Abstract
Background
Preoperative pain and depression predispose patients to delirium. Our goal was to determine whether pain and depressive symptoms interact to increase delirium risk.
Methods
We enrolled 459 persons without dementia aged ≥70 years scheduled for elective orthopedic surgery. At baseline, participants reported their worst and average pain within seven days and current pain on a 0–10 scale. Depressive symptoms were assessed using the 15-item Geriatric Depression Scale and chart. Delirium was assessed with the Confusion Assessment Method and chart. We examined the relationship between preoperative pain, depressive symptoms and delirium using multivariable analysis of pain and delirium stratified by presence of depressive symptoms.
Findings
Delirium, occurring in 23% of the sample, was significantly higher in those with depressive symptoms at baseline than those without (relative risk, RR, 1·6, 95% confidence interval, CI, 1·2–2·3). Preoperative pain was associated with an increased adjusted risk for delirium across all pain measures (RR from 1·07–1·08 per point of pain). In stratified analyses, patients with depressive symptoms had a 21% increased risk for delirium for each one-point increase in worst pain score, demonstrating a significant interaction (P=0·049). Similarly, a significant 13% increased risk for delirium was demonstrated for a one-point increase in average pain score, but the interaction did not achieve statistical significance.
Interpretation
Preoperative pain and depressive symptoms demonstrated increased risk for delirium independently and with substantial interaction, suggesting a cumulative impact. Thus, pain and depression are vulnerability factors for delirium that should be assessed before surgery.
Funding
U.S. National Institute on Aging.
Keywords: Delirium, depression, pain, elderly, surgery, effect modification
Introduction
Delirium, characterized by an acute decline in attention and cognitive function, is a common complication of surgery for older adults, with a reported incidence of 11 to 51 percent.1 Adverse outcomes associated with postoperative delirium include prolonged length of stay, institutionalization, mortality, functional decline, and long-lasting cognitive impairment.1,2 As the population of older adults undergoing surgical procedures continues to grow,3 advancing the understanding of risk factors for delirium assumes heightened importance.
Depression is a well-recognized delirium risk factor, associated with a two-to threefold greater risk of delirium in previous studies.4,5 Comorbid depression and delirium have been shown to be associated with worse outcomes than either syndrome alone.6 Depression also correlates with higher levels of pain.7 Pain is underdiagnosed and undertreated in older persons despite being linked to poor outcomes.8 Its relationship with delirium has mostly been described in studies of acute pain experienced during hospitalization, with reports of up to nine-fold increased delirium risk for patients with high levels of reported pain.9 Only two studies have examined the association between preoperative pain and delirium.10,11
Given the known associations between pain and depression, delirium and depression, and delirium and acute pain, an in-depth examination of the interrelationship of preoperative pain, depression, and delirium appears warranted. Our goal was to evaluate preoperative pain and depression as baseline vulnerability factors for delirium, an important initial step in understanding their respective contributions. 12,13 Thus, we conducted a prospective study of 459 older adults undergoing elective orthopedic surgery for painful conditions to examine the interrelationship of preoperative pain and depressive symptoms and risk of delirium following surgery. We hypothesized that the combination of more severe symptoms of pain and depression prior to surgery would be associated with an enhanced risk for postoperative delirium.
Methods
Study population
This study is based on a secondary analysis of the data collected for the Successful Aging After Elective Surgery (SAGES) study, a prospective cohort study of 566 elective surgical patients designed to examine delirium and its outcomes which has been described in detail previously. 14 In brief, eligible participants were age 70 years or older, and had no clinically documented evidence of prior delirium or dementia, and were scheduled for major elective surgery at two academic medical centers in the Boston area. Enrollment began in June, 2010 and ended in August, 2013. Patients were excluded for evidence of dementia if their medical records contained clinical documentation of the disease, or if they self-reported a previous clinical diagnosis, failed to pass a capacity assessment for providing informed consent, or scored below 69 or its education-adjusted equivalent on the Modified Mini-Mental State (3MS) 15 examination, administered before surgery. Ultimately, 566 patients met eligibility criteria and were enrolled in the cohort. The sample for this report is restricted to participants scheduled for orthopedic procedures (hip or knee replacement and laminectomy) predominantly for painful conditions; thus, 106 patients scheduled for vascular or general surgical procedures were excluded. In addition, one patient with a missing rating of depressive symptoms was excluded, yielding a final sample of 459 for the present report. Informed consent for study participation was obtained from all subjects according to procedures approved by the institutional review boards of Beth Israel Deaconess Medical Center and Brigham and Women’s Hospital, the two surgical sites, and Hebrew SeniorLife, the study coordinating center, all located in Boston, Massachusetts.
