p40 component (lack both IL-12 and IL-23) or in IFN-γ are highly susceptible to M. tuberculosis infection (6-9). Sustained production of IL-12 throughout the infection is necessary but not sufficient to maintain antibacterial immunity in the host (9). Moreover, the absence of IL-12 alone (p35 subunit mutant) is not as detrimental to the host for response to M. tuberculosis as the absence of p40 (10). Finally, the study of the Mendelian susceptibility to mycobacterial diseases (MSMD) syndrome (MIM 209950) which is associated with severe clinical disease in otherwise healthy individuals caused by weakly virulent mycobacterial species, such as BCG (bacilli Calmette-Guérin) vaccines and non-tuberculous, environmental mycobacteria (EM), as well as virulent species M. tuberculosis, has identified loss of function mutations in the IFN-γ receptors 1 and 2, IL12-p40, IL12 receptor β1, STAT1 and NEMO (reviewed in (11)).
Epidemiological data including geographical distribution, familial aggregation, and twin studies have suggested that a complex set of additional genetic factors may play a role in the outcome of infection with M. tuberculosis (3). Such genetic effects may reflect modulation of important proteins and biochemical pathways required for effective defenses against M. tuberculosis. The complex genetic component of susceptibility to TB in humans has been extensively studied in mouse models, using either virulent M. tuberculosis or avirulent M. bovis (BCG), as infectious agents. Differential susceptibility to M. tuberculosis in inbred mouse strains is genetically complex, and phenotypically expressed as different rates of pulmonary microbial replication, distinct histopathology, robustness of inflammatory response in situ, and survival time. The high susceptibility of C3HeB/FeJ mice to pulmonary TB (sst1 locus; super-susceptibility tuberculosis) has been shown to be caused by rearrangement in the Ipr1 (Intracellular pathogen resistance 1) gene that codes for a protein (Ifi75; interferon-induced protein 75) regulating apoptotic response of macrophage following phagocytosis of M. tuberculosis (12). In addition, the study of differential susceptibility of strain pairs DBA/2 (S):C57Bl/6J (R) and I/St (S):A/Sn (R) to pulmonary TB has led to the mapping of multiple Trl (Trl1-4; Tuberculosis Resistance Loci) and Tbs (Tbs1-2; Tuberculosis Severity) loci, respectively (13-15). On the other hand, differential susceptibility of inbred mouse strains to infection with less virulent mycobacteria including M. bovis (BCG) is caused by a two alleles system at the Nramp1/Slc11a1 gene (Nramp1G169, R; Nramp1D169, S), that codes for a metal efflux pump (e.g., Mn2+, Fe2+ and Zn2+) expressed at the phagosomal membrane (16, 17).
BXH-2 is a recombinant inbred mouse strain derived from C3H/HeJ (C3H) and C57BL/6J (B6) (18). Despite a C3H-derived resistance Nramp1G169 allele, BXH-2 is susceptible to infection with M. bovis (BCG), suggesting the presence of a genetic modifier of Nramp1 fixed in this strain (19). In addition, BXH-2 mice develop by one year of age a progressive and fatal myeloid leukemia caused by replication and genomic insertions of a B-ecotropic murine leukemia virus, suggesting a further defect in viral immune surveillance in these animals (20-23). We previously showed that both phenotypes are caused by a mutation in the Icsbp1 (interferon consensus sequence–binding protein 1) gene, also known as interferon regulatory factor 8 (IRF-8) (19). Members of the IRF family of transcriptional co-activators are composed of an N-terminal DNA binding domain and a C-terminal IRF association domain (IAD). IRF-8 acts as a co-activator with IRF-1 to stimulate transcription of IFN-γ responsive genes that bear an ISRE (Interferon Stimulated Response Element) sequence element in their regulatory regions, including IL12-p40, but can also act as a co-repressor with IRF-2 to antagonize IRF-1 dependent transcriptional activation. IRF-8 can further heterodimerize with PU.1 and other Ets proteins to activate transcription of genes containing GAS (gamma interferon activation site) or EICE (Ets/IRF composite element) promotor elements, including Igκ, p67phox, p91phox, CD20, IL1, Tlr4, and members of the macrophage scavenger receptor family (for review, see Levi et al. (24)). BXH-2 mice carry a R294C mutation within the predicted IRF-association domain of the protein. The R294C allele is associated with a complete failure of BXH-2 splenocytes to produce IL-12 and IFN-γ in vitro in response to activating stimuli (19). In vivo, homozygosity for the C294 allele at IRF-8 abrogates the protective effect of Nramp1G169 alleles against M. bovis (BCG) infection, with continuous growth in the spleen associated with absence of granuloma formation (19). In addition, the effect of the IRF-8R294C mutation appears pleiotropic as BXH-2 mice also show susceptibility to infection with unrelated pathogens such as Salmonella typhimurium and Plasmodium chabaudi (25).
We have evaluated the effect of the IRF-8R294C mutation on response of BXH-2 mice to intravenous and aerosol challenges with virulent M. tuberculosis. These experiments show extreme susceptibility of BXH-2 to M. tuberculosis, including uncontrolled replication, quick dissemination, and rapidly fatal disease. These findings identify IRF-8 as an important regulator of host defenses against TB.
Materials and Methods
Animals
C57BL/6J (B6) and C3H/HeJ (C3H) mice were from the Trudeau Institute (Saranac Lake, NY). Recombinant inbred BXH-2 mice were obtained from N. Copeland and N. Jenkin (National Cancer Institute, Frederick, MD) and subsequently maintained as a breeding colony at McGill University. All mice were housed under standard laboratory conditions and were free of common viral pathogens. Male and female mice 8 to 10 weeks of age were used for infection. All experimental procedures involving mice were approved by the Institutional Animal Care and Use Committee of the Trudeau Institute.
Infection with M. tuberculosis
M. tuberculosis strain H37Rv was obtained from the Trudeau Mycobacterial Culture Collection as a frozen (-70°C) stock dispersed in Proskauer and Beck medium (Difco) containing 0.01% Tween 80. A vial was thawed, subjected to 5 seconds ultrasound to break up aggregates, and diluted in PBS containing 0.01% Tween 80 for inoculation (104 colony-forming units; CFUs) via a lateral tail vein. Infection via the respiratory route (102 CFUs) was achieved by subjecting the mice to aerosolized bacilli in a Middlebrook airborne infection apparatus (Tri Instruments, Jamaica, NY). Bacilli were enumerated in major organs (lungs, spleen and liver) at different times after inoculation by preparing homogenates in PBS containing 0.05% Tween 80 and by plating 10-fold serial dilutions of the homogenates on Middlebrook 7H11 agar (Difco).