Preoperative evaluation and study variables
All subjects underwent a structured interview and medical record review approximately two weeks before surgery. Interviews were administered by experienced research associates who underwent four weeks of intensive training and standardization, which was repeated biannually. A trained physician abstractor conducted medical record reviews using standardized procedures.
Information on sex, race, educational attainment, marital status, and physical function were obtained by self-report. Cognitive function was assessed with the 3MS and a comprehensive neuropsychological battery described in detail elsewhere.14 Physical function was assessed with basic and Instrumental Activities of Daily Living (ADL and IADL) scales.16,17 Age and comorbidities were determined from the medical record. The Charlson index was used to measure comorbidity burden.18
The severity of preoperative pain was assessed with three items from the Brief Pain Inventory (BPI),19 which has been previously validated. Subjects were asked to rate on a scale of 0 (no pain) to 10 (worst pain imaginable) their current level of pain as well as their average and worst levels within the past seven days. Pain levels were categorized according to the World Health Organization classification scheme, where a rating of 0 corresponds to no pain, ratings of 1–3 correspond to mild pain, ratings of 4–6 indicate moderate pain, and ratings of 7–10 correspond to severe pain.20
Depressive symptoms were measured using the short form of the Geriatric Depression Scale (GDS-15).21 Scores range from 0 to 15, with higher scores reflecting a higher severity of depressive symptoms. A score ≥6, a commonly used threshold,22 was used to indicate substantial depressive symptoms for the present study. Information on depression was also abstracted from the medical record, including chart notation of active depression by a physician or nurse practitioner in preadmission screening, available outpatient and psychiatric notes before surgery, or by documentation of depression in the patient’s active problem list. Both screening questionnaire and medical record sources were used as evidence of preoperative depressive symptoms, as recommended by previous studies.23
Postoperative delirium
Delirium was rated according to the Confusion Assessment Method (CAM) criteria, which defines delirium present if a patient exhibits an acute change or fluctuation in mental status, inattention, and either disorganized thinking or an altered level of consciousness.24 The CAM is the most widely used bedside instrument for detecting delirium, and has been validated against psychiatrists’ diagnoses, with high sensitivity (94–100%), specificity (90–95%), and reliability (kappa=0·70–1·00).25,26 The CAM was rated at baseline and one time daily during the postoperative period starting the day following surgery. Scoring was informed by a standardized interview consisting of brief cognitive testing (i.e., orientation, recall, and sustained attention tasks), conversations with caregivers and nursing staff, and the Delirium Symptom Interview (DSI) which captures delirium symptoms reported by the patient.27 In our study, the CAM ratings were highly reliable with a kappa of 0·92 for the overall identification of delirium in 71 paired ratings (including 16 delirious patients). The daily evaluation was augmented with a validated chart-based instrument that identifies the signs and symptoms of delirium between interviews or during times when structured assessments are unfeasible (e.g. during night shift).28 Patients were classified as delirious if either CAM or chart-based criteria was satisfied on any postoperative day excluding the date of surgery. Patients were not considered delirious if symptoms of inattention, disorganized thinking, or level of consciousness did not change from the level at the baseline interview. No delirium interventions were conducted in the course of the study.
Statistical analysis
The characteristics of patients with and without high depressive symptomatology were described using standard statistics, including means, standard deviations, medians, inter-quartile ranges, and proportions. The distribution of worst, average, and current pain was described in each group, and differences between those with depressive symptoms and those without were compared using analysis of variance.
Robust Poisson regression was used to estimate the associations between each measure of preoperative pain and delirium. To enhance clinical interpretability, we present relative risks (RRs) and 95% confidence intervals (CIs) comparing delirium risk for patients in moderate and severe levels of pain to patients with mild or no pain. The Cochran-Armitage test was used to evaluate for trends in the rates of delirium as a function of pain level.
To determine whether depressive symptoms and pain interact in their association with delirium, analyses were stratified by presence of depressive symptoms. We first calculated the delirium incidence for each point of pain on the 10-point numeric rating scale. We then estimated the adjusted relative risk for delirium associated with a one unit between-person difference in pain. Significant effect modification by depressive symptoms was estimated by examining the multiplicative interaction term between the pain score and depressive symptoms in models without stratification.
Multivariate models were adjusted for age, sex, race, education level, and comorbidity, which were considered important confounders based on expert opinion and previous studies. Data on medication use were not available for these analyses. Data were analyzed with Stata MP version 13·0. Null hypotheses were evaluated with a two-tailed alpha level of 0·05.
To evaluate the robustness of our delirium outcome, we conducted sensitivity analyses which considered only delirium of high severity. Delirium symptom severity was measured using the peak CAM-S (long form). 29 All analyses were repeated including only patients with peak CAMS ≥5, indicating severe delirium. Because our primary goal was to examine baseline vulnerability factors and to avoid over-controlling for intermediate variables which might be on the causal pathway to delirium,30,31 we did not examine any hospital-based precipitating factors in these analyses. However, as another sensitivity analysis to verify the robustness of our findings, we added either the count of days the patient was on opioids (by class) or treatment with opioids for three or more days.
Role of the funding source
The study sponsor had no role in the study design, data collection, data analysis, data interpretation, or the writing of this report. The first (CMK) and senior author (SKI) had full access to the data and had final responsibility for the decision to submit for publication.
Results
The baseline characteristics and delirium rate for the cohort of 459 patients are presented in Table 1. Participants’ median age was 76 years (interquartile range [IQR], 73 to 80 years); 60% of the participants were women, 92% were white and non-Hispanic, and 58% were married at the time of enrollment. A quarter of surgical procedures were hip replacements; 46% were knee replacements, and 29% were laminectomies. Overall, the sample was well-educated and had low comorbidity burden, with a median of 15 years of completed schooling (IQR, 12 to 18 years) and a mean Charlson score (±SD) of 0·9 ± 1·2. Delirium developed in 23% of patients (106 of 459 patients) following surgery.
Table 1.
SAGES Sample: Overall and by Presence of Depressive Symptoms
| Variable | Full Sample (N=459) | Depressive symptoms (n=113) | No Depressive Symptoms (n=346) |
|---|---|---|---|
| Age--median years (IQR) | 76 (73, 80) | 76 (73, 81) | 76 (73, 80) |
| Female sex--n (%) | 274 (60) | 73 (65) | 201 (58) |
| Non-white race--n (%) | 38 (8) | 10 (9) | 28 (8) |
| Education-- median years (IQR) | 14 (12, 18) | 14 (12, 18) | 15 (12, 18) |
| Married, n (%) | 266 (58) | 58 (51) | 208 (60) |
| Charlson comorbidity--mean score (SD) | 0·9 (1·2) | 1·1 (1·3) | 0·8 (1·1) |
| Surgery Type | |||
| Hip Replacement | 115 (25) | 24 (21) | 91 (26) |
| Knee Replacement | 209 (46) | 44 (39) | 165 (48) |
| Laminectomy | 135 (29) | 45 (40) | 90 (26) |
| 3MS Score - mean (SD) | 93·4 (5·4) | 92·7 (5·8) | 93·7 (5·3) |
| Any impairment in basic activities of daily living--n (%)* | 38 (8) | 12 (11) | 26 (8) |
| Any impairment in instrumental activities of daily living--n (%)* | 137 (30) | 40 (35) | 97 (28) |
| Delirium during hospitalization, n (%) | 106 (23) | 37 (33) | 69 (20)± |
SAGES=Successful Aging after Elective Surgery Study; SD=standard deviation. 3MS=Modified Mini-Mental State examination (range 0–100, lower=worse).
Impairment defined as any impairment in one or more basic (or instrumental) activity of daily living
The relative risk for delirium associated with depressive symptoms is 1·6, 95% confidence interval, 1·2–2·3 (P=0·004). See text for details.
Higher levels of depressive symptoms were identified in 25% of patients (113 of 459 patients). The subsample of patients with depressive symptoms included more females and unmarried individuals, and had a higher rate of spine surgery, functional impairment and comorbidity burden than those without depressive symptoms. Delirium rates were significantly higher in patients with depressive symptoms, with a rate of 33% compared with 20% in patients without depressive symptoms (RR=1·6; 95% CI 1·2–2·3).
The distribution of worst, average, and current pre-surgical pain are described in Table 2. Worst pain, as expected, received the highest numeric rating, with a mean score of 6·6 ± 2·6 (median = 7). Average pain had a mean score of 4·7 ± 2·4 (median = 5) followed by current pain, with a mean score of 2·3 ± 2·6 (median = 2).
Table 2.