Microarray analysis
Total lung RNA (three individual samples per experimental group; 9 in total) from controls and from M. tuberculosis-infected (for 30 and 70 days) B6 mice were used for transcriptional profiling with Affymetrix oligos chips (Mouse Genome 430 2.0 array), according to the manufacturer's recommendations. To minimize technical variability, RNA processing steps (RNA extraction, probe labeling and microarray hybridization) were executed in parallel for all samples. The GeneSifter™ microarray data analysis system (VizX Labs, Seattle, WA, USA; www.genesifter.net) was used to analyze data generated from comparisons between control (uninfected) and M. tuberculosis-infected (Day 30 and 70) groups for B6 mice. Data were normalized by the robust multi-array average (RMA) algorithm. Ratios of expression (fold change in comparison to uninfected controls) were individually determined for B6 mice following 30 and 70 days of infection. All genes evaluated were statistically significant with a t test p value < 0.05 and a q value of 0.005. Complete microarray data (accession number: E-MEXP-1899) has been deposited in the ArrayExpress database (www.ebi.ac.uk/microarray-as/ae/).
mRNA expression studies by RT-PCR
Mice were sacrificed, and their lungs were rapidly homogenized in TRI REAGENT™ (SIGMA) using a polytron. Total RNA was extracted according to the manufacturer's recommendations, and RNA integrity was verified by electrophoresis on a 1% agarose gel containing formaldehyde. The expression of individual mRNAs was measured by PCR amplification of cDNA transcripts generated by reverse transcriptase (RT-PCR) as previously described (26). For PCR amplification (Taq DNA Polymerase; Invitrogen), 2.5% of the RT template was used with the following thermocycler parameters: initial denaturation step (3 min at 94°C), 25-30 amplification cycles (30 s at 94°C, 30 s at 55°C, 45 s at 72°C) and a final elongation step (7 min at 72°C). PCR primer pairs were designed according to the reported gene sequences for IRF-8, IFN-γ, IL-12p40, TNF-α, Nos2, and β-actin (internal control). PCR products were resolved by electrophoresis on 1.2% agarose gels containing ethidium bromide and were photographed under UV.
Determination of cytokine production
The serum levels of IFN-γ, IL-12(p70), TNF-α, MCP-1 and other cytokines (total of 13 tested) 30 days following M. tuberculosis infection were quantified using the Luminex xMAP technology (Milliplex™, Mouse Cytokine Panel – 13 Plex, Millipore; www.millipore.com/drugdiscovery). This technology simultaneously detects multiple analytes in a small sample volume (5-50 μl) and cross-reactivity between the antibodies and any of the other analytes in this panel is negligible.
Enumeration of CD4 and CD8 T cells by Flow cytometry and Elispot assay
Mice were euthanized, and their lungs were perfused with PBS containing 10 U/ml of heparin to remove intravascular leukocytes. The lungs were then perfused with an enzyme cocktail consisting of 150 U/ml of collagenase, 0.2 U/ml of elastase (Roche Applied Science) and 1 mg/ml of DNase (Sigma) in RPMI. The lungs were removed, diced into small fragments, subjected to further enzyme digestion before being mechanically disrupted to form a single cell suspension (27). Total lung cells were suspended in RPMI-FCS in two 5 ml tubes at 1 × 107/ml, and incubated with Brefeldin A (10μg/ml, 5 h at 37°C; Epicenter Technologies). They were then stained for flow cytometry with FITC-anti-CD3, R-phycoerythrin-anti-CD4, and Peridinin chlorophyll protein-anti-CD8 mAbs. After fixation overnight in 0.5% paraformaldehyde they were stained for intracellular IFN-γ with allophycocyanin-anti-IFN-γ mAb, as described previously (27), and analyzed by FACS (Calibur flow cytometer; BD Biosciences) using Cell Quest software (BD Biosciences). The total number of cells recovered from lungs (at day 15 and day 30) was very similar for all mouse strains (with a maximum difference of 1.9 fold seen for C3H lungs at day 15). The number of CD4+ cells and CD8+ cells in the lungs were also equivalent in all mouse strains. Changes in the total number of Ag-specific T cells in the lungs capable of making IFN-γ in response to M. tuberculosis antigens were determined using the Elispot assay (Mouse IFN-γ ELISPOT Set, BD Biosciences), according to the manufacturer's instructions (27, 28). The M. tuberculosis antigen preparation used to stimulate IFN-γ production was a sonicated extract of a M. tuberculosis culture (27). In all cases, the results were obtained with pooled lung cells from 4 mice per experimental group and measurements were performed in triplicates.
Immunocytochemistry
Lungs were fixed by intratracheal infusion of 10% buffered formalin followed by immersion in the same fixative for 24 h at room temperature. Livers were also fixed in 10% buffered formalin for 24 h. Lung lobes and livers were embedded in wax and sectioned according to standard procedures. Immunocytochemistry was performed to detect NOS2 in lung and liver sections, after which the sections were stained for acid-fast bacteria, as described previously (29). Briefly, immunocytochemistry involved reacting tissue sections with affinity-purified, monospecific rabbit anti-mouse NOS2 Ig, as the primary reagent, with biotinylated goat Ig anti-rabbit Ig as the second reagent, and with avidin-coupled biotinylated horse radish peroxidase with diaminobenzidine as the substrate to produce a brown reaction product. Sections were stained for acid-fast bacilli (30) and counterstained with methylene blue. Controls consisted of sections treated in the same way except that control rabbit Ig was substituted for rabbit anti-NOS2 Ig as the primary reagent. Photomicrography was performed with a Nikon Microphot-Fx microscope fitted with a Spot RT Slider camera (Diagnostic Instruments Inc.) using Spot RT Software for image acquisition.
Results
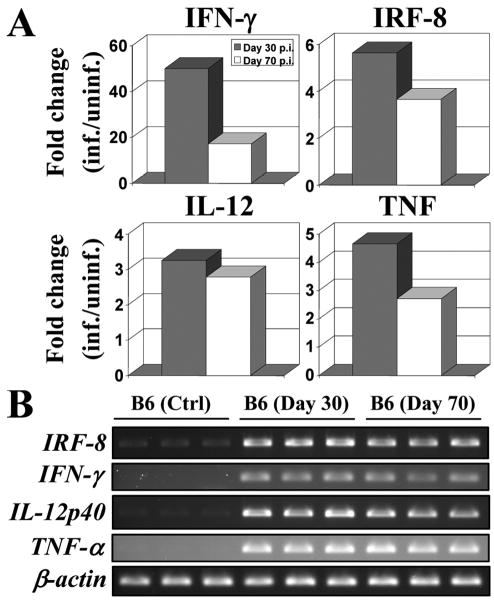
Following aerosol infection, pulmonary replication of M. tuberculosis in innately resistant (e.g. C57BL/6J; B6) and susceptible (e.g. DBA/2J; D2) mouse strains follows a biphasic course. During the first 30 days, M. tuberculosis replicates rapidly in the lungs of both types of mice. Subsequently, there is continuing microbial replication in susceptible strains accompanied by strong inflammatory response, severe tissue injury and premature death. By contrast, the infection is held stationary during that period (>30d) in resistant mice, a phenomenon associated with proficient granuloma formation, less severe pathology, and increased survival (5). To search for cell populations, physiological responses and biochemical pathways underlying protective anti-TB host responses in resistant B6 mice, we carried out transcriptional profiling analysis of normal and M. tuberculosis-infected lungs at different times following infection. We initially investigated day 30 lung RNAs (infected vs. non-infected), as resistant B6 mice start to restrict pulmonary bacterial replication at that time, and therefore are likely to express genes contributing to protection. Of the large number of 4,240 genes significantly regulated 30 days following M. tuberculosis infection (t test p value < 0.05, cutoff at 2-fold change; q value of 0.005), gene ontology analysis identified 232 genes associated with “immune response” (data not shown). A parallel gene list was generated from day 70 lung RNAs, a time point where resistant B6 mice still control infection, and therefore are likely to retain expression of protective genes. Comparison of the day 30 and day 70 gene lists revealed 63% overlap between the two in immune response-associated genes. Although this transcripts list included obvious actors such as IFN-γ, TNF, and IL-12 (IL-12p40), it also identified IRF-8 (Icsbp1) as a gene which pulmonary expression is up-regulated in response to, and throughout the course of M. tuberculosis infection (Fig. 1A). Up-regulation of IRF-8 (and IFN-γ, TNF, IL-12p40) in response to M. tuberculosis exposure was validated by RT-PCR (Fig. 1B). The observed induction of IRF-8 mRNA in response to M. tuberculosis infection together with the previously noted susceptibility of BXH-2 mice to infection with attenuated M. bovis (BCG) (25), suggested that IRF-8 may play a critical role in host defenses against M. tuberculosis.