Distribution of Preoperative Pain Scores*
| Full Sample (N=459) | Depressive symptoms (n=113) | No Depressive Symptoms (n=346) | |
|---|---|---|---|
| Worst Pain within 7 Days
| |||
| Mean (SD) | 6·6 (2·6) | 7·1 (2·2) | 6·5 (2·7) |
| Median (IQR) | 7 (5–8) | 7 (6–9) | 7 (4–8) |
| Level of worst pain, n (%) | |||
| None/Mild | 71 (15) | 6 (5) | 65 (19) |
| Moderate | 104 (23) | 26 (23) | 78 (23) |
| Severe | 284 (62) | 81(72) | 203 (59) |
|
| |||
| Average Pain within 7 Days†
| |||
| Mean (SD) | 4·7 (2·4) | 5·3 (2·0) | 4·5 (2·5) |
| Median (IQR) | 5 (3–6) | 5 (4–7) | 5 (3–6) |
| Level of average pain, n (%) | |||
| None/Mild | 145 (32) | 20 (18) | 125 (36) |
| Moderate | 205 (45) | 60 (54) | 145 (42) |
| Severe | 105 (23) | 32 (29) | 73 (21) |
|
| |||
| Current Pain | |||
| Mean (SD) | 2·3 (2·6) | 2·7 (2·8) | 2·2 (2·5) |
| Median (IQR) | 2 (0–4) | 2 (0–5) | 1 (0–4) |
| Level of current pain, n (%) | |||
| None/Mild | 331 (72) | 73 (65) | 258 (75) |
| Moderate | 85 (19) | 26 (23) | 59 (17) |
| Severe | 43 (9) | 14 (12) | 29 (8) |
SD=standard deviation. IQR=inter-quartile range.
Pain is rated on a scale of 0–10. Scores of 0=no pain, scores of 1–3=mild pain, scores of 4–6=moderate pain, and scores of 7–10=severe pain. None and mild pain levels were combined because few subjects rated worst or average pain with scores of 0.
Excludes three participants unable to recall their average pain and one who refused to answer.
Patients with high level of depressive symptoms reported higher pain intensity across all three pain measures. In patients with depressive symptoms, the mean score for worst pain was 7·1 ± 2·2 compared with 6·5 ± 2·7 in patients without depressive symptoms (P=0·017). Patients with depressive symptoms reported a mean score for average pain of 5·3 ± 2·0 while patients without depressive symptoms reported a mean score of 4·5 ± 2·5 (P=0·003). Mean scores for current pain were 2·7 ± 2·8 in patients with depressive symptoms and 2·2 ± 2·5 in those without (P=0·050). Worst and average pain scores were more variable in patients without depressive symptoms, with a larger standard deviation and inter-quartile range span, although the median scores were the same as patients with depressive symptoms.
Table 3 shows the incidence of delirium across levels of pain and the adjusted risk for delirium associated with moderate and severe pain relative to mild or absent pain. The incidence of delirium across levels of worst pain increased from 18% (no/mild pain) to 25% (severe pain). Across levels of average pain, delirium increased from 20% to 31%. The incidence of delirium increased from 21% in those with mild or no current pain to 44% in those with severe current pain. The test for trend across levels of average and current pain was statistically significant (P=0·045 for average pain P=0·013 for current pain), but not for worst pain. Notably, the estimated risk for delirium was higher at severe levels of all three pain measures compared to lower or levels of pain. However, only severe current pain demonstrated a statistically significant association (RR=2·0; 95% CI=1·4–3·0).
Table 3.
Adjusted Associations of Preoperative Pain Levels and Risk for Delirium*
| Pain Measure | Worst | Average | Current | |||
|---|---|---|---|---|---|---|
| Level | n/N (%) | Adjusted RR (95% CI) | n/N (%) | Adjusted RR (95% CI) | n/N (%) | Adjusted RR (95% CI) |
| None/Mild† | 13/71 (18) | Referent | 29/145 (20) | Referent | 71/331 (21) | Referent |
| Moderate | 21/104 (20) | 1·1 (0·6–2·0) | 44/205 (21) | 1·0 (0·7–1·6) | 16/85 (19) | 0·9 (0·5–1·4) |
| Severe | 72/284 (25) | 1·3 (0·8–2·2) | 33/105 (31) | 1·4 (0·9–2·3) | 19/43 (44) | 2·0 (1·4–3·0) |
|
| ||||||
| Ptrend: | 0·146 | --- | 0·045 | --- | 0·013 | --- |
RR= Relative risk for delirium. Pain is modeled as a three-level categorical variable. Risk estimates are adjusted for age, gender, race, education level, and comorbidity. CI=confidence interval. n/N=fraction of delirious patients in pain category. The Cochran-Armitage test was used to whether the proportion of delirium is constant across pain categories. A significant Ptrend (≤0·05) indicates that rate of delirium changes linearly across increasing levels of pain.
None and mild pain levels were combined because few subjects rated worst or average pain with scores of 0.