Figure 1. IRF-8, IFN-γ, IL-12, and TNF gene expression following aerosol infection (30 and 70 days) with M. tuberculosis.
(A) Genes (IRF-8, IFN-γ, IL-12, and TNF) expression ratios (fold change in comparison to uninfected controls) for C57BL/6J (B6) mice 30 (grey bars) and 70 (white bars) days following infection. A ratio of expression with a relative unit of 1 indicates no gene modulation. All genes evaluated were statistically significant with a t test p value < 0.05 and a q value of 0.005. (B) RNA samples (prepared from three mice separately) from uninfected control and M. tuberculosis infected lungs of B6 mice were used for RT-PCR amplification using 25-30 PCR cycles. β-actin mRNA expression was used as an internal control. All PCR products were analyzed by agarose gel electrophoresis.
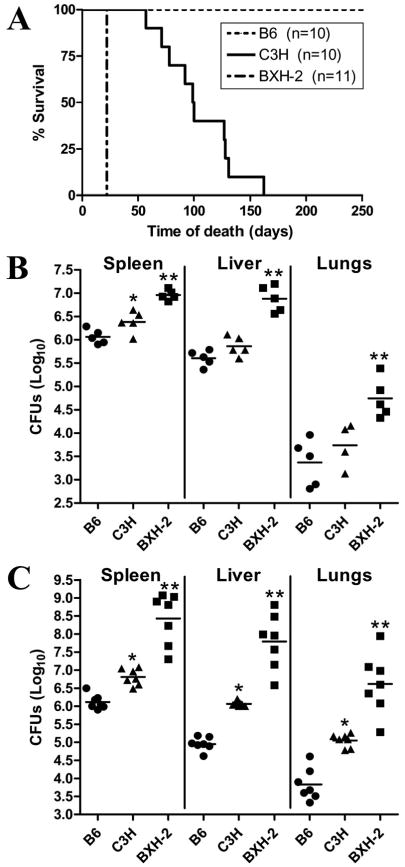
This was directly tested by infecting IRF-8-deficient BXH-2 mice (IRF-8R294C) with M. tuberculosis via different routes, and by following the infection with respect to microbial replication in different organs, cytokine response, type of pathology developed and survival time. In these experiments, innately resistant C57BL/6J (B6, IRF-8R294) and susceptible C3H/HeJ (C3H, IRF-8R294) mice were used as parental controls for BXH-2 (IRF-8C294). In a first set of experiments, mice were infected intravenously (i.v) with low-dose (104 CFUs) of M. tuberculosis H37Rv, and survival to infection was monitored. At this infectious dose, all B6 animals survived the 250 days observation period, while susceptible C3H controls showed a mean survival time (MST) of approximately 100 days, with all animals succumbing from infection by day 160. Surprisingly, there was uniform and precipitous death in BXH-2, with all animals succumbing within 22 days following i.v infection, suggesting extreme susceptibility of these animals to M. tuberculosis (Fig. 2A). In a second set of experiments, bacterial replication following i.v infection (104 CFUs) was monitored at day 10 and day 19 in spleen, liver and lungs in two independent experiments (Fig. 2, B and C). At day 10 (and day 19), significantly greater numbers of bacilli were recovered from liver and spleen of all groups compared to lungs, in agreement with published observations that only 0.1% of M. tuberculosis introduced i.v implants in the lungs, compared to 99% in liver and spleen (5). Although CFU counts were comparable in all organs in B6 and C3H at this early time point (day 10), BXH-2 mice already showed a significant increase (∼1-1.5 log) in microbial replication in the three organs (Fig. 2B). At day 19 post-infection (Fig. 2C), a modest but notable 1 log increase in CFU counts was detected in organs of permissive C3H mice compared to resistant B6 controls. However, over the same period, there was a dramatic 3 logs increase (7-8.5 logs total) in M. tuberculosis load in the spleen, liver and lungs of BXH-2 mice. These early results indicate that BXH-2 mice are hyper-susceptible to infection with M. tuberculosis. Loss of IRF-8 function in BXH-2 is expressed very early following infection as impaired innate response to the pathogen, associated with uncontrolled M. tuberculosis replication in all organs, leading to precipitous death of the animals.
Figure 2. Effect of the IRF-8R294C mutation on replication of M. tuberculosis and on survival following infection via the intravenous route.
B6, C3H, and BXH-2 mice were infected i.v with 104 M. tuberculosis H37Rv, and survival was monitored during 250 days (A). The number of mice in each group is indicated (n). B6, C3H, and BXH-2 mice were infected i.v with 104 M. tuberculosis H37Rv and the numbers of M. tuberculosis bacilli were enumerated in the spleen, the liver, and lungs (log10 CFU) at 10 days (B) and 19 days (C) post-infection. Horizontal bars represent means of CFU counts for each group. Asterisks indicate that the number of CFU counts between B6 and C3H (*) and between C3H and BXH-2 (**) are statistically significant (P<0.05, unpaired Student's t test).
In a third set of experiments, BXH-2 mice (and B6 and C3H controls) were infected by the aerosol route with a low inoculum of M. tuberculosis (102 CFUs), and the extent of microbial replication at day 45 post-infection was assessed in the lungs, liver and spleen (Fig. 3B). Day 45 was selected arbitrarily, as the day following the first death recorded in the infected BXH-2 group. In control B6 and C3H mice, there was active and similar level of M. tuberculosis replication in the lungs (∼6.5 log), with additional dissemination and replication in liver (4-4.5 logs) and spleen (4.5-5 logs) both accounting for less than 1-5% of total microbial loads in these mice. The picture was strikingly different in BXH-2: Not only was there superior pulmonary M. tuberculosis replication (∼7.5 logs) compared to controls (∼ 6.5 logs), but massive bacterial loads were also detected in BXH-2 livers (∼7 logs) and spleens (∼7 logs), 100 to 1000 times larger than those seen in controls. The same experiment was repeated, but this time bacterial counts were monitored in spleen, liver and lungs at an earlier 30 days time point (Fig. 3A). Very similar results were obtained, with BXH-2 mice showing spleen and liver CFU counts at least 10-50 times higher than those monitored in control B6 and C3H mice (Fig. 3A). These results suggest that loss of IRF-8 function in BXH-2 impairs not only their ability to control microbial replication at the site of infection, but also their capacity to contain the infection at the entry point resulting in rapid dissemination to distant sites.
Figure 3. Effect of the IRF-8R294C mutation on replication of M. tuberculosis following infection via the aerosol route.