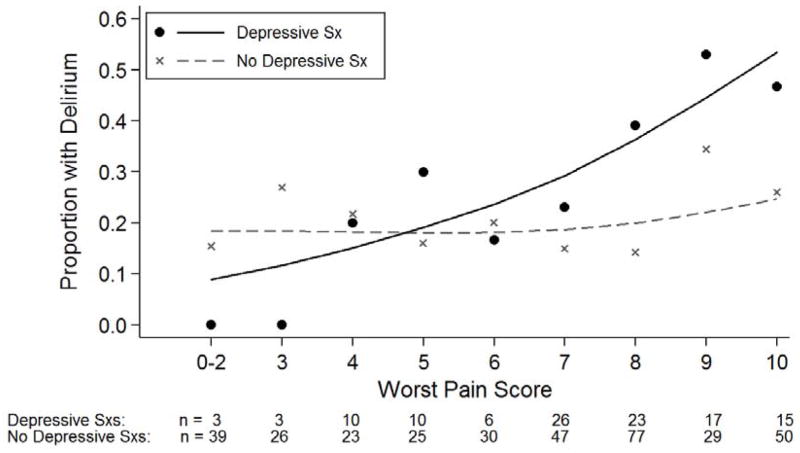
Evidence for an enhanced relationship between pain and delirium in the presence of depressive symptoms is illustrated in Figure 1. The incidence of delirium in patients with and without depressive symptoms is plotted along the numeric rating scale (0–10) for worst pain. The rate of delirium increases in a curvilinear fashion with increasing pain in the group with depressive symptoms, starting from 0% at worst pain scores of 0–2 to 47% at scores of 10 (the highest level). In patients without depressive symptoms, the rate of delirium increases only marginally with higher levels of pain, with 15% of patients at the lowest pain scores developing delirium, increasing to 26% at scores of 10 (highest pain).
Figure 1.
The Relationship of Pain and Delirium by Presence of Depressive Symptoms
Note: No participants with depressive symptoms (Sx) reported worst pain scores of 1, thus for illustrative purposes scores of 0–2 were combined. Trend lines are calculated with a generalized additive model with a Poisson error distribution and cubic smoothing splines.
The adjusted association between preoperative pain and delirium stratified by presence or absence of depressive symptoms is shown in Table 4. In the overall cohort, increasing reports of pain on all three of the pain scores (worst, average, and, current) were associated with a significantly increased risk of delirium. Among patients with depressive symptoms, the risk of delirium increased multiplicatively by 21% for each one-point increase in worst pain (RR=1·21; 95% CI=1·05–1·40), contrasting with a 3% increased risk per one-point increase among patients without depressive symptoms (RR=1·03; 95% CI=0·95–1·12). For a 5-point difference in worst pain, this corresponds to 2·6-fold risk of delirium for patients with depressive symptoms. The interaction of worst pain score and depressive symptoms was statistically significant (P=0·049), indicating that the association of pain and delirium was significantly different between patients with and without depressive symptoms, with pain exerting a stronger impact in patients with depressive symptoms.
Table 4.
Adjusted Associations of Preoperative Pain and Delirium Stratified by Presence of Depressive Symptoms*
| Pain Measure | Worst | Average† | Current |
|---|---|---|---|
| Group | Adjusted RR (95% CI) | Adjusted RR (95% CI) | Adjusted RR (95% CI) |
| Overall (N=459) | 1·08 (1·01–1·16) | 1·08 (1·01–1·16) | 1·07 (1·01–1·13) |
| Depressive Symptoms (n=113) | 1·21 (1·05–1·40) | 1·13 (1·01–1·29) | 1·07 (0·98–1·17) |
| No Depressive Symptoms (n=346) | 1·03 (0·95–1·12) | 1·05 (0·97–1·15) | 1·06 (0·98–1·14) |
RR=Relative risk for delirium. Pain is modeled as a continuous variable. Relative risks refer to a 1-point change on the 0–10 pain rating scale. Risk estimates are adjusted for age, gender, race, education level, and comorbidity. CI=confidence interval. CIs were rounded to the nearest hundredth. To avoid confusion, values that were significant before rounding are indicated as 1·01
Excludes three participants unable to recall their average pain and one who refused to answer.
Among patients with depressive symptoms, delirium risk increased multiplicatively by 13% for each one-point increase in average pain (RR=1·13; 95% CI=1·01–1·29) compared with a 5% increase among patients without depressive symptoms (RR=1·05; 95% CI=0·97–1·15). Although this risk difference suggests possible effect modification by depressive symptoms, the interaction did not achieve statistical significance. Current pain was not significantly associated with delirium in the stratified analyses. \
Sensitivity Analyses
When analyses were repeated including only patients with severe delirium (CAM-S scores ≥5), results remained similar. The proportion of delirious patients across levels of pain (Table 3) remained similar, with comparable tests for trend. The adjusted associations of pain and delirium depicted in Tables 3 and 4 were unchanged. Similarly, when repeating analyses adding either the count of days the patient is on opioids or treatment with opioids for three or more days, the results remain virtually unchanged with no impact on findings or conclusions (results not shown).