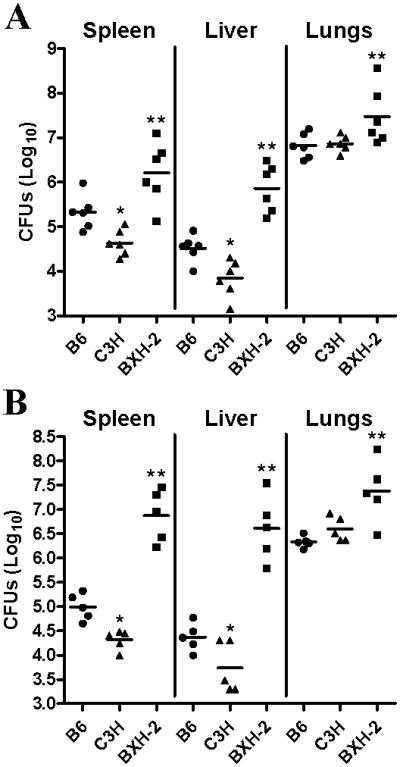
B6, C3H, and BXH-2 mice were infected via the aerosol route with 102 M. tuberculosis H37Rv, and the numbers of M. tuberculosis were enumerated in the spleen, the liver, and lungs (log10 CFU) at 30 days (A) and 45 days (B) post-infection. Horizontal bars represent means of CFU counts for each group. Asterisks indicate that the number of CFU counts between B6 and C3H (*) and between C3H and BXH-2 (**) are statistically significant (P<0.05, unpaired Student's t test).
An intact IFN-γ/IL-12 cytokine loop is required for granuloma formation in vivo, a key protective response to M. tuberculosis infection. IL-12 (and to a certain extent, IL-23 and IL-27) stimulates IFN-γ production. IFN-γ stimulates expression of genes containing ISRE promotor elements (including IRF-8) and is also critical for the expression of a number of protective responses including effector molecules and bactericidal enzymes synthesized by cells within the granuloma (31). To try to better understand what aspect of anti-tuberculous host response is impaired in BXH-2 and hence directed by IRF-8, we monitored the level of mRNA expression in infected lungs of certain genes known to play a role in protection against TB, including IL-12p40, IFN-γ, TNF-α, NOS2, and IRF-8 itself, in the lungs of mutant and control mice prior to and following M. tuberculosis infection (day 45) (Fig. 4). There was robust induction of IFN-γ, TNF-α, and NOS2 mRNA expression in infected lungs of BXH-2, as well as B6 and C3H controls, suggesting that this normal response was not affected by the absence of functional IRF-8. IRF-8 expression was elevated in response to M. tuberculosis infection in all mouse strains, although the endogenous level of non-functional IRF-8R294C mRNA was somewhat elevated in BXH-2 (possible compensatory mechanism in response to a non-functional protein). This suggests that transcriptional activation of target genes such as IRF-8 and NOS2 is intact in all strains. However, although infected lungs from B6 and C3H mice showed robust IL-12p40 mRNA up-regulation following M. tuberculosis infection, there was a complete absence of IL-12p40 induction in BXH-2 (Fig. 4). Similar RNA expression profiles were also detected at an earlier day 30 time point (data not shown).
Figure 4. IRF-8, IL-12p40, IFN-γ, Nos2, and TNF-α mRNA expression in lungs following aerosol infection with M. tuberculosis.
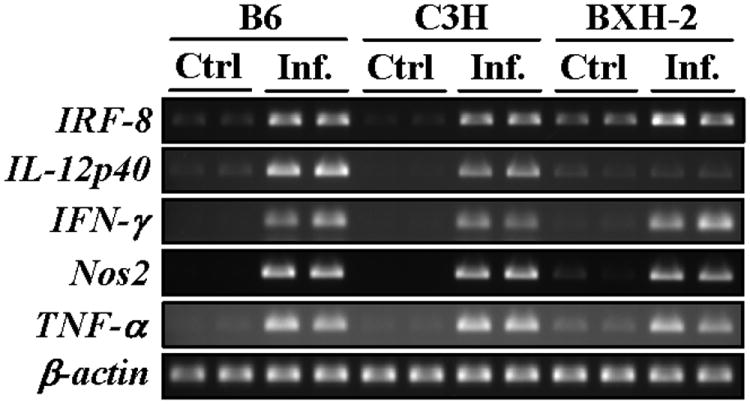
Total lung RNA (2 individual samples per experimental group; 12 in total) from controls and from M. tuberculosis infected B6, C3H, and BXH-2 mice (at day 45) was prepared and used for RT-PCR (25-30 cycles). β-actin mRNA expression was used as an internal control. All PCR products were analyzed by agarose gel electrophoresis.
The serum level of 13 cytokines, including IFN-γ, IL-12(p70), MCP-1 and TNF-α was monitored at day 30 post-infection in control and BXH-2 mice (Fig. 5, and data not shown). IFN-γ production was clearly detected in the serum of B6 (resistant), but not in the serum of either BXH-2 (super-susceptible) or C3H (susceptible) mice. Likewise, there was complete absence of IL-12(p70) production in BXH-2 mice, while low and robust levels of this key cytokine were detected in C3H and B6 controls (Fig. 5). On the other hand, the BXH-2 mice produced higher levels of MCP-1 (Monocyte Chemoattractant Protein-1) in response to infection than did B6 and C3H mice (Fig.5). This may reflect a compensatory inflammatory mechanism in response to the mounting bacterial loads rapid dissemination of infection in BXH-2. Finally, low levels of TNF-α (Tumor Necrosis Factor alpha) were equally detected in all 3 strains (Fig. 5). These results suggest that systemic production of IL-12(p70) and IFN-γ is impaired in BXH-2 mice.
Figure 5. Serum cytokine production by M. tuberculosis-infected B6, C3H, and BXH-2 mice.
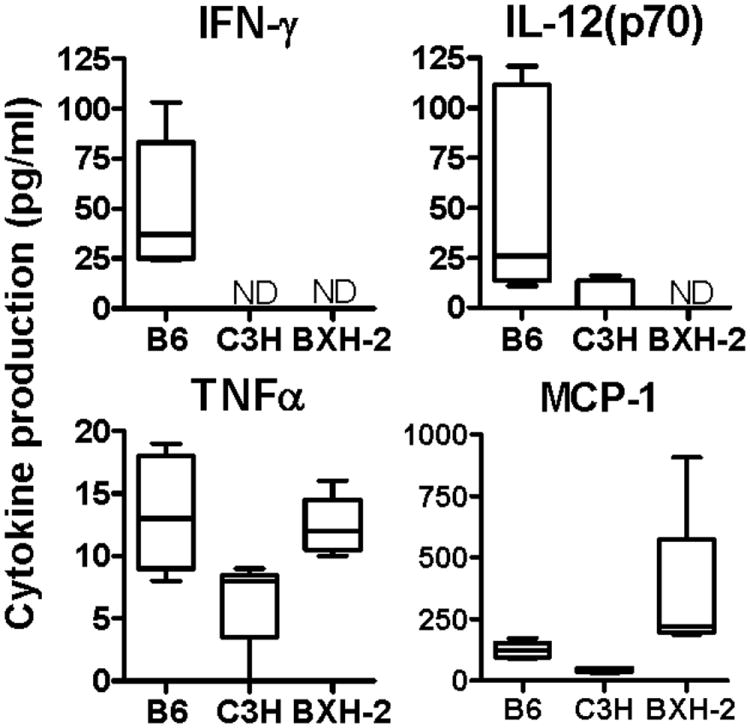
Mice were infected with 102 M. tuberculosis H37Rv by the aerosol route and serum cytokine production was evaluated at 30 days post-infection. Quantitation of serum IFN-γ, IL-12(p70), TNF-α, and MCP-1 was determined by a Milliplex™ assay. The results were obtained from 4-5 individual mice per experimental group. ND, non-detectable.
We also investigated the effect of loss of IRF-8 function on the type and extent of Th1 response during M. tuberculosis aerosol infection. In this experiment, lungs were harvested from a separate set of B6, C3H and BXH-2 infected animals and the number of IFN-γ producing CD4+ and CD8+ T cells were enumerated at 15 and 30 days post-infection by FACS analysis (Fig. 6A). In parallel, the capacity of these T cell populations to secrete IFN-γ in response to stimulation with M. tuberculosis antigens was also evaluated by Elispot assay using total lung cells (Fig. 6B). There was a very robust accumulation of IFN-γ producing CD4+ T cells in B6 lungs (7.8 × 104) at day 30, but significant numbers of these T cells were also detected in the lungs of infected C3H (1.8 × 104) and BXH-2 (1.5 × 104) mice (Fig. 6A, left axis). This IFN-γ producing CD4+ T cells response was stronger at day 30 than day 15 post-infection (Fig. 6A, left axis). Similar profiles of IFN-γ producing CD8+ T cells were also observed at day 30 post-infection, but overall numbers were lower than IFN-γ producing CD4+ T cells (Fig. 6A, right axis). However, when tested by Elispot assay, total lung cells isolated from B6 and C3H mice readily produced IFN-γ at detectable levels in response to M. tuberculosis antigens, while cells from BXH-2 mice produced amounts barely above background (7.8 and 14.5 times lower than for C3H and B6 cells, respectively) (Fig. 6B). These results suggest that in BXH-2, T cells produced during M. tuberculosis infection do not secrete IFN-γ when stimulated with M. tuberculosis antigens. Together, this suggest that a major consequence of loss of IRF-8 function in BXH-2 mice, which leads to super-susceptibility to M. tuberculosis, is associated with the absence of production/secretion of either IL-12p40 (IL-12p70) or IFN-γ or both, in response to infection. The severely reduced or absent levels of IFN-γ secretion seems to be associated with the inability of CD4+ or CD8+ T cells to release this cytokine in response to specific antigenic stimulation, which suggests impaired T cells priming in these mice.
Figure 6. Kinetics of T cell response in the lungs of M. tuberculosis-infected B6, C3H, and BXH-2 mice.
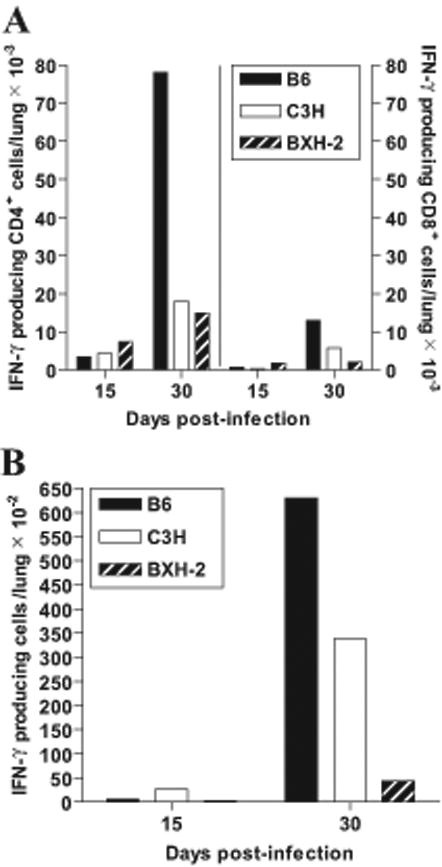
Mice were infected with 102 M. tuberculosis H37Rv by the aerosol route and the number of IFN-γ-producing CD4+ and CD8+ T cells were enumerated in the lungs at 15 and 30 days post-infection (A). In parallel, the number of antigen-specific, IFN-γ-secreting T cells in the lungs (total cells) was evaluated by Elispot assay (B), using a crude M. tuberculosis sonicate as antigenic stimulus, and RPMI culture medium as control. Results are from pooled lung cells obtained from 4 mice per experimental group. Individual experimental points are representative of measurements performed in triplicate.
The effect of loss of IRF-8 function on response to M. tuberculosis infection was further examined at the cell and tissue levels by histochemistry, with a special emphasis on the number, type and cellular composition of granulomas, including presence of acid-fast bacilli in Nos2+ and Nos2- cells. For this, lungs (Fig. 7, A-D) and liver (Fig. 7, E-H) sections of B6 and BXH-2 mice were examined 45 days following aerosol infection with M. tuberculosis. In infected B6 lungs, lesions were seen as regions of macrophage-dominated alveolitis in proximity to or surrounding large aggregates of lymphoid cells (Fig. 7A). Infected macrophages were large with a foamy cytoplasm, stained positively for Nos2, and contained acid-fast bacilli in small number (Fig. 7B). In infected BXH-2 lungs (Fig. 7C), lesions were larger and more diffuse than in B6 lungs, and consisted of an alveolitis dominated by Nos2+ infected macrophages; areas of alveolitis contained macrophages replete with acid-fast bacilli (Fig. 7D). Distinctly, lymphoid aggregates were much less present in BXH-2 lesions (Fig. 7C) compared to those seen in B6 mice (Fig. 7A). In agreement with results of lung CFU counts (Fig. 2, B and C; and Fig. 3), BXH-2 pulmonary macrophages appear much less capable of controlling M. tuberculosis growth than those of B6 mice. In the livers of infected B6 mice, a few well-structured granulomas were observed (Fig. 7E). They consisted of a small core of Nos2+ macrophages containing very few acid fast bacilli, and surrounded by a mantel of smaller mononuclear cells, likely to be lymphocytes (Fig. 7F). The situation was very different in infected BXH-2 livers, which pathology was dominated by areas of necrosis and extensive myeloid cells infiltration that replaced liver parenchyma that originally occupied these spaces (Fig. 7G and data not shown). Few granulomas were also seen in BXH-2 livers, but they were less structured and strikingly different from those seen in B6 livers, consisting of a core of heavily-infected Nos2+ macrophages surrounded by a mantle of mononuclear cells and neutrophils. Other types of lesions were detected in BXH-2 livers, consisting of large areas of myelomonocytic cell infiltration containing a mixture of infected Nos2+ or Nos2- macrophages (Fig. 7H) surrounded by neutrophils. These lesions were associated with necrosis of surrounding hepatic tissues (data not shown). Finally, acid fast bacilli were commonly seen scattered throughout the liver sinusoids inside Kuppfer cells or in small collections of monocytes or neutrophils. Taken together, these results suggest that BXH-2 macrophages cannot contain M. tuberculosis replication, and are found within unique lesions that are distinct from those seen in control B6 organs.
Figure 7. Histopathology of B6 and BXH-2 mice following aerosol infection with M. tuberculosis.