Discussion
The results from this prospective cohort of older adults show that levels of preoperative pain and depressive symptoms are both independently associated with the development of postoperative delirium. Delirium, occurring in 23% of the sample, was significantly higher in those with depressive symptoms at baseline than those without (relative risk, RR, 1·6, 95% confidence interval, CI, 1·2–2·3). Higher levels of preoperative pain intensity were found in patients with depressive symptoms across all pain measures. Overall, preoperative pain was associated with an increased adjusted risk for delirium across all three pain measures (RR from 1·07–1·08 per point of pain), with a significant trend for increasing categorical levels of pain for average and current pain. With certain dimensions of pain, the association with delirium was evident only in the presence of depressive symptoms. For instance, the borderline associations of delirium with worst and average pain levels without regard to depressive symptoms (Table 3) become evident when depressive symptoms are considered (Figure 1, Table 4). In stratified analyses, patients with depressive symptoms had a multiplicative 21% increased risk for delirium for each one-point increase in worst pain score, demonstrating a significant interaction (P=0·049). Similarly, a significant 13% increased risk for delirium was demonstrated for a one-point increase in average pain score, but the interaction did not achieve statistical significance.
Our findings corroborate and extend prior research examining the associations between pain, depression and delirium separately, but to our knowledge this is the first study to describe the development of delirium associated with the interaction of preoperative depressive symptoms and pain. As in other studies,4,5 patients with depressive symptoms in our sample were both more likely to report higher pain (Table 2) and also to develop delirium (Table 1). However, we found that while the between-group differences in reported pain level were small, even a one-point difference in worst pain score within patients with depressive symptoms was associated with a 21% increase in delirium risk. The finding that current pain is associated with delirium irrespective of depressive symptom status is consistent with prior work examining preoperative pain and delirium.10,11 Depressive symptoms may have not influenced this association, however, perhaps because current pain at a single point in time may be less affected by the influences of a persistent mood disorder.
Strengths of our study include the prospective design and large sample allowing for a stratified examination of pain and delirium by depressive symptoms. The measurement of pain and depressive symptoms before the onset of delirium assures temporal precedence and enhances examination of these variables as true risk factors for delirium. The use of state-of-the-art epidemiologic methods for delirium identification and well-established approaches to measuring both pain and depressive symptoms, with minimal missing data (<1%), strengthen the validity of our results.
Several limitations of this study deserve comment. Currently, we did not have available comprehensive data on individual analgesics (only class of opioids) and antidepressants and were therefore unable to evaluate whether the heighted delirium risk we observed was driven by medication use or withdrawal. We also did not examine the role of postoperative pain, which has been associated with delirium. An important area of future investigation will be to expand our current findings to examine the interaction of postoperative pain, analgesics, and other precipitating factors with preoperative pain and depression on risk for delirium. While we used widely-applied epidemiologic methods to detect delirium and depressive symptoms, our results are not based on a formal diagnosis for either condition, such as by a psychiatrist’s evaluation. We also acknowledge that the GDS may be limited as a depression screening instrument in older persons with somatic complaints and multi-morbidity.32 Our sample consisted of predominantly white, well-educated, high-functioning participants recruited from two Harvard-affiliated sites in a single geographic region, potentially limiting the generalizability of our findings. Therefore, our results require validation in population-based samples. Finally, our estimates of delirium risk may not necessarily reflect causal effects of preoperative pain or depressive symptoms. However, we believe the present results are important to help identify high risk patients for delirium whose surgical course merits close monitoring.
The mechanisms underlying the association between preoperative pain and delirium are likely to be multifactorial and complex. For example, patients with elevated preoperative pain may experience higher levels of postoperative pain and be over-treated with agents that are potentially delirium-inducing. Alternatively, the burden of elevated pain and depressive symptoms may produce an underlying cerebral dysfunction, such as through abnormal stress response or triggering of brain inflammatory responses, predisposing patients to an exaggerated risk of postoperative delirium.33 Disturbances in circadian function due to pain, depression, or analgesia may also play an important role. Clarifying the mechanisms underlying the association between preoperative pain, depression, and delirium is a much-needed area of future research.
In conclusion, our data provide evidence for a combined effect of preoperative pain and depressive symptoms on delirium. Our results highlight the need for increased assessment of both preoperative pain and depressive symptoms prior to surgery, with particular attention given to patients endorsing higher levels of both symptoms.
Panel: Research in Context
Systematic Review
Our exploration of the interrelationship of pain, depressive symptoms was motivated by recent delirium prevention guidelines by the National Institute for Health and Clinical Excellence (NICE) in the United Kingdom, which identified pain as a modifiable risk factor. 34 Our aim was to identify studies of persistent, non-acute pain and delirium, which are currently lacking. We systematically searched PubMed for reports published in English with no date restriction, combining the search terms “pain” with “delirium” and “confusion.” We eliminated studies where the combination of terms did not appear in the study title, abstract, or key terms. Most studies of pain and delirium focus indeed focused on acute pain developed during hospitalization (e.g. after hip fracture) or during postoperative periods. Two earlier studies did report an association between preoperative pain (resting pain and pain with movement) and postoperative delirium.10,11 The role of depression was not examined in these studies.