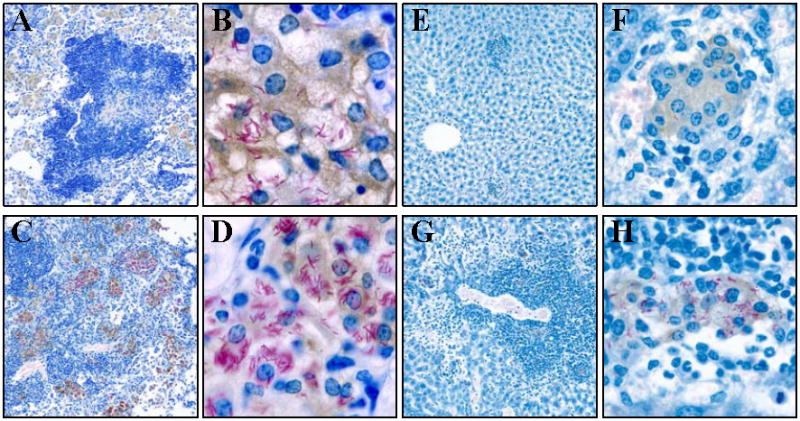
Control B6 and BXH-2 mice were infected by the aerosol route with 102 CFUs of M. tuberculosis, and 45 days later lungs and livers were harvested, and processed for immunocytochemistry as described in Materials and Methods. Briefly, tissue sections were stained for acid-fast bacilli (red), while nitric oxide synthase (NOS2) was detected by immunocytochemistry (brown); sections were counterstained with methylene blue. (A) Low power (55×) of B6 lungs; (B) High power (563×) of B6 lungs; (C) Low power (55×) of BXH-2 lungs; (D) High power (563×) of BXH-2 lungs; (E) Low power (55×) of B6 liver; (F) High power (326×) of B6 liver; (G) Low power (55×) of BXH-2 liver; (H) High power (326×) of BXH-2 liver.
Discussion
A large body of published literature indicates that the IFN-γ/IL-12 signaling loop plays a critical role in defenses against intracellular infections, including M. tuberculosis. Following phagocytosis of M. tuberculosis, macrophages produce IL-12 and IL-23, two cytokines composed of a common p40 subunit and specific p35 and p19 subunits, respectively. IL-12 (and to lesser extent IL-23) bind to cell surface receptors expressed on T-lymphocytes and NK cells, and act as a powerful stimulator of IFN-γ production by these cells. In turn, IFN-γ binds to specific receptors (IFNγR1, IFNγR2) on macrophages and dendritic cells, causing STAT1 activation and nuclear translocation. STAT1 binds to GAS (gamma interferon activation site) elements in the promotor region and activates transcription of the IRF-8 gene; IRF-8 heterodimerizes with IRF-1 to bind to ISRE promotor elements and stimulate transcription of the IL-12p40 gene leading to further activation of CD4+ T cells and NK cells to produce IFN-γ, thereby amplifying the initial signal (we have noted up-regulation of both IRF-1 and IRF-8 mRNAs in the lungs throughout M. tuberculosis infection; data not shown). This results in Th1 polarization of the early immune response to infection. In addition, STAT1 and IRF-8 (through heterodimerization with other IRF proteins or with members of the ETS family) act to transcriptionally activate a number of genes and proteins, including microbicidal pathways and effector molecules, thereby triggering a potent biological response to increase bactericidal activity of phagocytes. IL-12p40 and IFN-γ are both required for immune defenses against TB, and mutant animals lacking either genes are susceptible to M. tuberculosis (6-9). Likewise, IRF-1 and STAT1 mutants show increased susceptibility to TB (32, 33). Finally, in humans, mutations in IL-12p40 and its receptor, in IFN-γ and its receptor, as well as in STAT1 and Iκβ kinase γ have been detected in rare patients developing mycobacteriosis following M. bovis (BCG) immunization (reviewed in (11)).
Our results now demonstrate that mice bearing a loss-of-function mutation in IRF-8 are extremely susceptible to infection with M. tuberculosis. This may be linked in part to their loss of production of IL-12p40 mRNA and protein, as observed either in vivo in response to M. tuberculosis (this study), or in vitro in splenocytes stimulated with CpG and LPS (25). However, the observation that BXH-2 mice seem to be as or more susceptible to M. tuberculosis infection than either mouse knock-out for IL-12p40 (34) or IFN-γ (7, 35, 36) requires additional consideration. Indeed, following i.v infection with M. tuberculosis, BXH-2 mice succumb earlier (MST, day22) than IL-12p40 (MST, day55) or even IFN-γ (MST, day35) mutants (the latter known as the most TB susceptible mouse mutant published), even though the infectious dose used to challenge BXH-2 mice was 10 fold lower than that used to test the IL-12p40 (34) and IFN-γ (36) mutants. This raises the interesting possibility that IRF-8 may regulate additional host defense mechanisms (in addition to IL-12p40) that are essential for protection against TB, and that are lost in BXH-2. As a result, the R294C mutation may therefore abrogate transcriptional activation with co-activators IRF-1 and PU-1 (11, 24) of additional genes and proteins beneficial for host response against TB. Such candidates for IRF-8 and IRF-1 transcriptional activation may be included in the list recently published by Dror and colleagues and obtained by transcriptional profiling (37). Interestingly, BXH-2 are also more susceptible to TB than mouse mutants lacking IRF-1 (33). Alternatively, the R294C mutation may abrogate negative regulation of certain targets which expression is normally repressed by IRF-8 upon hetero-dimerization with IRF-2 and IRF-4 (11, 24, 25). Identifying such pathways and genes would be a great interest to understand protective immunity against TB.
Interestingly, and based on RT-PCR results (Fig. 4 and data not shown), the extreme susceptibility of BXH-2 seems to occur despite expression of IFN-γ mRNA in infected lungs. FACS analyses further reveals recruitment of IFN-γ positive CD4+ and CD8+ T-cells in BXH-2 lungs in response to M. tuberculosis infection (Fig. 6A), possibly representing the source of IFN-γ mRNA detected in this tissue by RT-PCR (Fig. 4 and data not shown). However, we also observed that total cells purified from BXH-2 infected lungs fail to release IFN-γ when stimulated by M. tuberculosis antigens in vitro (Fig. 6B), which is also in agreement with the lack of IFN-γ production in the serum of these mice (Fig. 5). This leads us to conclude that IRF-8 plays a critical role in a) directly regulating expression of certain genes and proteins (such as IL-12p40) that are required for IFN-γ production by antigen-specific T cells in response to infection, or b) influencing the maturation of specific myeloid or lymphocytic subsets that are required for proper T cell priming for IFN-γ production. Indeed, it has been demonstrated that CD8α+ DCs and pDCs (plasmacytoid dendritic cells) are largely missing in IRF-8-/- mice (38-41). Furthermore, it has been reported that IRF-8-/- DCs fail to produce IL-12p40 or IFN-α in response to various pathogen-associated molecular patterns (PAMPs) or Newcastle disease virus (NDV) (38-40, 42). Since total lung cells were used in our Elispot assays, such a deficit in DC populations in BXH-2 (IRF-8 mutant) mice is likely to contribute to their impaired T cells priming, and consequently to their incapacity to release IFN-γ following specific antigenic stimulation. However, we cannot formally rule out the possibility that there is low level production of IFN-γ production in situ in infected BXH-2 lungs which may be sufficient to stimulate iNOS expression in macrophages. However, this low level IFN-γ production is clearly inferior to B6 and C3H controls, and is certainly insufficient to restrict replication and dissemination of M. tuberculosis in these infected mice.
At the cellular level, our findings suggest a dual effect of the R294C mutation in IRF-8 on host response. First, there is a striking early defect which is detected within a few days following infection, and which is manifested by increased CFU counts in infected tissues following i.v infection (Fig. 2, B and C). This defect would appear to be intrinsic to infected phagocytes (macrophages, dendritic cells) and to result from a failure to suppress intracellular replication of M. tuberculosis. We have noted a similar failure of BXH-2 macrophages to control intracellular replication of Legionella following infection ex vivo (Fortier and Gros, unpublished observations). Although IRF-8 plays a role in development of the immune system, including cell development and differentiation, the hypothesis that the BXH-2 defect could be imputable to a lower number of phagocytes in these mice has been excluded since we observed similar numbers of macrophages in B6 and BXH-2 mice (data not shown). Therefore IRF-8 may control expression of certain macrophage proteins essential for defenses against intracellular pathogens. The second defect is detected later in infection, and is reflected by the unique lesions developed by BXH-2 mice at the site of infection (Fig. 7). Formation of well structured granulomas around infected macrophages is a critical host response against M. tuberculosis in order to restrict microbial replication, and importantly prevent dissemination. Cytokines such as IFN-γ, IL-12 and TNF-α are essential for granuloma formation (43). Examination of lesions in BXH-2 mice identifies extensive microbial replication in macrophages that are positive for the key bactericidal enzyme Nos2. The granulomas formed in BXH-2 are strikingly different, with different size, and cell composition, with extensive damage to surrounding tissues with extracellular bacilli often seen in liver sections. Further, there appears to be rapid and extensive dissemination of the infection from the lungs to spleen and liver. These observations suggest that IL-12 dependent polarization of the T-cell response is abrogated in BXH-2, leading to failure to contain infection.
Together, our findings in the BXH-2 mouse identify IRF-8 as a previously unsuspected but critical regulator of host response to the tuberculous bacillus. Studies are underway to determine if susceptibility to mycobacterial infections in MSMD (Mendelian susceptibility to mycobacterial diseases) patients are associated with loss-of-function mutations in the human IRF-8 gene.
Abbreviations used in this paper
- BCG
Bacilli Calmette-Guérin
- IRF
interferon regulatory factor
- ISRE
interferon stimulated response element
- NOS
nitric oxide synthase
- MST
mean survival time
- TB
tuberculosis
Footnotes
P.G. is a James McGill Professor of Biochemistry and a Distinguished Scientist of the Canadian Institutes of Health Research (CIHR). J.-F.M. is supported by a fellowship from the Fonds de Recherche en Santé du Québec. This work was supported by grant AI035237 to PG from the National Institutes of Health USA (NIAID), and grant AI069161 to RJN also from the NIAID.
References
- 1.WHO. Health Systems: Improving Performance. Geneva: WHO; 2000. The World Health Report 2000. [Google Scholar]
- 2.WHO. WHO report. Geneva: WHO; 2002. Global Tuberculosis Control: Surveillance, Planning Financing. [Google Scholar]
- 3.Levin M, Newport M. Inherited predisposition to mycobacterial infection: historical considerations. Microbes Infect. 2000;2:1549–1552. doi: 10.1016/s1286-4579(00)01310-1. [DOI] [PubMed] [Google Scholar]
- 4.Russell DG. Mycobacterium tuberculosis: here today, and here tomorrow. Nat Rev Mol Cell Biol. 2001;2:569–577. doi: 10.1038/35085034. [DOI] [PubMed] [Google Scholar]
- 5.North RJ, Jung YJ. Immunity to tuberculosis. Annu Rev Immunol. 2004;22:599–623. doi: 10.1146/annurev.immunol.22.012703.104635. [DOI] [PubMed] [Google Scholar]
- 6.Cooper AM, Magram J, Ferrante J, Orme IM. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J Exp Med. 1997;186:39–45. doi: 10.1084/jem.186.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flynn JL, Goldstein MM, Triebold KJ, Sypek J, Wolf S, Bloom BR. IL-12 increases resistance of BALB/c mice to Mycobacterium tuberculosis infection. J Immunol. 1995;155:2515–2524. [PubMed] [Google Scholar]
- 9.Feng CG, Jankovic D, Kullberg M, Cheever A, Scanga CA, Hieny S, Caspar P, Yap GS, Sher A. Maintenance of pulmonary Th1 effector function in chronic tuberculosis requires persistent IL-12 production. J Immunol. 2005;174:4185–4192. doi: 10.4049/jimmunol.174.7.4185. [DOI] [PubMed] [Google Scholar]
- 10.Cooper AM, Kipnis A, Turner J, Magram J, Ferrante J, Orme IM. Mice lacking bioactive IL-12 can generate protective, antigen-specific cellular responses to mycobacterial infection only if the IL-12 p40 subunit is present. J Immunol. 2002;168:1322–1327. doi: 10.4049/jimmunol.168.3.1322. [DOI] [PubMed] [Google Scholar]
- 11.Fortin A, Abel L, Casanova JL, Gros P. Host Genetics of Mycobacterial Diseases in Mice and Men: Forward Genetic Studies of BCG-osis and Tuberculosis. Annu Rev Genomics Hum Genet. 2007;8:163–192. doi: 10.1146/annurev.genom.8.080706.092315. [DOI] [PubMed] [Google Scholar]
- 12.Pan H, Yan BS, Rojas M, Shebzukhov YV, Zhou H, Kobzik L, Higgins DE, Daly MJ, Bloom BR, Kramnik I. Ipr1 gene mediates innate immunity to tuberculosis. Nature. 2005;434:767–772. doi: 10.1038/nature03419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanchez F, Radaeva TV, Nikonenko BV, Persson AS, Sengul S, Schalling M, Schurr E, Apt AS, Lavebratt C. Multigenic control of disease severity after virulent Mycobacterium tuberculosis infection in mice. Infect Immun. 2003;71:126–131. doi: 10.1128/IAI.71.1.126-131.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitsos LM, Cardon LR, Fortin A, Ryan L, LaCourse R, North RJ, Gros P. Genetic control of susceptibility to infection with Mycobacterium tuberculosis in mice. Genes Immun. 2000;1:467–477. doi: 10.1038/sj.gene.6363712. [DOI] [PubMed] [Google Scholar]
- 15.Mitsos LM, Cardon LR, Ryan L, LaCourse R, North RJ, Gros P. Susceptibility to tuberculosis: a locus on mouse chromosome 19 (Trl-4) regulates Mycobacterium tuberculosis replication in the lungs. Proc Natl Acad Sci USA. 2003;100:6610–6615. doi: 10.1073/pnas.1031727100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gros P, Schurr E. Immunogenetics of host response to bacteria in mice. In: Kaufman S, Ahmed R, editors. Immunology of infectious diseases. ASM Press; 2002. pp. 407–419. [Google Scholar]