Interpretation
Ours is the first study to examine the interaction of depressive symptoms and pain and its association with delirium. Using different dimensions of pain from other studies, we have both confirmed that preoperative pain increases the risk of delirium and discovered that this risk is exacerbated among patients with elevated depressive symptoms. Our results highlight the need for increased assessment of pain and depressive symptoms prior to surgery. This research provides groundwork for further exploration of preoperative pain, depressive symptoms, and pathways to postoperative delirium. Areas to explore in future research include the role of pre-and postoperative medication use as well as postoperative pain as mediating factors, and whether better treatment of preoperative pain and depressive symptoms would lower delirium risk.
Acknowledgments
Grant funding: This study was funded by P01AG031720 and K07AG041835 from the U.S. National Institute on Aging (SKI). Dr. Marcantonio's effort was supported by the National Institute of Aging grants R01AG030618 and K24AG035075. Dr. Saczynski was supported by grants National Institute of Aging grants K01AG33643 and U01HL105268. Dr. Inouye holds the Milton and Shirley F. Levy Family Chair at Hebrew SeniorLife/Harvard Medical School.
We thank the patients, family members, nurses, and physicians who participated, as well as the study staff at Beth Israel Deaconess Medical Center, Brigham and Women’s Hospital and Hebrew SeniorLife. This work is dedicated to the memory of Joshua Bryan Inouye Helfand.
Footnotes
Conflicts of Interest
We declare that we have no conflicts of interest.
Contributors
CMK, PAT, MCR, and SKI conceived and designed the study. CMK, EMS, ERM, and SKI acquired and assembled the data. CMK, PAT, TGT, RNJ, and SKI analyzed the data. CMK, PAT, TGT, RNJ, JSS, ERM, MCR and SKI interpreted the data. CMK and SKI drafted the report. All authors provided critical revisions to the report for intellectual content and provided final approval. Funding was obtained by SKI.
References
- 1.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383:911–22. doi: 10.1016/S0140-6736(13)60688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304:443–51. doi: 10.1001/jama.2010.1013. [DOI] [PubMed] [Google Scholar]
- 3.Chow WB, Rosenthal RA, Merkow RP, Ko CY, Esnaola NF. Optimal preoperative assessment of the geriatric surgical patient: a best practices guideline from the American College of Surgeons National Surgical Quality Improvement Program and the American Geriatrics Society. J Am Coll Surg. 2012;215:453–66. doi: 10.1016/j.jamcollsurg.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 4.Galanakis P, Bickel H, Gradinger R, Von Gumppenberg S, Forstl H. Acute confusional state in the elderly following hip surgery: incidence, risk factors and complications. Int J Geriatr Psychiatry. 2001;16:349–55. doi: 10.1002/gps.327. [DOI] [PubMed] [Google Scholar]
- 5.Leung JM, Sands LP, Mullen EA, Wang Y, Vaurio L. Are preoperative depressive symptoms associated with postoperative delirium in geriatric surgical patients? J Gerontol A Biol Sci Med Sci. 2005;60:1563–68. doi: 10.1093/gerona/60.12.1563. [DOI] [PubMed] [Google Scholar]
- 6.Givens JL, Jones RN, Inouye SK. The overlap syndrome of depression and delirium in older hospitalized patients. J Am Geriatr Soc. 2009;57:1347–53. doi: 10.1111/j.1532-5415.2009.02342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamb SE, Guralnik JM, Buchner DM, et al. Factors that modify the association between knee pain and mobility limitation in older women: the Women’s Health and Aging Study. Annals Ann Rheum Dis. 2000;59:331–37. doi: 10.1136/ard.59.5.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Institute of Medicine. Relieving pain in America: A blueprint for transforming prevention, care, education and Research. National Academies Press; Washington, D.C: 2011. [PubMed] [Google Scholar]
- 9.Morrison RS, Siu AL. A comparison of pain and its treatment in advanced dementia and cognitively intact patients with hip fracture. J Pain Symptom Manage. 2000;19:240–48. doi: 10.1016/s0885-3924(00)00113-5. [DOI] [PubMed] [Google Scholar]
- 10.Leung JM, Sands LP, Lim E, Tsai TL, Kinjo S. Does preoperative risk for delirium moderate the effects of postoperative pain and opiate use on postoperative delirium? Am J Geriatr Psychiatry. 2013;21:946–56. doi: 10.1016/j.jagp.2013.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaurio LE, Sands LP, Wang Y, Mullen EA, Leung JM. Postoperative delirium: the importance of pain and pain management. Anesth Analg. 2006;102:1267–73. doi: 10.1213/01.ane.0000199156.59226.af. [DOI] [PubMed] [Google Scholar]