- 17.Jabado N, Jankowski A, Dougaparsad S, Picard V, Grinstein S, Gros P. Natural resistance to intracellular infections: natural resistance-associated macrophage protein 1 (Nramp1) functions as a pH-dependent manganese transporter at the phagosomal membrane. J Exp Med. 2000;192:1237–1248. doi: 10.1084/jem.192.9.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor BA. Recombinant inbred strains: use in gene mapping. In: HV M III, editor. In Origins of inbred mice. Academic Press; New York: 1978. pp. 423–438. [Google Scholar]
- 19.Turcotte K, Gauthier S, Tuite A, Mullick A, Malo D, Gros P. A mutation in the Icsbp1 gene causes susceptibility to infection and a chronic myeloid leukemia-like syndrome in BXH-2 mice. J Exp Med. 2005;201:881–890. doi: 10.1084/jem.20042170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bedigian HG, Taylor BA, Meier H. Expression of murine leukemia viruses in the highly lymphomatous BXH-2 recombinant inbred mouse strain. J Virol. 1981;39:632–640. doi: 10.1128/jvi.39.2.632-640.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bedigian HG, Johnson DA, Jenkins NA, Copeland NG, Evans R. Spontaneous and induced leukemias of myeloid origin in recombinant inbred BXH mice. J Virol. 1984;51:586–594. doi: 10.1128/jvi.51.3.586-594.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bedigian HG, Shepel LA, Hoppe PC. Transplacental transmission of a leukemogenic murine leukemia virus. J Virol. 1993;67:6105–6109. doi: 10.1128/jvi.67.10.6105-6109.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jenkins NA, Copeland NG, Taylor BA, Bedigian HG, Lee BK. Ecotropic murine leukemia virus DNA content of normal and lymphomatous tissues of BXH-2 recombinant inbred mice. J Virol. 1982;42:379–388. doi: 10.1128/jvi.42.2.379-388.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levi BZ, Hashmueli S, Gleit-Kielmanowicz M, Azriel A, Meraro D. ICSBP/IRF-8 transactivation: a tale of protein-protein interaction. J Interferon Cytokine Res. 2002;22:153–160. doi: 10.1089/107999002753452764. [DOI] [PubMed] [Google Scholar]
- 25.Turcotte K, Gauthier S, Malo D, Tam M, Stevenson MM, Gros P. Icsbp1/IRF-8 Is Required for Innate and Adaptive Immune Responses against Intracellular Pathogens. J Immunol. 2007;179:2467–2476. doi: 10.4049/jimmunol.179.4.2467. [DOI] [PubMed] [Google Scholar]
- 26.Marquis JF, Nantel A, Lacourse R, Ryan L, North RJ, Gros P. Fibrotic response as a distinguishing feature of resistance and susceptibility to pulmonary infection with Mycobacterium tuberculosis in mice. Infect Immun. 2008;76:78–88. doi: 10.1128/IAI.00369-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung YJ, Ryan L, LaCourse R, North RJ. Properties and protective value of the secondary versus primary T helper type 1 response to airborne Mycobacterium tuberculosis infection in mice. J Exp Med. 2005;201:1915–1924. doi: 10.1084/jem.20050265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogerson BJ, Jung YJ, LaCourse R, Ryan L, Enright N, North RJ. Expression levels of Mycobacterium tuberculosis antigen-encoding genes versus production levels of antigen-specific T cells during stationary level lung infection in mice. Immunology. 2006;118:195–201. doi: 10.1111/j.1365-2567.2006.02355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mogues T, Goodrich ME, Ryan L, LaCourse R, North RJ. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J Exp Med. 2001;193:271–280. doi: 10.1084/jem.193.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ellis RC, Zabrowarny LA. Safer staining method for acid fast bacilli. J Clin Pathol. 1993;46:559–560. doi: 10.1136/jcp.46.6.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ozato K, Tailor P, Kubota T. The interferon regulatory factor family in host defense: mechanism of action. J Biol Chem. 2007;282:20065–20069. doi: 10.1074/jbc.R700003200. [DOI] [PubMed] [Google Scholar]
- 32.Sugawara I, Yamada H, Mizuno S. STAT1 knockout mice are highly susceptible to pulmonary mycobacterial infection. Tohoku J Exp Med. 2004;202:41–50. doi: 10.1620/tjem.202.41. [DOI] [PubMed] [Google Scholar]
- 33.Yamada H, Mizuno S, Sugawara I. Interferon regulatory factor 1 in mycobacterial infection. Microbiol Immunol. 2002;46:751–760. doi: 10.1111/j.1348-0421.2002.tb02760.x. [DOI] [PubMed] [Google Scholar]
- 34.Kinjo Y, Kawakami K, Uezu K, Yara S, Miyagi K, Koguchi Y, Hoshino T, Okamoto M, Kawase Y, Yokota K, Yoshino K, Takeda K, Akira S, Saito A. Contribution of IL-18 to Th1 response and host defense against infection by Mycobacterium tuberculosis: a comparative study with IL-12p40. J Immunol. 2002;169:323–329. doi: 10.4049/jimmunol.169.1.323. [DOI] [PubMed] [Google Scholar]
- 35.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawakami K, Kinjo Y, Uezu K, Miyagi K, Kinjo T, Yara S, Koguchi Y, Miyazato A, Shibuya K, Iwakura Y, Takeda K, Akira S, Saito A. Interferon-gamma production and host protective response against Mycobacterium tuberculosis in mice lacking both IL-12p40 and IL-18. Microbes Infect. 2004;6:339–349. doi: 10.1016/j.micinf.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 37.Dror N, Alter-Koltunoff M, Azriel A, Amariglio N, Jacob-Hirsch J, Zeligson S, Morgenstern A, Tamura T, Hauser H, Rechavi G, Ozato K, Levi BZ. Identification of IRF-8 and IRF-1 target genes in activated macrophages. Mol Immunol. 2007;44:338–346. doi: 10.1016/j.molimm.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 38.Schiavoni G, Mattei F, Sestili P, Borghi P, Venditti M, Morse HC, 3rd, Belardelli F, Gabriele L. ICSBP is essential for the development of mouse type I interferon-producing cells and for the generation and activation of CD8alpha(+) dendritic cells. J Exp Med. 2002;196:1415–1425. doi: 10.1084/jem.20021263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tamura T, Tailor P, Yamaoka K, Kong HJ, Tsujimura H, O'Shea JJ, Singh H, Ozato K. IFN regulatory factor-4 and -8 govern dendritic cell subset development and their functional diversity. J Immunol. 2005;174:2573–2581. doi: 10.4049/jimmunol.174.5.2573. [DOI] [PubMed] [Google Scholar]
- 40.Tsujimura H, Tamura T, Ozato K. Cutting edge: IFN consensus sequence binding protein/IFN regulatory factor 8 drives the development of type I IFN-producing plasmacytoid dendritic cells. J Immunol. 2003;170:1131–1135. doi: 10.4049/jimmunol.170.3.1131. [DOI] [PubMed] [Google Scholar]
- 41.Aliberti J, Schulz O, Pennington DJ, Tsujimura H, Reis e Sousa C, Ozato K, Sher A. Essential role for ICSBP in the in vivo development of murine CD8alpha + dendritic cells. Blood. 2003;101:305–310. doi: 10.1182/blood-2002-04-1088. [DOI] [PubMed] [Google Scholar]
- 42.Tsujimura H, Tamura T, Gongora C, Aliberti J, Reis e Sousa C, Sher A, Ozato K. ICSBP/IRF-8 retrovirus transduction rescues dendritic cell development in vitro. Blood. 2003;101:961–969. doi: 10.1182/blood-2002-05-1327. [DOI] [PubMed] [Google Scholar]
- 43.Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]