- 12.Inouye SK, Viscoli CM, Horwitz RI, Hurst LD, Tinetti ME. A predictive model for delirium in hospitalized elderly medical persons based on admission characteristics. Ann Intern Med. 1993;119:474–481. doi: 10.7326/0003-4819-119-6-199309150-00005. [DOI] [PubMed] [Google Scholar]
- 13.Inouye SK. Prevention of delirium in hospitalized older patients: risk factors and targeted intervention strategies. Ann Med. 2000;32:257–263. doi: 10.3109/07853890009011770. [DOI] [PubMed] [Google Scholar]
- 14.Schmitt EM, Marcantonio ER, Alsop DC, et al. Novel risk markers and long-term outcomes of delirium: the successful aging after elective surgery (SAGES) study design and methods. J Am Med Dir Assoc. 2012;13:818, e1–10. doi: 10.1016/j.jamda.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–18. [PubMed] [Google Scholar]
- 16.Katz S. Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31:721–27. doi: 10.1111/j.1532-5415.1983.tb03391.x. [DOI] [PubMed] [Google Scholar]
- 17.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–86. [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 19.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–38. [PubMed] [Google Scholar]
- 20.World Health Organization. Cancer pain relief. 2. Geneva: World Health Organization; 1996. [Google Scholar]
- 21.Yesavage JA, Sheikh JI. Geriatric Depression Scale (GDS) Recent Evidence and Development of a Shorter Version. Clin Gerontol. 1986;5:165–73. [Google Scholar]
- 22.Almeida OP, Almeida SA. Short versions of the geriatric depression scale: a study of their validity for the diagnosis of a major depressive episode according to ICD-10 and DSM-IV. Int J Geriatr Psychiatry. 1999;14:858–65. doi: 10.1002/(sici)1099-1166(199910)14:10<858::aid-gps35>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 23.Smith PJ, Attix DK, Weldon BC, Greene NH, Monk TG. Executive function and depression as independent risk factors for postoperative delirium. Anesthesiology. 2009;110:781–87. doi: 10.1097/aln.0b013e31819b5bc2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–48. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 25.Wong CL, Holroyd-Leduc J, Simel DL, Straus SE. Does this patient have delirium?: value of bedside instruments. JAMA. 2010;304:779–86. doi: 10.1001/jama.2010.1182. [DOI] [PubMed] [Google Scholar]
- 26.Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008;56:823–30. doi: 10.1111/j.1532-5415.2008.01674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albert MS, Levkoff SE, Reilly C, et al. The delirium symptom interview: an interview for the detection of delirium symptoms in hospitalized patients. J Geriatr Psychiatry Neurol. 1992;5:14–21. doi: 10.1177/002383099200500103. [DOI] [PubMed] [Google Scholar]
- 28.Inouye SK, Leo-Summers L, Zhang Y, Bogardus ST, Jr, Leslie DL, Agostini JV. A chart-based method for identification of delirium: validation compared with interviewer ratings using the confusion assessment method. J Am Geriatr Soc. 2005;53:312–18. doi: 10.1111/j.1532-5415.2005.53120.x. [DOI] [PubMed] [Google Scholar]
- 29.Inouye SK, Kosar CM, Tommet D, et al. The CAM-S: development and validation of a new scoring system for delirium severity in 2 cohorts. Ann Intern Med. 2014;8:526–33. doi: 10.7326/M13-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glymour MM, Greenland S. Causal diagrams. In: Rothman KJ, Greenland S, Lash TL, editors. Modern Epidemiology. 3. New York: Wolters Kluwer, Lippincott Williams & Wilkins; 2008. pp. 183–209. [Google Scholar]
- 31.Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20:488–495. doi: 10.1097/EDE.0b013e3181a819a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karp KF, Rudy T, Weiner DK. Persistent Pain Biases Item Responses on the Geriatric Depression Scale (GDS): Preliminary Evidence for Validity of the GDS-Pain. Pain Med. 2008;9:33–43. doi: 10.1111/j.1526-4637.2007.00406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maclullich AM, Ferguson KJ, Miller T, de Rooij SE, Cunningham C. Unravelling the pathophysiology of delirium: a focus on the role of aberrant stress responses. J Psychosom Res. 2008;65:229–38. doi: 10.1016/j.jpsychores.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Institute for Health and Clinical Excellence. [Accessed January 6, 2014];Delirium: Diagnosis, Prevention and Management (Clinical Guideline 103) 2010 http://www.nice.org.uk/guidance/cg103/resources/guidance-delirium-pdf.